Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.6 Lisboa dez. 2019
https://doi.org/10.1159/000497248
CLINICAL CASE STUDY
Long-Term Gastrocolocutaneous Fistula after Endoscopic Gastrostomy: How Concerned Should We Be?
Fístula gastrocolocutânea crónica após gastrostomia endoscópica: Que gravidade?
Gonçalo Nunesa, Gabriel Paiva de Oliveirab, João Cruzc, Carla Adriana Santosa, Jorge Fonsecad
aGastroenterology Department, Hospital Garcia de Orta, Almada, Portugal; bSurgery Department, Hospital Garcia de Orta, Almada, Portugal; cRadiology Department, Hospital Garcia de Orta, Almada, Portugal; dCiiEM – Centro de Investigação Interdisciplinar Egas Moniz, Monte da Caparica, Portugal
* Corresponding author.
ABSTRACT
Percutaneous endoscopic gastrostomy (PEG) is a safe technique for long-term enteral feeding. The most common PEGassociated adverse events are minor. Gastrocolocutaneous fistula (GCCF) results from misplacement of the PEG tube through the colon. The importance of this complication is not currently defined, and there is no clearly established therapeutic algorithm. The authors report a series of 3 cases of GCCF diagnosed and treated in a tertiary center. Case 1: An 88-year-old man underwent PEG due to head and neck cancer. The procedure was uneventful, and the patient remained asymptomatic. After the first PEG tube substitution performed at 6 months, stool drainage through the stoma was observed. Computed tomography (CT) showed a GCCF. After tube removal, the fistula spontaneously closed, and the patient remained under nasogastric feeding until death. Case 2: A 31-year-old man with hereditary spastic paraplegia was submitted to PEG without early complications. The patient remained asymptomatic, and 7 months later, replacement of the PEG tube was planned. Under endoscopic control, the primary tube was removed, but the balloon replacement tube, introduced through the skin, was not observed in the gastric lumen. CT displayed a GCCF that spontaneously closed after a few days. A combined laparoscopic and endoscopic approach was used to resect the fistula tracts and perform a new gastrostomy. Case 3: A 45-year-old man with cerebral palsy was referred to PEG. Skin transillumination was only observed transiently, and the abdominal puncture was performed obliquely. The patient remained asymptomatic until the 7th month, when the primary PEG tube replacement was performed. The percutaneously placed substitution tube did not reach the stomach. GCCF was evident on CT. The fistula spontaneously closed, and the patient was referred to elective surgery for laparoscopic gastrostomy. GCCF is an uncommon complication of PEG. Its clinical course seems to be benign with patients remaining asymptomatic under ambulatory enteral feeding for long periods until PEG tube replacement. Spontaneous fistula closure is the rule in this setting. Laparoscopic gastrostomy should be considered when a new PEG is advised and cannot be safely performed due to colon interposition.
Keywords: Gastrocolocutaneous fistula, Endoscopic gastrostomy, PEG
RESUMO
A gastrostomia endoscópica percutânea (PEG) é uma técnica segura, utilizada como acesso para nutrição entérica de longa duração. A maioria dos eventos adversos mais frequentemente associados a esta técnica endoscópica são geralmente de gravidade ligeira. A fístula gastrocolocutânea (FGCC) resulta da transfixação cólica durante o procedimento de PEG. A importância desta complicação não está atualmente definida e nenhum algoritmo terapêutico está validado. Os autores relatam uma série de casos de FGCC diagnosticados e tratados num centro hospitalar terciário. Caso 1: Homem de 88 anos, submetido a PEG por cancro cervicofacial. O procedimento decorreu sem intercorrências e o doente permaneceu assintomático durante o follow-up. Após a primeira substituição do tubo de PEG realizada aos seis meses, constatou-se drenagem de conteúdo fecal pelo estoma. A tomografia computorizada (TC) mostrou uma FGCC. Após a remoção do tubo a fístula encerrou espontaneamente e o doente permaneceu sob nutrição entérica por sonda nasogástrica até ao óbito. Caso 2: Homem de 31 anos com Paraplegia Espástica Hereditária, submetido a PEG sem intercorrências imediatas. O doente permaneceu assintomático e sete meses depois foi realizada substituição do tubo primário de PEG. Sob controlo endoscópico, o tubo inicial foi removido mas o tubo secundário com balão, introduzido através da pele, não foi identificado no lúmen gástrico. A TC mostrou uma FGCC que encerrou espontaneamente após alguns dias. Uma abordagem laparoscópica e endoscópica combinada foi posteriormente utilizada para ressecar os trajetos fistulosos e realizar uma nova gastrostomia. Caso 3: Homem de 45 anos com Paralisia Cerebral, referenciado para PEG. Durante o procedimento a transiluminação da parede abdominal apenas foi observada transitoriamente e a punção realizada com orientação oblíqua. O doente permaneceu assintomático até ao sétimo mês, altura em que foi realizada substituição do tubo primário. O tubo de substituição inserido percutaneamente não atingiu o estômago. Uma FGCC foi observada na TC. A fístula encerrou espontaneamente e o doente foi referenciado para gastrostomia laparoscópica eletiva. A FGCC é uma complicação invulgar da PEG. O seu curso clínico aparenta ser benigno e os doentes permanecem assintomáticos sob nutrição entérica domiciliária por longos períodos até à substituição do tubo primário. O seu encerramento espontâneo é a regra. A gastrostomia laparoscópica deve ser considerada quando uma nova PEG está recomendada e não pode ser efetuada com segurança por interposição cólica.
Palavras-Chave: Fístula gastrocolocutânea, Gastrostomia endoscópica, PEG
Introduction
Percutaneous endoscopic gastrostomy (PEG) is a safe and well-tolerated method for long-term enteral feeding. It is recommended when a period of inadequate oral intake exceeding 3–4 weeks is expected [1, 2]. The main indication for PEG is persistent dysphagia caused mainly by neurologic disorders and head or neck cancer, both increasing in western countries [3].
Survival after PEG is usually dependent on the patient’s underlying disorders and baseline nutritional status. Actually, major adverse events associated with this technique are uncommon. However, massive bleeding, internal organ injury, aspiration pneumonia, necrotizing fasciitis, buried bumper syndrome, and stoma cancer seeding are potentially life-threatening complications, which may impair patients’ clinical outcome [3]. On the other hand, minor complications, including local wound infection, granuloma formation, peristomal leakage, and tube dislodgement, are much more frequent and usually promptly managed by trained staff [3].
Gastrocolocutaneous fistula (GCCF) is a rare complication of PEG with an estimated prevalence of 0.5–3% [4]. It is caused by incidental colon transfixation at the time of the initial PEG tube insertion due to colon interposition between the stomach and the abdominal wall. Gastric hyperinflation during the procedure, postoperative adhesions associated with previous abdominal surgery, and spine deformation are important risk factors [5]. Although GCCF may present as an acute event with peritonitis or a significant pneumoperitoneum developing immediately after the procedure, the absence of clinical manifestations is the rule until the fistula maturates and the internal bumper remains in the gastric lumen. When the PEG tube migrates through the fistula or, more commonly, is replaced by secondary balloon tubes, symptoms such as diarrhea after food administration, fecaloid vomiting, and stool drainage through the tube may arise [5]. Nevertheless, management of GCCF is not clearly de fined in the current literature as most data come from a few anecdotal reports [4–14].
The present article reviews 3 cases of GCCF developed in a tertiary hospital with a high number of PEGs performed every year. The authors aim to describe the indolent course of GCCF and report their good experience managing this uncommon complication.
Case 1
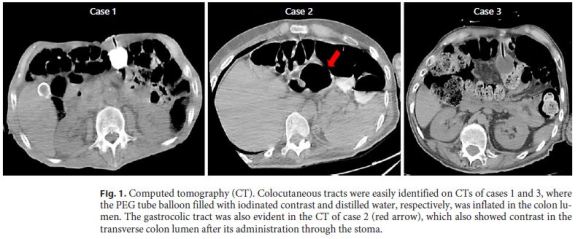
An 88-year-old man was referred to the artificial nutrition outpatient clinic in March 2007 for PEG. The patient was bedridden due to severe dyskinesia caused by Parkinson disease and was further diagnosed with an advanced larynx neoplasia causing airway obstruction and dysphagia, being not suitable for surgical resection. A tracheostomy was performed before PEG. The gastrostomy procedure was uneventful, and ambulatory enteral nutrition was maintained for several weeks. The patient remained asymptomatic and improved nutritional status during PEG feeding. Six months later, tube replacement was scheduled due to tube deterioration. In upper gastrointestinal endoscopy, the internal bumper was correctly positioned in the gastric body. It was looped using a polypectomy snare and removed by mouth according to the standard protocol of our center for replacing the initial PEG tube. A replacement tube with a distal inflation balloon was easily introduced through the stoma without resistance. No endoscopic control of the replacing tube was performed. One day later, the patient was admitted due to extravasation of fecaloid content through the tube. Abdominal computed tomography (CT) showed the PEG tube located in the colon and an iatrogenic colocutaneous fistula (Fig. 1). During hospital admission, parenteral nutrition was performed and stool drainage through the skin spontaneously resolved. Since there was no intraperitoneal leakage and the gastrocolic tract was not identified in the control CT, the patient was discharged under nasogastric feeding. A few weeks later, the skin orifice had completely closed without additional intervention. The risk of a new incidental colonic puncture caused by the complete colon interposition between the abdominal wall and the stomach led the team to avoid a new PEG. Given the patient’s anesthetic risk and his short life expectancy, a surgical gastrostomy was not performed. The patient remained using a long-term nasogastric tube until death, which occurred 1 month after hospital discharge.
Case 2
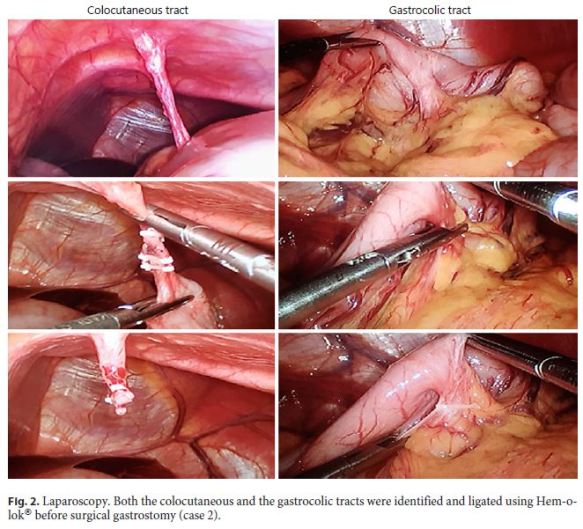
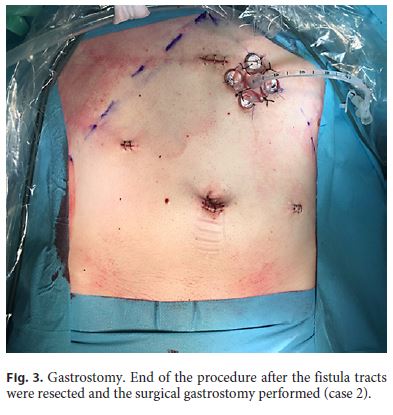
A 31-year-old man with hereditary spastic paraplegia was referred to the artificial nutrition outpatient clinic in July 2017 due to prolonged dysphagia and need of a nasogastric tube for nutritional support. A PEG was scheduled for long-term enteral nutrition. During the procedure, abdominal transillumination was easily obtained and the PEG tube could be placed without immediate complications. The patient was followed in the ambulatory, remaining asymptomatic for several months. Seven months later, PEG tube deterioration was evident, and the replacement was planned. Endoscopy confirmed the intragastric position of the internal bumper, which was removed. Under endoscopic control, the replacement tube was easily inserted through the stoma; however, it did not reach the gastric lumen. Gastric cannulation was impossible even after using a rigid guidewire, and, therefore, the procedure was postponed and the patient admitted for investigation. CT showed complete interposition of the transverse colon between the stomach and the abdominal wall. After diluted loperamide contrast (Ultravist® 370, Bayer) administration through the stoma, colon opacification was observed without intraperitoneal extravasation. The gastrocolic tract was also identified (Fig. 1). The diagnosis of GCCF was confirmed. Since leakage through the skin was not observed as the orifice partially closed, the patient was immediately discharged under nasogastric feeding, being referred for surgical gastrostomy. After observing the patient and the CT images, the surgical team proposed a minimally invasive laparoscopic ap proach. The colocutaneous and gastrocolic tracts were easily identified and ligated using Hem-o-lok® (Fig. 2). The colocutaneous tract was resected and the cutaneous end excised from the outside. To ensure that the new gastrostomy was placed far from the gastric end of the gastrocolic tract, a percutaneous combined assisted laparoscopic and endoscopic gastrostomy was performed using the push method (Ballard introducer Kit Mic Key 20F) with gastropexy (Fig. 3). The patient was discharged after resuming enteral nutrition and maintained follow-up without additional complications.
Case 3
A 45-year-old man with cerebral palsy was referred to the artificial nutrition outpatient clinic in January 2018 for PEG due to long-term dysphagia and nasogastric tube feeding. Although the patient presented a normal body mass index, abdominal transillumination was quite difficult to obtain, only being transiently observed below the xiphoid process. Gastric access was achieved, and the PEG tube could be placed after abdominal puncture, performed not completely perpendicular to the skin. Feeding tube transparietal thickness assessed at the end of the procedure was 5.5 cm. The patient remained asymptomatic under PEG feeding for several months. Seven months later, PEG tube replacement was proposed due to deterioration. Endoscopy showed the internal bumper in the stomach, which was removed. Under endoscopic control, the replacement tube inserted through the stoma did not reach the gastric lumen. Some stool vestiges could be observed in the tube at this time. CT showed the balloon inflated in the colon, even without contrast injection, and transverse colon interposition between the stomach and the abdominal wall (Fig. 1). The diagnosis of GCCF was assumed, and the patient was admitted to the gastroenterology ward for surveillance. After PEG tube removal, the skin orifice was partly closed avoiding stool drainage. The patient was further discharged under nasogastric feeding, being referred for surgical gastrostomy. A laparoscopic approach was selected by the surgical team. During the procedure, the colocutaneous and gastrocolic tracts were ligated and easily resected. Laparoscopic gastrostomy was performed using the push method with gastropexy. Gastrostomy feeding was resumed and nutritional follow-up maintained.
Discussion
Since its introduction in clinical practice, PEG has become a widespread endoscopic procedure performed when long-term enteral feeding is recommended, saving patients from the discomfort and risks of the chronic use of nasogastric tubes. It can be quickly performed in ambulatory patients under conscious sedation through a minimally invasive approach with a success rate over 95% [6].
Iatrogenic GCCF after PEG is considered an uncommon condition with scarce literature data. Among more than 1,000 PEGs performed by our artificial nutrition team in the last 15 years, only 3 cases were detected. Even considering that a large number of patients died a few months after the gastrostomy procedure and some few GCCF patients may be undiagnosed, the prevalence of this complication remains very low. Although a standard management protocol is not currently established, most patients seem to present a benign clinical evolution with the fistula closing spontaneously after PEG tube removal [4, 5]. Some authors suggest the possibility of endoscopic treatment when the fistula does not spontaneously close within several weeks. It may be a valid approach if aiming to accelerate closure, especially when large orifices with significant drainage causing skin damage are present and/or the patient is malnourished and exhibits deficient wound healing [4]. Clipping the colonic and/or the gastric side of the fistula is a possible intervention with favorable results, especially when the modern overthe-scope clips are applied [4, 9]. Fistula plugs may also be an attractive approach; however, its specific application in PEG patients with GCCF has not been described. Anecdotal reports of fistula closure using other devices, such as those used to manage congenital cardiac shunts, have also been reported [15]. Urgent surgery is only required when intraabdominal leakage is present causing peritonitis [4].
The 3 cases of GCCF reported by our team support the favorable outcome of this complication even when no specific intervention was performed. In all cases, nutritional status could be improved after PEG as patients remained asymptomatic under ambulatory enteral feeding for long periods and GCCF diagnosis was only performed at the time of the first tube replacement. Actually, blind cannulation of the gastrocolic tract through the skin after primary PEG tube removal is extremely unlikely. After the diagnosis, in all patients, the colocutaneous fistula tract spontaneously closed after removal of the PEG tube. Also, the gastrocolic tract seemed to have closed given the absence of abdominal pain and diarrhea when enteral nutrition was resumed early. Therefore, no endoscopic intervention was considered for these patients. In the first patient, no procedure was planned after fistula closure given the poor short-term outcome associated with his underlying condition. The second and third patients were referred for elective surgery considering their young age and the need of a new gastrostomy as the abdominal wall precluded a percutaneous approach. Actually, surgical gastrostomy is, nowadays, rarely used as we have recently reported [16]. It is currently reserved for patients without abdominal wall transillumination due to overweight, prior operated stomach or large hiatal hernias, and for patients without endoscopic access [16]. Our option for the last 2 patients not only provided a safe and effective gastrostomy but also allowed the complete resection of the fistula tracts avoiding any remote possibility of reopening and further drainage.
This small case series highlights a rare but usually not life-threatening complication of PEG. Eventually, most cases of GCCF may present this benign course and do not require specific intervention as it was assessed by Friedmann et al. [17] in a retrospective review of 28 patients. Nevertheless, in order to minimize its occurrence, we recommend a 4-step strategy: (1) achieve abdominal transillumination during PEG; (2) use finger pressing and en doscopic indentation of the gastric wall; (3) perform the needle aspiration test routinely and confirm that air enters the syringe at the same time as the needle is seen endoscopically in the gastric lumen but does not enter when the needle is outside the stomach, excluding visceral interposition; and (4) achieve a perpendicular puncture of the abdominal wall. When any of these steps cannot be guaranteed, the PEG should be aborted or postponed due to GCCF risk. A CT may also be considered in this scenario to assess if there is colon interposition, avoiding further inutile attempts and helping to define the best alternative approach.
Most GCCFs seem to close spontaneously and, therefore, this should be considered the first-line approach in stable and pauci-symptomatic patients. The routine use of contrast through the stoma at the time of the diagnostic CT is a safe way to define both gastrocolic and colocutaneous tracts better and to rule out intraperitoneal extravasation. In the few cases in which GCCF persists over time, endoscopic management may be advised, particularly using mechanic devices like endoscopic clips to close the fistula orifices. If a new gastrostomy is indicated and cannot be done using a percutaneous route, a minimally invasive laparoscopic approach is a good alternative. The authors expected to find profuse adherences surrounding the fistula tracts and difficulty accessing the stomach during laparoscopy. However, there were few adherences and the colocutaneous tract was long and well individualized, allowing for full stomach access to establish the new gastrostomy. The gastrocolic tract was somewhat shorter but easily dissected and ligated. Based on the last 2 cases, we expect that laparoscopy in GCCF might not be as troublesome as was anticipated. Thus, combined laparoscopic and endoscopic control may be recommended in selected cases to improve the safety of the procedure.
In conclusion, gastroenterologists should be aware of GCCF as a late-onset complication of PEGs, especially at the time of the first tube replacement, although excessive concern seems to be unwarranted considering the authors’ experience that describes its benign clinical outcome and spontaneous resolution.
References
1 Loeser C, von Herz U, Küchler T, Rzehak P, Müller MJ. Quality of life and nutritional state in patients on home enteral tube feeding. Nutrition. 2003 Jul-Aug;19(7-8):605–11. [ Links ]
2 Löser C, Aschl G, Hébuterne X, Mathus-Vliegen EM, Muscaritoli M, Niv Y, et al. ESPEN guidelines on artificial enteral nutrition – percutaneous endoscopic gastrostomy (PEG). Clin Nutr. 2005 Oct;24(5):848–61. [ Links ]
3 Rahnemai-Azar AA, Rahnemaiazar AA, Naghshizadian R, Kurtz A, Farkas DT. Percutaneous endoscopic gastrostomy: indications, technique, complications and management. World J Gastroenterol. 2014 Jun;20(24):7739–51. [ Links ]
4 Lee J, Kim J, Kim HI, Oh CR, Choi S, Noh S, et al. Gastrocolocutaneous Fistula: An Unusual Case of Gastrostomy Tube Malfunction with Diarrhea. Clin Endosc. 2018 Mar;51(2):196–200. [ Links ]
5 Ruiz Ruiz JM, Rando Muñoz JF, Salvá Villar P, Lamarca Hurtado JC, Sánchez Molinero MD, Sanjurgo Molezun E, et al. [Gastrocolocutaneous fistula: an uncommon complication of percutaneous endoscopic gastrostomy]. Nutr Hosp. 2012 Jan-Feb;27(1):306–9. [ Links ]
6 Lee HJ, Choung RS, Park MS, Pyo JH, Kim SY, Hyun JJ, et al. Two cases of uncommon complication during percutaneous endoscopic gastrostomy tube replacement and treatment. Korean J Gastroenterol. 2014 Feb;63(2):120– 4. [ Links ]
7 Büyükaşık K, Tatar C, Arı A, Sarı S, Bektaş H. Successful endoscopic treatment of a gastrocolocutaneous fistula due to PEG tube. Turk J Gastroenterol. 2017 May;28(3):231–2. [ Links ]
8 Kim HS, Bang CS, Kim YS, Kwon OK, Park MS, Eom JH, et al. Two cases of gastrocolocutaneous fistula with a long asymptomatic period after percutaneous endoscopic gastrostomy. Intest Res. 2014 Jul;12(3):251–5. [ Links ]
9 Bertolini R, Meyenberger C, Sulz MC. First report of colonoscopic closure of a gastrocolocutaneous PEG migration with over-thescope-clip-system. World J Gastroenterol. 2014 Aug;20(32):11439–42. [ Links ]
10 Hwang JH, Kim HW, Kang DH, Choi CW, Park SB, Park TI, et al. A case of endoscopic treatment for gastrocolocutaneous fistula as a complication of percutaneous endoscopic gastrostomy. Clin Endosc. 2012 Mar;45(1):95–8. [ Links ]
11 Okutani D, Kotani K, Makihara S. A case of gastrocolocutaneous fistula as a complication of percutaneous endoscopic gastrostomy. Acta Med Okayama. 2008 Apr;62(2):135–8. [ Links ]
12 Chen Y, Ni YH, Lai HS. Gastrocolocutaneous fistula in a child with congenital short bowel syndrome: a rare complication of percutaneous endoscopic gastrostomy. J Formos Med Assoc. 2004 Apr; 103(4): 306–10. [ Links ]
13 Roozrokh HC, Ripepi A, Stahlfeld K. Gastrocolocutaneous Fistula as a complication of peg tube placement. Surg Endosc. 2002 Mar;16(3):538–9. [ Links ]
14 Lohiya GS, Tan-Figueroa L, Krishna V. Intermittent diarrhea as a delayed presentation of percutaneous endoscopic gastrostomy (PEG)-associated fistula. J Am Board Fam Med. 2010 Sep-Oct;23(5):681–4. [ Links ]
15 Melmed GY, Kar S, Geft I, Lo SK. A new method for endoscopic closure of gastrocolonic fistula: novel application of a cardiac septal defect closure device (with video). Gastrointest Endosc. 2009 Sep;70(3):542–5. [ Links ]
16 Oliveira GP, Santos CA, Fonseca J. The role of surgical gastrostomy in the age of endoscopic gastrostomy: a 13 years and 543 patients retrospective study. Rev Esp Enferm Dig. 2016 Dec;108(12):776–9. [ Links ]
17 Friedmann R, Feldman H, Sonnenblick M. Misplacement of percutaneously inserted gastrostomy tube into the colon: report of 6 cases and review of the literature. JPEN J Parenter Enteral Nutr. 2007 Nov-Dec; 31(6): 469– 76. [ Links ]
18 Nunes G, Oliveira G, Cortez-Pinto J, Cruz J, Fonseca J. Gastrocolocutaneous fistula: an undetected complication of colon transfixation during percutaneous endoscopic gastrostomy. Turk J Gastroenterol. 2018;doi: 10.5152/tjg.2018.18552. [ Links ]
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The present article includes the description of 3 clinical cases. One of them (case 2) was previously reported in another journal [see 18]. The current manuscript includes most authors involved in the previous case report. Different iconography was selected.
Funding Sources
The authors have no funding sources to disclose.
* Corresponding author.
Gonçalo Nunes
Gastroenterology Department
Hospital Garcia de Orta
Av. Torrado da Silva, PT–2805-267 Almada (Portugal)
E-Mail goncalo.n@hotmail.com
Received: December 1, 2018; Accepted after revision: January 21, 2019
Author Contributions
Gonçalo Nunes: conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, and final approval of the version to be published. Gabriel Paiva de Oliveira, João Cruz, Carla Adriana Santos, and Jorge Fonseca: analysis and interpretation of data, critical revision of the article, study supervision, and final approval of the version to be published.














