Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.27 no.4 Lisboa Aug. 2020
https://doi.org/10.1159/000504720
RESEARCH ARTICLE
Dieulafoy Lesion: Predictive Factors of Early Relapse and Long-Term Follow-Up
Lesão de Dieulafoy: factores preditivos de recidiva precoce e follow up a longo prazo
Paulo Massinhaa,b, Inês Cunhac, Luís Toméc,d
aGastroenterology Department, Garcia de Orta Hospital, E.P.E., Almada, Portugal; bUniversità Cattolica del Sacro Cuore, Rome, Italy; cGastroenterology Department, Coimbra Hospital and Universitary Centre, E.P.E., Coimbra, Portugal; dFaculty of Medicine of the University of Coimbra, Coimbra, Portugal
* Corresponding author.
ABSTRACT
Introduction: The Dieulafoy lesion (DL) is a rare cause of gastrointestinal bleeding. Advances in the endoscopy field have allowed an increased rate of detection and therapeutic efficacy. However, doubts remain about the most effective hemostatic approach, the affecting variables of therapeutic failure, and early relapse, as well as in the long-term followup. Aims: To assess the efficacy of endoscopic treatment of DL and to identify possible risk factors for early relapse and long-term results. Methods: All patients with DL admitted to a tertiary hospital between 01/01/2007 and 12/31/2018 were evaluated. The form of presentation, associated pathologies, chronic medication, therapeutic approach, and eventual relapse were determined. A telephone interview was conducted for all patients to find out the long-term results. Results: We identified 73 patients with DL, 45 (61.6%) males, with a mean age of 74 ± 15 years. Thirty-nine patients presented the DL in the stomach, 15 in the duodenum, 2 in the small bowel, 3 in the colon, and 11 in the rectum. The median number of endoscopic examinations required for diagnosis was 2. Median Rockall was 4 (range 2–7). After endoscopic treatment, in 95% of the cases, no active bleeding was evident. Only 2 patients required interventional radiology procedures and 1 needed surgery. Fourteen patients (19%) had a rebleeding, 12 during hospitalization and 2 after a median time of 51 months (range 1–117). There was no difference between the groups with and without early relapse in relation to age, gender, hemoglobin values at presentation, presence of shock, associated pathologies, and anticoagulation. Antiplatelet agents intake had a statistically significant relationship with early relapse (p = 0.003). Conclusion: Endoscopic therapy is safe and effective in DL. Patients under antiplatelet therapy are more likely to have an early relapse. The long-term prognosis is excellent, even in patients only treated with endoscopic methods.
Keywords: Dieulafoy lesion, Exulceratio simplex, Gastrointestinal bleeding, Endoscopy
RESUMO
Introdução: A lesão de Dieulafoy (LD) é uma causa pouco frequente de hemorragia digestiva. Os avanços na endoscopia permitiram um aumento na taxa de deteção e na eficácia terapêutica, contudo, permanecem dúvidas na abordagem hemostática mais eficaz, nas causas de falência terapêutica e de recidiva precoce, assim como no follow up a longo prazo. Objectivos: Avaliar a eficácia do tratamento endoscópico para a LD, identificar eventuais factores de risco para a recidiva precoce e os resultados a longo prazo. Métodos: Avaliaram-se todos os pacientes com LD, admitidos num hospital terciário, entre 01/01/2007 e 31/12/2018. Determinou-se a forma de apresentação, patologias associadas, medicação habitual, abordagem terapêutica e eventual recidiva. Uma entrevista telefónica foi realizada a todos os doentes para averiguar os resultados a longo prazo. Resultados: Identificaram- se 73 doentes com LD, 45 (61.6%) do sexo masculino, idade média no diagnóstico 74 ± 15 anos. Trinta e nove apresentavam a LD no estômago, 15 no duodeno, dois no delgado, três no cólon e 11 no recto. Foram necessarios um número mediano de 2 exames endoscópicos para diagnóstico. O Rockall médio, na hemorragia digestiva alta, foi de 4 (range 2–7). Em 95% dos casos não se verificou hemorragia activa após tratamento endoscópico. Apenas dois doentes necessitaram de radiologia de intervenção e um de cirurgia. 14 doentes (19%) apresentaram recidiva, 12 durante o internamento e dois num periodo de follow up mediano de 51 meses (range 1–117). Não houve diferença entre os grupos com e sem recidiva precoce em relação à idade, género, valores de hemoglobina à apresentação, presença de choque, patologias associadas e anticoagulação. A toma de antiagregantes teve uma relação estatisticamente significativa com a recidiva precoce (p = 0.003). Conclusão: A terapêutica endoscópica é segura e eficaz na LD. Pacientes antiagregados têm maior propabilidade de recidiva precoce. O prognóstico a longo prazo é excelente, mesmo nos pacientes apenas tratados por métodos endoscópicos.
Palavras-Chave: Lesão de Dieulafoy, Exulceratio simplex, Hemorragia gastrointestinal, Endoscopia
Introduction
Dieulafoy lesion (DL) is an unusually sizeable and tortuous artery that runs along the muscularis mucosae of the gastrointestinal tract, occurring mainly in the proximal stomach (Fig. 1). This protruding vessel can erupt at the lumen as a minor mucosal imperfection, although the pathogenic mechanisms are not yet completely understood [1].
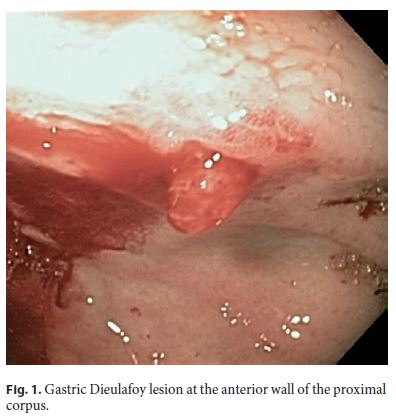
This injury is an uncommon but important cause of gastrointestinal hemorrhage (GIH), with an incidence of 2% for all causes of GIH, and up to 6% of nonvaricose bleeding in the upper gastrointestinal tract, but despite its dramatic presentation, mortality from DL is similar compared to other causes of GIH [2].
Although DL was first described by Gallard in 1884 [3], it was more precisely described 14 years later by the French surgeon, Georges Dieulafoy, reporting fatal GIH in three patients caused by sizeable, actively bleeding blood vessels in the stomach due to minor ulcers, which he named “exulceratio simplex,” as he mistakenly assumed these injuries were small peptic ulcers [4]. Since these first descriptions, several cases of DL have been described in the medical community. Before the endoscopy era, the mortality from bleeding due to DL was high, as the only existing treatment was surgical ligation of the aberrant vessel or subtotal (or total) gastrectomy. Thus, the progress of hemostatic endoscopic techniques offered a different and effective therapeutic approach, with a reduction in mortality rate to around 10% [5–8].
Despite the undeniable benefits of endoscopic treatment, little has been reported on the long-term follow-up of DL. In this study, we assessed the clinical features and long-term outcome in a large series of patients with DL who were managed in a tertiary center.
Methods
In this study, we retrospectively evaluated all patients admitted to a tertiary hospital, with gastrointestinal bleeding from a DL, between January 1, 2007 and December 31, 2018. The form of presentation, associated pathologies, usual medication, therapeutic approach, rebleeding, and mortality (related or unrelated to DL) were determined.
All patients routinely underwent emergency endoscopic evaluation, performed within 12 h of hospital admission, and a secondlook endoscopy was performed 24–48 h in selected patients. Some patients were admitted to a gastroenterological intensive care unit and transferred to a standard yard after a period of at least 48 h without bleeding since the last endoscopy.
When endoscopic therapy failed or was inappropriate, with persistent rebleeding or the presence of a nonbleeding large-caliber vessel, patients were referred for surgery or interventional radiologic intervention.
A telephone interview was conducted with all patients who survived the initial episode to find out the long-term results. A standard questionnaire was used for all patients, inquiring if any episode of bleed occurred after initial hospitalization and, in the positive cases, data of occurrence and hospital where the observation occurred.
Diagnosis of DL was established when endoscopy presented any active bleeding (spurting or oozing) or recent bleeding stigmata (visible vessel or adherent clot) from a minimal mucosal defect (< 3 mm) and normal surrounding mucosa.
Hemostatic failure was defined as sustainable active bleeding despite primary endoscopic management or any indication of active bleeding, such as hematemesis, hematochezia, or fresh blood aspirated from a nasogastric tube, or hemodynamic instability within 12 h of the first endoscopic hemostasis. Rebleeding was defined as the occurrence of other episodes of hematemesis or persistent melena, hemodynamic instability, or a reduction in hemoglobin concentration of a minimum of 2 g/dL, at least 1 day after therapy and diagnosed when the endoscopy showed bleeding from a previously treated DL. Early relapse was defined, when rebleeding occurred between the first 24 h and the first 5 days after the initial episode.
Statistical Analysis
Data were tested statistically by means of tests of normality. Descriptive data are presented as mean } standard deviation, median (range), or percentage, where appropriate. To compare characteristics between the groups with and without early relapse, we used the t test for normally distributed variables, the Mann-Whitney U test for skewed variables, and χ2 test for categorical data. When indicated, a binary logistic regression or a multivariate linear regression was applied. The log-rank test was used to compare the survival distributions. All analyses were performed using SPSS 20.0 software (SPSS, Chicago, IL, USA). All reported p values were two-tailed, and p values of less than 0.05 were considered statistically significant.
Results
Patients Characteristics and Bleeding Episode
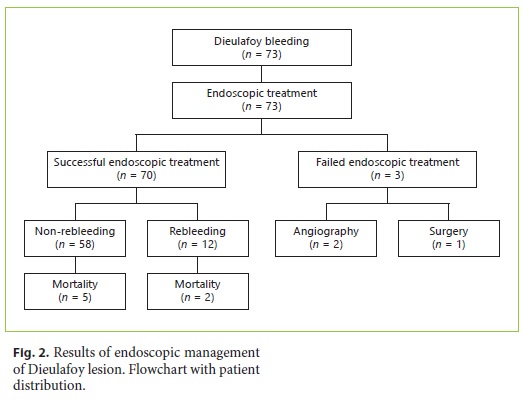
During the study period, from a total of 9,125 gastrointestinal bleeding episodes, we identified 73 patients with DL, 45 males (61.6%), with a median age at diagnosis of 74 years (range 25–94) (Fig. 2).
Almost all patients presented with acute GIH, except one patient who underwent endoscopy due to anemia. The most frequent form of presentation was melena in 26 patients (35.6%), followed by hematemesis in 21 patients (28.7%). Eleven patients (15%) presented with hematemesis and melena and in 14 patients (19.1%), isolated hematochezia occurred. In 42 (58%), active bleeding was evident at the time of the diagnosis: 14 had spurting and 28 had an oozing hemorrhage. The remaining patients had a nonbleeding visible vessel or an adherent cloth. Patients characteristics are summarized in Table 1.
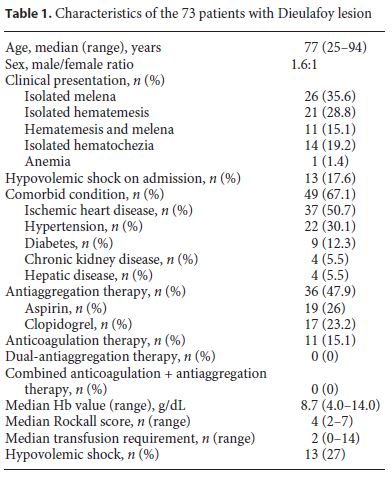
At presentation, the median hemoglobin value was 8.7 g/dL (range 4.0–14.0), with a median transfusion requirement of 2 units of red blood cells (range 0–14). Regarding hemodynamic status, 13 patients were in hypovolemic shock, with good response to fluid therapy and transfusion. Median Rockall score of patients included in our cohort was 4 (range 2–7), corresponding to intermediate risk (4.2 in patients who died and 3.9 in the survivors).
Significant comorbidity was present in 67% of the patients, most commonly ischemic heart disease (76%), hypertension (45%), diabetes (19%), chronic kidney disease (8%), and hepatic disease (6%). From all patients, 47 were taking medications that interfere with coagulation: 36 (49.3%) were under platelet agent and 11 (15%) under anticoagulants.
Endoscopy Data
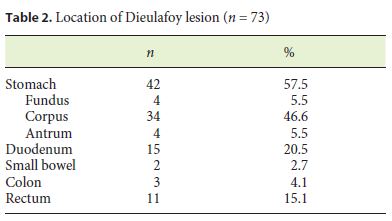
The locations of the bleeding lesion were as follows: 42 with DL in the stomach (4 occurring in the fundus, 34 in the body, and 4 in the antrum), 15 in the duodenum, 2 in the small intestine, 3 in the colon, and 11 in the rectum (Table 2).
Notwithstanding the fact that in 84% of the patients, the diagnosis of DL was made in the emergency department, the median number of endoscopic examinations required for a sure diagnosis was 2 endoscopies (range 1–6). Endoscopic therapy was successful in achieving initial hemostasis in 96% (n = 70) of the cases.
After an initial unsuccessful attempt of endoscopic resolution, two patients required interventional radiology and one patient required surgery, all with clinical success. Two of these patients had a DL in gastric body, and one in proximal jejunum. None of them was under anticoagulation agents, and only one was medicated with an antiplatelet agent (75 mg of clopidogrel i.d.). In all patients, an initial endoscopic treatment with adrenalin 1: 10,000, absolute alcohol, and hemoclip was tried.
The initial endoscopic hemostatic method used to control bleeding was in 44 patients a combination of adrenaline 1: 10,000 and hemoclips, in 14 patients adrenaline 1: 10,000 and absolute alcohol were used , in 9 triple therapy with adrenaline 1: 10,000, absolute alcohol, and hemoclips, argon plasma was used in three patients, and hemoclip alone was used in one patient (Table 3).
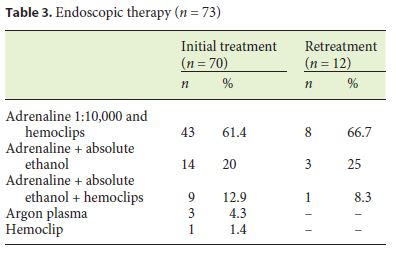
Follow-Up after Initial Bleeding Episode
From the 70 patients with initial successful endoscopy therapy, 14 patients (20%) had rebleeding, 12 an early rebleeding during the initial hospitalization and two in an median follow-up period of 51 months (range 1–127). All underwent emergency endoscopy and retreatment with effective endoscopic hemostasis.
The median time to discharge was 6 days (range 3–44) and the 30-day mortality was 9.7% (7 patients), all occurring during hospitalization. From the seven patient who died, 5 had DL in gastric corpus, one in duodenum, and one in rectum. After application of χ2 test, no statistical difference between the location of DL and mortality was found. Causes of death included acute myocardial infarction (2), cardiopulmonary failure (2), pneumonia (1), stroke (1), and pulmonary thromboembolism (1).
Long-Term Results
From the 65 surviving patients, it was possible to contact 53 of them through a phone interview. Median follow- up was 51 months (range 1–117). Only 2 patients had relapse of the same DL, and a successful endoscopy approach was possible.
Predictive Factors of Early Relapse
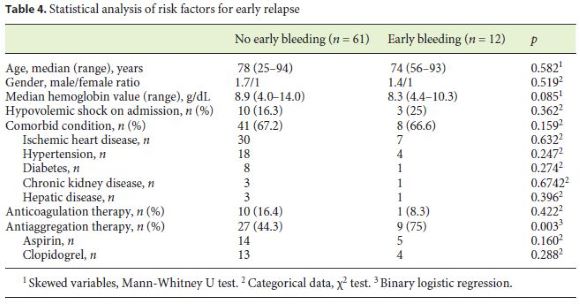
After statistical analysis, we found no difference between the groups with and without early relapse in relation to age, gender, hemoglobin values at presentation, presence of shock, associated pathologies, and anticoagulation. The antiplatelet aggregation had a statistically significant relationship with early relapse (p = 0.003). No differences were found between the different types of antiplatelet agents. (Table 4).
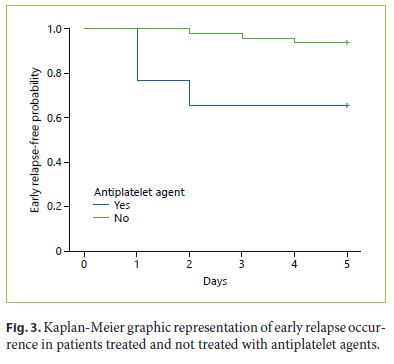
The mean time to event was 1.6 ±} 0.9 days (1.2 ±} 0.4 days in patients with antiplatelet therapy and 3 ±} 1 days in patients without antiplatelet therapy). After analysis of Kaplan-Meier distribution (Fig. 3) and application of logrank test, we observed a statistically significant difference between the curves of the early relapse patients, treated and not treated with antiplatelet agents, where the patients under antiplatelet treatments showed an early occurrence of early relapse (p = 0.001).
Discussion
Several series have shown that DL is apparently an underdiagnosed rather than a rare condition [7–10]. It is the cause of 0.3–12% of acute GI bleeding [9]. In our study, the DL was responsible for 0.8% of acute GIH. Epidemiologically, the patients of our study are in consensus among those of other publications. In the study by Jamanca-Poma et al. [10], with 39 cases, a preponderance in men was reported, with a ratio of 3: 1, compared with our 1.6: 1 ratio. Our patients were older compared with the majority of studies [9–11]. Nevertheless, every series corroborates the wide-ranging age distribution, with patient age covering the second or third decade to the tenth decade.
In our study, 36 (49%) of the patients were medicated with antiplatelet agents including aspirin, similar to what was described by Shin et al. [11], where among 42 patients, 19 (45%) were under the effects of antiplatelet agents. This high value is not surprising due to the high median age of our study population, many with cardiovascular disease. Despite not yet enlightened, some studies defend that nonsteroidal anti-inflammatory drug inducing gastritis with subsequent vessel wall erosion has a role in the pathogenesis of the DL rupture [12]. In our study, 17 patients were medicated with aspirin, and only antiplatelet therapy was a statistically relevant factor in early relapse. No differences were found between the different types of antiplatelet agents.
Despite endoscopy unquestionably being the first-line method to diagnose DL, its sensitivity is relatively low, around 82% [13]. In our series, 84% were identified at initial endoscopy; notwithstanding this, a median 2 endoscopies (range 1–6) were necessary to a conclusive diagnostic. In a non-conclusive endoscopy, in an intestinal bleeding patient, DL should always be part of the diagnostic differential. Due to the intermittent type of bleeding from the DL, the sensitivity of endoscopic diagnosis is probable to be improved throughout an early endoscopy in acute GI bleeding.
Although several studies reported a small proportion of extragastric DL [12, 14], this location of DL occurred in 43% of our patients. This could be explained by the fact that these are often classified as ulcer disease in the duodenum or vascular malformation in the duodenum or other sites.
In our series, endoscopic management was highly effective, with primary hemostasis accomplished in 96%, with no complications related to the procedure. This was slightly higher than the rate of primary hemostasis reported by Lim et al. [15]. Despite the fact that in the majority of our patients, a combination of adrenaline 1: 10,000 and hemoclips was the chosen method, sclerotheraphy and argon plasma are alternative procedures. Many series have portrayed effective hemostasis applying a variety of endoscopic modalities, such as injection with cyanoacrylate glue, thermal ablation with a heat probe, monopolar or bipolar probe, and band ligation. The most experienced and described procedure is with sclerosant injection and combination of adrenaline 1: 10,000 and hemoclips [12–18]. In a meta-analysis by Barakat et al. [19], who aim to assess and compare the efficacy of endoscopic band ligation and endoscopic hemoclip placement in achieving primary hemostasis of actively bleeding DL and their rates of rebleeding, the two procedures were effective, achieving primary hemostasis in 91–96% and with few cases of rebleeding (6–17%). No statistical significance between the two techniques was found [19].
Despite the initial success, 17% of patients had a relapse in hospitalization period following endoscopic treatment. Nevertheless, a second endoscopic treatment was enough to control bleeding. Our results are comparable to the rates of early relapse in other series, as 17.9% in the series by Lim et al. [15], 11% in the study by Shin et al. [11], and 22% in the cohort of Jamanca-Poma et al. [2]. In our study, only one patient (1.4%) required surgery. This low value was consistent with several recent studies in which the rate of surgical intervention was between 2 and 6% [2, 11, 15]. This fact could be justified by the two patients who underwent angiography embolization, which successfully controlled bleeding.
Some series have shown angiography as a successful method to control bleeding. Park et al. [16] described one successful angiographic embolization in one patient with failed initial hemostatic endoscopy, and in the study by Lim et al. [15], 3 patients were successfully treated without complications. This method can be especially valuable once endoscopic therapy has been unsuccessful and the patient is a poor candidate for surgery.
In our study, the mortality rate was 9.7%, a value that is in line with other series [17]. Median Rockall score was 4 (range 2–7), a value that corresponds to an intermediate risk. Curiously, the Rockall score was similar in patients who died and survivors, suggesting that this score may not be able to predict mortality in this particular situation. The fact that the more unstable and ill patients were admitted in a gastroenterological intensive care unit may have some influence.
There are a few large series of patients with prolonged follow-up. In our study, in a median follow-up time of 51 months (range 1–117), only 2 patients suffered a relapse of bleeding from a DL after discharge, which is similar to what is described in other studies [2, 11, 13, 15].
After statistical analysis, there was no difference between the groups with and without early relapse in relation to age, gender, hemoglobin values at presentation, presence of shock, associated pathologies, and anticoagulation. Nonetheless, we found that antiplatelet agents had a statistically significant relationship with early relapse (p = 0.003). This fact is particularly important due to a rather high mean age of patients with DL, with important comorbidities, and a high percentage being under antiplatelet agents effect. Therefore, antiplatelet therapy should be a decisive factor for a second-look endoscopy decision.
We admit some limitations to our study. The retrospective design, with medical records that are not designed for research, could lead to obvious difficulties. The drugs and methods of hemostatic therapy were selected by the inclinations of the clinical physicians; nonetheless, we consider this did not manipulate the results of the study. Also, the fact that 12 patients lost long-term follow-up adds some restraints in evaluation of long-term rebleeding. Notwithstanding these limitations, this study remains critical for validating the efficacy of endoscopic treatment for DL and identifying the associated factors with early rebleeding.
In summary, DL appears to account for approximately 1% of acute GIH. Endoscopic therapy is effective and well tolerated and is of long-term benefit. Antiplatelet therapy is a risk factor for early relapse. No patients in our series have bled from more than one DL. Thirty-day and subsequent mortality following hemorrhage from a DL is high but relates almost exclusively to the patient’s comorbid status.
References
1 Nojkov B, Cappell MS. Gastrointestinal bleeding from Dieulafoy’s lesion: clinical presentation, endoscopic findings, and endoscopic therapy. World J Gastrointest Endosc. 2015 Apr;7(4):295–307.
2 Jamanca-Poma Y, Velasco-Guardado A, Piñero-Pérez C, Calderón-Begazo R, Umaña-Mejía J, Geijo-Martínez F, et al. Prognostic factors for recurrence of gastrointestinal bleeding due to Dieulafoy’s lesion. World J Gastroenterol. 2012 Oct;18(40):5734–8.
3 Gallard T. Anéurysmesmiliaires de l’estomac donnant lieu des hematemesis mortalles. Bull Mem Soc Med Hop Paris. 1884;1:84–91.
4 Dieulafoy G. Exulceratio simplex. Bull Acad Med. 1898;39:49–84.
5 Lara LF, Sreenarasimhaiah J, Tang SJ, Afonso BB, Rockey DC. Dieulafoy lesions of the GI tract: localization and therapeutic outcomes. Dig Dis Sci. 2010 Dec;55(12):3436–41.
6 Saleh R, Lucerna A, Espinosa J, Scali V. Dieulafoy lesion: the little known sleeping giant of gastrointestinal bleeds. Am J Emerg Med. 2016 Dec;34(12):2464.e3–5.
7 Kanth R, Mali P, Roy PK. Outcomes in Dieulafoy’s lesion: a 10-year clinical review. Dig Dis Sci. 2015 Jul;60(7):2097–103.
8 Chung IK, Kim EJ, Lee MS, Kim HS, Park SH, Lee MH, et al. Bleeding Dieulafoy’s lesions and the choice of endoscopic method: comparing the hemostatic efficacy of mechanical and injection methods. Gastrointest Endosc. 2000 Dec;52(6):721–4.
9 Karaahmet F, Kılıncalp S, Coskun Y, Hamamci M, Akinci H, Ustun Y, et al. The efficiency of endoclips in maintaining the gastrointestinal bleeding-related Dieulafoy’s lesion. Wien Klin Wochenschr. 2016 Oct;128(19-20):700–5.
10 Jamanca-Poma Y, Velasco-Guardado A, Piñero-Pérez C, Calderón-Begazo R, Umaña-Mejía J, Geijo-Martínez F, et al. Prognostic factors for recurrence of gastrointestinal bleeding due to Dieulafoy’s lesion. World J Gastroenterol. 2012 Oct;18(40):5734–8.
11 Shin HJ, Ju JS, Kim KD, Kim SW, Kang SH, Kang SH, et al. Risk factors for Dieulafoy lesion in the upper gastrointestinal tract. Clin Endosc. 2015 May;48(3):228–33.
12 Inayat F, Ullah W, Hussain Q, Hurairah A. Dieulafoy’s lesion of the oesophagus: a case series and literature review. BMJ Case Rep. 2017 Jan;2017:bcr2016218100.
13 Romãozinho JM, Pontes JM, Lérias C, Ferreira M, Freitas D. Dieulafoy’s lesion: management and long-term outcome. Endoscopy. 2004 May;36(5):416–20.
14 Inayat F, Ullah W, Hussain Q, et al. Dieulafoy’s lesion of the colon and rectum: a case series and literature review. BMJ Case Rep. 2017 Oct 25;2017.
15 Lim W, Kim TO, Park SB, Rhee HR, Park JH, Bae JH, et al. Endoscopic treatment of dieulafoy lesions and risk factors for rebleeding. Korean J Intern Med (Korean Assoc Intern Med). 2009 Dec;24(4):318–22.
16 Park SH, Lee DH, Park CH, Jeon J, Lee HJ, Lim SU, et al. Predictors of rebleeding in upper gastrointestinal Dieulafoy lesion. Clin Endosc. 2015 Sep;48(5):385–91.
17 Baxter M, Aly EH. Dieulafoy’s lesion: current trends in diagnosis and management. Ann R Coll Surg Engl. 2010 Oct;92(7):548–54.
18 Jiang Y, Hu J, Li P, Jiang W, Liang W, Wei H. A Retrospective Analysis of Cyanoacrylate Injection versus Hemoclip Placement for Bleeding Dieulafoy’s Lesion in Duodenum. Gastroenterol Res Pract. 2018 Mar;2018:3208690.
19 Barakat M, Hamed A, Shady A, Homsi M, Eskaros S. Endoscopic band ligation versus endoscopic hemoclip placement for Dieulafoy’s lesion: a meta-analysis. Eur J Gastroenterol Hepatol. 2018 Sep;30(9):995–6.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Dr. Paulo Massinha
Garcia de Orta Hospital E.P.E.
Av. Torrado da Silva
PT–2805-267 Almada (Portugal)
E-Mail paulomassinha@icloud.com
Received: July 9, 2019; Accepted after revision: October 18, 2019
Author Contributions
All authors participated in the conception, writing and revision of the manuscript. The final version was approved by the authors.














