Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.4 Lisboa dez. 2014
CASE REPORT
Add insult to injury: An unusual cause of renovascular hypertension
Da lesão à disfunção: um caso invulgar de hipertensão renovascular
Ana Pimentel1, Ana Cabrita1, Teresa Jeronimo1, Augusto Ministro2, Idalecio Bernardo1, Pedro Leao Neves1
1 Nephrology Department, Centro Hospitalar do Algarve, Unidade de Faro. Faro, Portugal
2 Vascular Surgery Department, Centro Hospitalar Lisboa Norte, Hospital de Santa Maria. Lisboa, Portugal
ABSTRACT
Resistant hypertension is a clinical condition that needs an aetiological investigation with the purpose of establishing if there is a secondary cause that can be treated. The authors describe a rare cause of secondary hypertension. We report a case of a 40-year-old Caucasian man followed in an outpatient nephrology clinic, since January 2012, with renal insufficiency and hypertension known for a year. An initial aetiological study was performed, including an endocrinological study, which revealed a secondary hyperaldosteronism, a renal ultrasound that further revealed a diminished left kidney and a renal artery Doppler ultrasound that described a normal arterial blood flow. The patient was admitted in the nephrology department presenting malignant hypertension that included hypertensive retinopathy with retinal haemorrhage.
At the time, the patient initiated several convulsive crises and had to be admitted in the intensive care unit, needing invasive mechanical ventilation. To exclude renovascular disease causing the clinical scenario, a computed tomography angiography was performed, showing extensive thrombosis of the distal aorta involving at least the renal left artery. The immunologic study showed positive antiphospholipid antibodies, anticardiolipin antibodies and anti-beta2-glycoprotein 1 antibodies.
Key Words: Antiphospholipid antibodies; Ashersons syndrome; distal aorta thrombosis; renovascular hypertension; secondary hypertension.
RESUMO
A hipertensão arterial resistente e uma condição clinica que carece de investigação etiológica, cujo objectivo e identificar causas secundarias passiveis de serem corrigidas. Os autores descrevem uma apresentação rara de hipertensão secundaria. O caso consiste num doente que e seguido em consulta externa desde Janeiro de 2012 por insuficiência renal e hipertensão diagnosticadas ha um ano. Foi realizado um estudo etiológico no qual constou um estudo endocrinológico que demonstrou um hiperaldosteronismo secundário e uma ecografia renal que revelou um rim esquerdo de dimensões reduzidas mas com uma ecografia com Doppler sem alterações do fluxo sanguíneo ao nivel das artérias renais. Posteriormente o doente foi internado no serviço de nefrologia por hipertensão maligna com retinopatia hipertensiva com sinais de hemorragia ao nível da retina. O doente apresentou vários episódios de crises convulsivas tendo sido admitido na Unidade de Cuidados Intensivos onde foi entubado e ventilado. Para exclusão de causas de hipertensão renovascular foi realizado uma tomografia axial computorizada que revelou extenso trombo envolvendo a aorta distal e a artéria renal esquerda. O estudo imunológico realizado demonstrou positividade para os anticorpos anti-fosfolipido, anti-cardiolipina
Palavras‑chave: Hipertensão renovascular; hipertensão secundaria; síndrome antifosfolipido; síndrome de Asherson; trombose da aorta distal.
INTRODUCTION
Resistant hypertension is defined by the American Heart Association (AHA) as blood pressure that is not properly controlled with the use of three antihypertensive agents from different classes, with one of them being a diuretic, and all agents should be prescribed at optimal dose amounts1. The AHA definition of resistant hypertension fails to differentiate the true resistant hypertensive patients from the other forms of hypertension, such as apparent or pseudo-resistant hypertension2.
Resistant hypertension does not always imply secondary hypertension but secondary causes are common in these patients although the overall prevalence is unknown3. To avoid potential for harm of the invasive diagnosis procedures, for example, contrast-induced nephropathy, the evaluation of the secondary hypertension should be performed only in patients with high likelihood of benefiting from the procedure and those who will most probably benefit from its correction4.
The most common causes of secondary hypertension that have to be investigated on the hypertensive patients initial study and that often coexist in the resistant hypertensive patient5, are primary aldosteronism, renovascular disease, renal parenchymal disease and obstructive sleep apnoea6.
The patients evaluation shows several clinical clues that suggest the presence of secondary hypertension, such as severe resistant hypertension, an acute rise in blood pressure that was previously stable under medication and a younger onset hypertension without a documented family history. If the patient presents an abrupt onset of accelerated hypertension, asymmetric renal size, elevated serum creatinine and severe or refractory hypertension, the clinician should suspect renovascular disease4.
CASE REPORT
We report a case of a 40-year-old Caucasian male followed in an outpatient nephrology clinic, since January 2012, for renal insufficiency and hypertension diagnosed for over a year. The patients personal history included three episodes of anaphylactic shock caused by the intake of a fruit component still not identified and a moderate smoking habit.
The initial aetiological study began in the outpatient clinic with biochemical evaluation of the treatment-resistant hypertension that included a routine metabolic profile: haemoglobin (12.9g/dL), parathyroid hormone (88pg/mL), calcium (9.9mg/dL), phosphorus (3.6mg/dL), sodium (137mmol/L), potassium (4.45mmol/L), chloride (103mmol/L), bicarbonate (28mmol/L), fasting glucose (82mg/dL), blood urea nitrogen (41mg/dL), and creatinine (2.39mg/dL). Morning plasma aldosterone was 32 ng/dL (normal range 1 to 16 ng/dL) and plasma active renin was 371.8 pg/mL (normal range up to 27.8 pg/mL), tested without medication with angiotensin converting enzyme inhibitors, angiotensin II receptor blockers and/or beta-blockers.
Thyroid hormones and vanilmandelic acid were in the normal range, as were serum 17-ketosteroids and 17-hydroxicorticosteroids.
The urinalysis was normal. A renal ultrasound with arterial doppler revealed asymmetric renal size (left kidney measuring 80mm and right kidney 125mm), but without describing renal blood flow impairment.
The patient started to present other clinical signs, such as worsening asthenia with progressive debility and weight loss, loosing up to 5Kg in the last 5 months, and further investigation had to be performed. Four months after starting the investigation, his monthly mean serum creatinine levels were around 2.6mg/dL.
The immune assays performed were normal or negative for anti-nuclear antibodies (ANA), anti-double stranded DNA antibodies (Anti-dsDNA), anti-neutrophil cytoplasmic antibodies (ANCA), anti-hepatitis C virus (HCV), anti-hepatitis B virus (HBV) and human immunodeficiency virus (HIV) 1 and 2 antibodies. Anticardiolipin antibodies (aCL) IgG were 75.10 GPL/mL (normal range up to 15.0 GPL/mL) and IgM were 33.4 MPL/mL (normal range up to 15 MPL/mL) and the anti-beta2-glycoprotein1 (anti-b2GPI) antibodies IgG were normal but the IgM were 23.5 U/mL (normal range up to 15U/mL). Complement levels including CH50, C4, C1q were between normal values, though C3 was slightly lower 0.74g/L (normal range between 0.79 and 1.52g/L). Serum immunoglobulins were normal.
The patient was submitted to abdominal magnetic resonance imaging (MRI) that showed slight hyperplasia of the left adrenal gland and confirmed the diminished volume and lack of parenchymal differentiation of the left kidney (measuring 76mm).
In August 2012, despite being medicated with furosemide, diltiazem, clonidine, minoxidil and a beta-blocker, acceptable blood pressure control was not achieved. Deterioration of the renal function was observed when inhibitors of the angiotensin-converting enzyme were administered at the time.
The patient was then admitted to the nephrology unit with malignant hypertension that included hypertensive retinopathy with retinal haemorrhage causing visual impairment of the left eye. He had several convulsive crises and was transferred to the Intensive Care Unit (ICU) where he was intubated and mechanically ventilated for five days. Although the renal function continued to worsen (serum creatinine rose up to 4.39mg/dL), a computed tomography angiography (CTA) was deemed necessary, to exclude renal artery stenosis. The CTA revealed thrombosis of the distal aorta from the insertion of the mesenteric artery to the bifurcation of the primitive iliac arteries, with complete obliteration for a longitudinal extension of 12 centimetres, involving at least the left renal artery.
The right renal artery presented thrombosis in the initial segment but it maintained normal blood flow.
The external and internal iliac arteries were supplied by collateral blood vessels (Fig. 1).
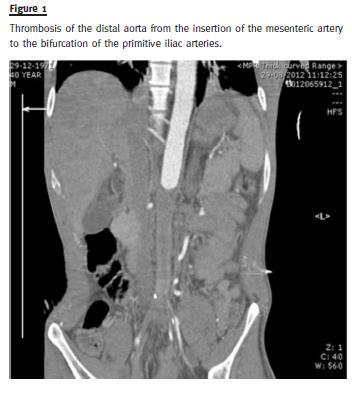
The patients blood pressure remained uncontrolled, reaching a maximum value of 210/90mmhg even with labetalol and dinitrate perfusions, clonidine, nifedipine, spironolactone and minoxidil given orally. While in the ICU, heparin perfusion was started and the patient was afterwards transferred to the Reference Vascular Surgery Department at the Hospital de Santa Maria, in Lisbon. He was submitted to thrombendarterectomy of the superior mesenteric artery and both of the renal arteries and arterial bypass from the visceral aorta to the left iliac artery and femoral artery.
He was discharged in September having a controlled blood pressure, medicated with a beta–blocker, a calcium channel blocker and oral anticoagulation.
The serum creatinine at discharge was 2.0mg/dL. The antiphospholipid antibodies tests were afterwards repeated when the patient had a clinical event and they confirmed that it was not a transitory elevation.
Anticardiolipin IgG and IgM antibodies remained elevated (60.5 GPL/mL and 69.6 MPL/mL, respectively) and the anti-b2GPI IgM antibodies had also high levels (26.5 U/mL), with normal IgG.
One year after the surgery the patient remained with controlled blood pressure.
DISCUSSION
The challenge, in this case was to find the secondary cause for the renovascular hypertension that was leading to the fast and progressive deterioration of the renal function and other end-organ signs. Its rare aetiology had great impact on its way of diagnosis and fast progressive course.
Factors that produce a false impression of resistance hypertension are common and should be excluded in patients that are initially identified with resistant hypertension7.
Several studies show that renovascular hypertension is found in about 20% to 50% of patients with acute, severe or resistant hypertension8 and in 1% to 5% of all cases of hypertension9.
In this patient, the first diagnostic approach revealed a small kidney and abnormal renal function suggesting a renovascular cause for the secondary hypertension.
There were several identifiable clinical findings that suggested increased likelihood for the hypertension to be partly secondary, such as the presence of resistant hypertension, an acute rise in blood pressure over a previously stable value, and a young onset hypertension with a negative family history4.
Renal artery stenosis (RAS) is the most common form of renovascular disease and it is known that more than 90% is from atherosclerotic origin10 leading to a reduction in the renal blood flow that can be identified by the increasing serum creatinine, as observed in our patient. In some cases, proteinuria or even nephrotic syndrome may be present4. The RAS can be designated as unilateral disease if the renal stenosis affects only one kidney and the other one retains normal function or bilateral if both kidneys are affected or a solitary kidney is affected.
In this particular case, an atherosclerotic origin wasexcluded as the primary cause of the renal artery obstruction due to the absence of several known cardiovascular risk factors, like age and diabetes, being a smoker the only risk factor identified10. It is known that the prevalence of atherosclerotic RAS rises with age and it clinically manifests as coronary (18%-20%), aortic or peripheral artery disease (35%-50%)11.
Testing for renovascular disease is associated with potential risks, particularly in patients with renal insufficiency. In our patient, the failure to control the blood pressure led to the further investigation to exclude renal artery stenosis, assuming that a corrective procedure would be considered if renovascular disease was confirmed12. The performance of diagnostic studies to identify clinically significant RAS is indicated in patients with the following characteristics: (a) accelerated hypertension with sudden and persistent worsening of previously controlled hypertension; (b) resistant hypertension; or (c) malignant hypertension (hypertension with coexistent evidence of acute end-organ damage, i.e., acute renal failure, acutely decompensated congestive heart failure, new visual or neurological disturbance, and/or advanced [grade III to IV] retinopathy)13.
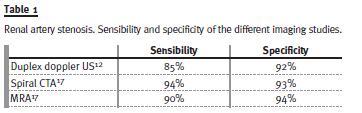
The gold standard imaging study to test for renal artery stenosis is the conventional renal angiography14, an invasive procedure. Duplex doppler ultrasonography (US) is the preferred initial non-invasive diagnostic test of renovascular disease15 and provided us the information about the initial renal size asymmetry, but not abnormal renal blood flow. Both CTA and magnetic resonance angiography (MRA) provide minimally invasive options for diagnostic evaluation and are most accurate for proximal disease, identifying anatomic abnormalities of the kidneys, aortic disease and stenosis12. The main limitations of these imaging techniques include the risk of contrast nephropathy with CTA and concerns regarding the potential for nephrogenic systemic fibrosis in patients with significant renal insufficiency16.
Table I shows the sensibility and specificity of the different imaging studies in the diagnosis of renal artery stenosis.
Other findings can lead more specifically to renovascular hypertension, like unexplained acute and sustained rise in serum creatinine of more than 30% after initiating a renin-angiotensin system inhibitor, moderate to severe hypertension in a patient with diffuse atherosclerosis, renal asymmetry, or recurrent episode of flash pulmonary oedema4, not present in this case.
Due to the potential high risk for cardiovascular events and high mortality rate it was promptly necessary to submit the patient to an invasive imaging study. A CTA was performed and it revealed thrombosis of the distal aorta involving at least the left renal artery. At this point, the low likelihood of atherosclerotic renal artery stenosis causing the thrombosis was reinforced. There were no previous records or exams that could confirm a known atherosclerotic disease, especially peripheral arterial disease10, so the investigation for the primary aetiology for the thrombosis had to be continued.
Immune assays indicated the presence of APS, which is an autoimmune prothrombotic disorder due to pathogenic autoantibodies directed against proteins that bind to phospholipids. The exact mechanism by which these autoantibodies inflict a prothrombotic tendency remains to be clarified18.
It leads to a global thrombotic predisposition and in this particular patient, the suspicion initially started due to the unique catastrophic clinical presentation.
Although the primary APS is not associated with other clinical or laboratory evidence of an associated clinical condition, multiple clinical presentations are possible due to the involvement of several vessels and multiple vascular occlusions.
At least one clinical (vascular thrombosis or pregnancy morbidity) and one laboratory (aCL, LA or anti-b2GPI antibodies) criteria have to be met for the classification of APS. In the general population, the prevalence range of antiphospholipid antibodies (aPL) is about 1% and 5%, but only a minority develops APS.
In addition to these APS classification criteria, the consensus paper provides specific definitions for commonly associated clinical manifestations of APS, such as livedo reticularis, cardiac valve disease, thrombocytopenia and nephropathy, present in 25% of primary APS19. In our patient, only nephropathy was present.
Definite antiphospholipid antibody syndrome is present if at least one clinical criterion and one of the laboratory criteria are met, with the first measurement of the laboratory test performed at least 12 weeks from the clinical manifestation20. In this case, the APS was diagnosed by the presence of considerable arterial thrombosis confirmed by imaging studies and the presence of aCL antibodies, the most common immunological abnormalities in the APS20. Because of the non-inflammatory occlusion, a broad spectrum of renal blood vessels can be affected, such as in this case, the aorta and renal artery.
This form of presentation of APS also meets the criteria for the Ashersons syndrome or the catastrophic antiphospholipid syndrome (CAPS), which consists of a catastrophic and potentially lethal form of the disease corresponding to 1% of all cases of APS21. The diagnosis criteria for preliminary classification for catastrophic APS were summed up in a statement consensus that was published in 200323.
The first criterion is the evidence of involvement of three or more organs, systems and/or tissues, usually clinical evidence of vessel occlusions, confirmed by imaging techniques when appropriate.
Renal involvement is defined by a 50% rise in serum creatinine, severe systemic hypertension (>180/100 mm Hg) and/or proteinuria (> 500 mg/24 h). The second criterion is the development of manifestations simultaneously or in less than a week. The third criterion is confirmation by histopathology of small vessel occlusion in at least one organ or tissue, or significant evidence of thrombosis, although vasculitis may coexist occasionally.
The fourth criterion involves a laboratory confirmation of the presence of antiphospholipid antibodies.
In our case, at least three of these criteria were fulfilled which suggests probable catastrophic APS.The patient had renal involvement without proteinuria and severe systemic hypertension, including hypertensive retinopathy with retinal haemorrhage. There was also evidence of vessel occlusions with significant thrombosis, confirmed on the CTA.
The most common manifestations of renal involvement in APS, as demonstrated in this case, are thrombosis or stenosis of renal artery, kidney infarction, thrombosis of the renal vein and end-stage renal disease/renal failure23, microvascular thrombosis being less common although it can potentially manifest itself as CAPS24. Histologically, the primary APS is classified mainly as a non-inflammatory occlusion of several blood vessels ranging from glomerular capillaries to the main renal artery and vein. The vascular events reflect the site and size of the involved vessels25.
Positive aCL antibodies at least 12 weeks before clinical manifestation were detected and confirmed after the thrombotic event. No biopsy was performed to confirm microthrombus due to the fast clinical deterioration and the evident imaging of the extensive aortic thrombus.
After the thrombendarterectomy, the patients blood pressure and kidney function improved. No surgical complications have been reported on the first year after surgery. Patients who require aortic reconstruction near the renal arteries, for example as the ones who have severe aortoiliac occlusive disease or aneurysm that need surgical repair, the preferred initial procedure is surgical revascularization, that seems to have a similar efficacy in controlling the blood pressure as the percutaneous transluminal renal angioplasty26. The treatment of arterial events in patients with APS continues to be a controversial subject and further investigation must be done27.
CONCLUSION
This case is a rare form of renovascular hypertension related to a primary antiphospholipid syndrome with catastrophic presentation.
Despite being a relatively common syndrome, APS can present itself as a life-threatening event that can be identified and treated if the differential diagnosis is done.
More prospective studies are necessary to establish the ideal treatment of the arterial thrombosis in the APS patient.
References
1. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008;51(6):1403-1419. [ Links ]
2. Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens 2014;28(8):463-468. [ Links ]
3. McAdam-Marx C, Ye X, Sung JC, Brixner DI, Khaler KH. Results of a retrospective, observational pilot study using electronic medical records to assess the prevalence and characteristics of patients with resistant hypertension in an ambulatory care setting. Clin Ther 2009;31(5):1116–1123. [ Links ]
4. Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens 2010;23(11):1159-1169. [ Links ]
5. Kumar N, Calhoun DA, Dudenbostel T. Management of patients with resistant hypertension: current treatment options. Integr Blood Press Control 2013;6:139-151. [ Links ]
6. Goodfriend TL, Calhoun DA. Resistant hypertension, obesity, sleep apnea, and aldosterone: theory and therapy. Hypertension 2004;43(3):518-524. [ Links ]
7. Sarafidis PA. Epidemiology of resistant hypertension. J Clin Hypertens (Greenwich) 2011;13(7):523-528. [ Links ]
8. Pedersen EB. New tools in diagnosing renal artery stenosis. Kidney Int 2000;57(6):2657-2677. [ Links ]
9. Derkx FH, Schalekamp MA. Renal artery stenosis and hypertension. Lancet 1994; 344(8917):237-239. [ Links ]
10. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med 2001;344(6):431–442. [ Links ]
11. De Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens 2009;27(7):1333–1340. [ Links ]
12. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association of Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113(11):e463-654. [ Links ]
13. Rooke TW, Hirsch AT, Misra S, et al. Management of Patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61(14):1555-1570. [ Links ]
14. Zucchelli PC. Hypertension and atherosclerotic renal artery stenosis: diagnostic approach. J Am Soc Nephrol 2002;13 Suppl 3:S184–S186. [ Links ]
15. Williams GJ, Mascaskill P, Chan SF, et al. Comparative accuracy of renal duplex sonographic parameters in the diagnosis of renal artery stenosis: paired and unpaired analysis. AJR Am J Roentgenol 2007;188(3):798-811. [ Links ]
16. Herrmann SMS and Textor SC. Diagnostic criteria for renovascular disease: where are we now? Nephrol Dial Transplant 2012;27(7):2657-2663. [ Links ]
17. Rountas C, Vlychou M, Vassiou K, et al. Imaging modalities for renal artery stenosis in suspected renovascular hypertension: prospective intraindividual comparison of color Doppler US, CT angiography, GD-enhanced MR angiography, and digital subtraction angiography. Ren Fail 2007;29(3):295-302. [ Links ]
18. Sikara PS, Eleftheria P, Vlachoyiannopoulos G, Vlachoyiannopoulos PG. Pathogenic Mechanisms of Thrombosis in Antiphospholipid Syndrome (APS). Tranquilli A (Ed), ISBN: 978-953-307-872-4. InTech, 2011; DOI: 10.5772/25522. [ Links ]
19. Petri M. Epidemiology of the antiphospholipid antibody syndrome. J Autoimmun 2000;15(2):145–151. [ Links ]
20. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholip syndrome (APS). J Thromb Haemost 2006;4(2):295-306. [ Links ]
21. Asherson RA, Cervera R, de Groot PG, et al for the Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrophic antiphosphospholipid syndrome: International consensus statement on classification criteria and treatment guidelines. Lupus 2003;12(7):530–534. [ Links ]
22. Cervera R, Piette JC, Font J, et al. Antiphospholipid syndrome. Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 2002;46(4):1019–1027. [ Links ]
23. Gigante A, Gasperini ML, Cianci R, et al. Antiphsopholipid antibodies and renal involvement. Am J Nephrol 2009;30(5):405-412. [ Links ]
24. Keeling D, Mackie I, Moore GW, et al. for the British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012;157(1):47-58. [ Links ]
25. Sciascia S, Cuadrado MJ, Khamashta M, Roccatello D. Renal involvement in antiphospholipid syndrome. Nat Rev Nephrol 2014;10(5):279-289. [ Links ]
26. Ronden RA, Houben AJ, Kessels AG, Stehouwer CD, de Leeuw PW, Kroon AA. Predictors of clinical outcome after stent placement in atherosclerotic renal artery stenosis: a systematic review and meta-analysis of prospective studies. J Hypertens 2010;28(12):23702377. [ Links ]
27. Cohen D, Berger SP, Steup-Beekman GM, Bloemenkamp KW, Bajema IM. Diagnosis and management of the antiphospholipid syndrome. BMJ 2010;340:c2541. [ Links ]
Dra Ana Pocinho Pimentel
Department of Nephrology, Centro Hospitalar do Algarve
Rua Leao Penedo
8000 Faro, Portugal
E-mail: anappimentel@gmail.com
Conflict of interest statement. None declared.
Received for publication: 21/08/2014
Accepted in revised form: 06/10/2014














