Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.4 Lisboa Aug. 2019
https://doi.org/10.1159/000493762
ORIGINAL ARTICLE
Prediction of Self-Limited Acute Pancreatitis Cases at Admission to Emergency Unit
Predição dos casos de pancreatite aguda auto-limitada na admissão na urgência hospitalar
Yusuf Kayara, Hakan Senturka, Mukaddes Tozlua, Birol Baysala, Musa Atayb, Ali Tuzun Incea
aDepartment of Internal Medicine, Division of Gastroenterology, Bezmialem Vakıf University, Istanbul, Turkey; bDepartment of Radiology, Bezmialem Vakıf University, Istanbul, Turkey
* Corresponding author.
ABSTRACT
Background: While acute pancreatitis (AP) resolves spontaneously´with supportive treatment in most patients, it may be life-threatening. Predicting the disease severity at onset dictates the management strategy. We aimed to define the patients with mild pancreatitis who may be considered for outpatient management with significant cost-savings. Materials and Methods: This prospective observational study included 180 patients with mild AP according to the harmless acute pancreatitis score (HAPS) and Imrie score. The relationships of biochemical parameters with the changes in the Balthazar score and clinical course were examined. Results: The study included 180 patients (111 females, 69 males; mean age: 53.9 ± 17.2 years; range: 17–92 years). The etiology was biliary in 118 (65%) patients and remained undetermined in 38 (21.1%) patients. Computed tomography (CT) performed within the first 12 h revealed mild and moderate AP in 159 (88.3%) and 21 (11.7%) patients, respectively. CT repeated at 72 h revealed mild, moderate, and severe AP in 155 (86.1%), 24 (13.3%), and 1 (0.6%) patients, respectively. Comparisons between stages A + B + C and D + E showed significant differences in the amylase levels on day 1 and 3, and in C-reactive protein on day 3. Also, in stage D and E disease, narcotic analgesic intake, oral intake onset time, and pain were significantly higher. Conclusion: There were no significant changes in the CT findings of patients with mild AP at 12 and 72 h. Most patients (n = 179; 99.4%) recovered uneventfully. Patients with mild pancreatitis according to the HAPS and Imrie scores can be considered for outpatient management. The recovery is longer in stage D and E disease.
Keywords: Acute pancreatitis, Balthazar score, Severity
RESUMO
Introdução: Apesar da pancreatite aguda resolver espontaneamente com medidas de suporte na maioria dos doentes, esta também pode ser grave e fatal. A predição inicial da gravidade da doença orienta a estratégia terapêutica. O nosso objetivo foi definir os doentes com pancreatite ligeira que podem ser considerados para terapêutica em ambulatório com redução dos custos. Material e Métodos: Estudo prospetivo observacional com 180 doentes com pancreatite aguda ligeira segundo os scores de HAPS e Imrie. As relações entre os parâmetros bioquímicos, as alterações no score de Balthazar e o curso clinico foram examinadas. Resultados: Este estudo incluiu 180 doentes (111 mulheres, 69 homens; idade média 53.9±17.2 anos). A etiologia foi biliar em 118 (65%) e permaneceu indeterminada em 38 (21.1%) doentes, respetivamente. A tomografia computorizada (TC) realizada nas primeiras 12 h revelou pancreatite ligeira e moderada em 159 (88.3%) e 21 (11.7%) doentes, respetivamente. A TC repetida às 72h revelou pancreatite aguda, moderada e grave em 155 (86.1%), 24 (13.3%), e 1 (0.6%) dos doentes, respetivamente. As comparações entre os estadios A+B+C e D+E mostraram diferenças significativas nos níveis de amílase nos dias 1 e 3, e na PCR no dia 3. Também nos estádios D e E, a toma de narcóticos, tempo de inicio da dieta oral e a dor foram significativamente superiores. Conclusão: Não se verificaram alterações significativas na TC dos doentes com pancreatite ligeira nem às 12 nem às 72h. A maioria dos doentes (99.4%) recuperou sem complicações. Doentes com pancreatite ligeira segundos os scores de HAPS e Imrie podem ser considerados para orientação em ambulatório. A recuperação é mais longa nos estadios D e E da doença.
Palavras-Chave: Pancreatite aguda, Score de Balthazar, Gravidade de doença
Introduction
The course of acute pancreatitis (AP) is highly heterogeneous. Although most patients with AP recover spontaneously with supportive treatment in a short period of time, it has life-threatening potential in a minority [1]. While over 80% of patients have mild, self-limiting AP requiring only brief hospitalization, less than 20% have severe AP, which may cause various complications [2]. The AP incidence has been reported as 30–45/100,000 person-years in large studies. All patients with AP are traditionally admitted to the ward after an initial diagnosis in the emergency room. It is the number 1 gastrointestinal disease requiring hospital admission and the most expensive one, with an associated cost of 2.6 billion USD per year in the USA [3, 4]. A life-threatening course occurs in 2–3% of the patients [5]. Early treatment in severe AP is known to decrease the morbidity and mortality [6].
Because of concerns about worsening course even in initially mild patients, and due to the lack of established home management strategies, physicians routinely admit all patients to the hospital [7]. Prediction of very mild courses with certainty in the emergency room would have a significant financial impact. Recently, the traditional approach was challenged, and the home management concept of AP was suggested for mild AP [7].
In order to determine the severity of AP, various classifications have been developed using laboratory and radiological parameters. Some of these most widely used ones are the Ranson, Imrie, Acute Physiology and Chronic Health Evaluation (APACHE) II, Bedside Index of Severity in AP (BISAP), harmless acute pancreatitis score (HAPS), and Balthazar scores [1, 6]. Despite certain limitations, the imaging scoring developed by Balthazar in 1990, which can be used to assess pancreatic and peripancreatic inflammation and pancreatic necrosis, appears to be the most accurate one [6]. However, it is considered that none of these scoring systems alone is prognostically sufficient in AP patients [1, 3, 6]. Numerous studies have shown that proinflammatory cytokines and C-reactive protein (CRP) levels are associated with AP severity [8, 9]. In contrast, studies investigating the relationship between pancreatic enzyme levels and AP severity have revealed conflicting results [10].
Kuo et al. [11] recommend that patients who appear well, show normal mental activity, and tolerate oral intake, with appropriate vital signs, with normal findings on physical examination, and with appropriate laboratory findings may not require hospitalization. However, there has been no study investigating the predictive value of several initial parameters for the decision for outpatient management. Previous studies have reported that computed tomography (CT) performed at the early stage shows normal appearance of the pancreas in 14–30% of AP patients, and it is advised not to perform it before 72 h [12]. However, this assumption is mainly based on the fact that necrosis is not apparent in early hours. There are few studies investigating the changes in imaging findings during the course of mild AP [13].
In this study, we evaluated the changes in the CT findings according to the Balthazar score between scans performed within the first 12 h and after 72 h in patients with mild AP, as classified by the HAPS and Imrie scores. We aimed to determine the subset of mild AP patients who may be managed on an outpatient basis.
Materials and Methods
The study was performed prospectively at our center after obtaining approval from the local ethics board. All study participants provided written consent prior to study enrollment.
Subjects
We included patients aged over 16 years who presented within 4–6 h after pain onset and who were admitted to our hospital with the diagnosis of AP between August 2014 and September 2015. The diagnosis was made according to the American College of Gastroenterology guidelines. At least 2 out of the following 3 criteria must be met for AP diagnosis: (1) presence of characteristic epigastric pain; (2) amylase and/or lipase values of > 3 times the upper limit of normal; and (3) presence of characteristic imaging findings of AP [14]. Actually, all patients fulfilled the first 2 criteria. Patients with a history of chronic pancreatitis, allergy to contrast material, in poor general condition, and with heart, kidney, or liver failure were excluded.
In the 12-month study period, a total of 242 patients were admitted to our hospital with the diagnosis of AP. The patients demographic data, including age and sex, were documented. The complete blood count, glucose, lactate dehydrogenase, aspartate transaminase, alanine transaminase, calcium, albumin, urea, creatinine, and CRP were analyzed in all patients after physical examination. Oxygen saturation was measured with a pulse oximeter. Patients with mild AP according to the HAPS and Imrie scores calculated at the time of admission were included in the study [15, 16]. Patients were considered as having mild pancreatitis according to the HAPS if all 3 criteria (defense/rebound on abdominal examination, abnormal hematocrit, and increased creatinine levels) were negative. In terms of the Imrie score, a total score ≤2 was considered to indicate mild AP. According to the Balthazar score, patients with scores of 0–3, 4–6, and 7–10 were identified as having mild, moderate, and severe AP, respectively [17]. The 7th, 39th, and 16th patients were excluded from the study due to pregnancy, nonmild pancreatitis according to the HAPS and Imrie scores, and unwillingness to participate in the study. There was no endoscopic retrograde cholangiopancreatography-related AP, probably because this is mostly an in-hospital condition. Finally, 180 patients with mild AP according to the HAPS and Imrie scores were enrolled. The relationships between biochemical parameters (amylase, lipase, creatinine, white blood cell count, hematocrit, and CRP) and the Balthazar score were examined within 12 h and after 72 h. For pain management, nonsteroidal anti-inflammatory agents and narcotic analgesics were used. Oral feeding was allowed in case nausea and/or vomiting was not present or subsided.
Scoring Systems
Several multi-factorial scoring systems based on clinical and biochemical data have been used over the past few decades. Each of these scoring systems has its own limitations, including low sensitivity and specificity. Early predictors of disease severity are important to help triage the patient to an appropriate management setting and to avoid over- or under-resuscitation of patients with an adverse outcome. An ideal predictor needs to be economical, safe, simple, highly sensitive and specific, and can be performed rapidly. Recently, the HAPS has been introduced to identify AP with a nonsevere course. The HAPS contains fewer parameters which can help stratify nonsevere disease within a short time after presentation and to decide whether to admit these patients to a general ward or the critical care unit [7, 18]. We preferred the Imrie and HAPS scoring systems over APACHE II and Ransons scoring systems because the predictive power of the latter scoring systems was shown to be poor in previous studies.
Timing of Cholecystectomy
High complication and mortality rates after early cholecystectomy in patients with severe pancreatitis have prompted guidelines recommending delaying cholecystectomy until all signs of inflammation have resolved. In patients with severe biliary pancreatitis, it is generally accepted to perform an interval cholecystectomy. In mild biliary pancreatitis, patients frequently do not undergo an early cholecystectomy, resulting in a high percentage of hospital readmissions due to recurrent biliary events. So, after mild biliary pancreatitis, early cholecystectomy is advised by the current guidelines [19]. We performed cholecystectomy in the first 2 weeks in case of mild AP and in weeks 4–6 in cases with moderate and severe AP.
Imaging Technique
All patients underwent helical CT (section 64, Aquilion; Toshiba Medical Systems, Tokyo) within the first 12 h and after 72 h. A contrast-enhanced CT scan (collimation, 4 × 2.5 mm; slice thickness, 5 mm; range of reconstruction, 5 mm) was obtained 65 s after the administration of 100 mL iohexol (Omnipaque 300) at a rate of 3 mL/s.
Image Analysis
All CT images were evaluated by a radiologist blinded to other findings. The pancreatic, peripancreatic, and extrapancreatic findings and complications were analyzed. Pancreatitis was graded and stratified according to the Balthazar classification [17].
Data Analysis
IBM SPSS 22 (IBM SPSS, Turkey) software was used for the statistical analysis. One-way ANOVA was used for comparisons of definitive statistical data (mean ± standard deviation, frequency), as well as quantitative data when normally distributed parameters were compared between more than 2 groups. The Kruskal-Wallis test was used for intergroup comparisons of parameters that were not normally distributed, and the Mann-Whitney U test was used for detecting the factors causing differences between the groups. The χ2 and McNemar tests were used for comparisons of qualitative data. The diagnostic accuracy and true and false positive rates of each test (sensitivity and specificity) were evaluated using receiver operating characteristics curves. The level of significance was set as p < 0.05.
Results
We included 180 patients (111 [61.7%] females and 69 [38.3%] males) in the present study. The age ranged from 17 to 92 years, with a mean age ± standard deviation of 53.86 ± 17.19 years. The etiology was determined to be of biliary origin in 118 (65%); malignant (periampullary tumor) in 7 (3.9%); due to a structural anomaly (pancreas divisum, annular pancreas) in 4 (2.2%); due to drugs in 4 (2.2%); and due to alcohol, hyperlipidemia, hypercalcemia, and infection in 2 (1.1%) cases each; and there was extraintestinal involvement of ulcerative colitis in 1 (0.6%) case. The etiology could not be determined in 38 (21.1%) cases, and these were hence considered idiopathic. Magnetic resonance (MR) and MR cholangiopancreatography images were performed to diagnose pancreas divisum and annular pancreas. The causes of AP were excluded in the patient with ulcerative colitis. It was seen that he had AP simultaneously with exacerbation of the disease twice within 1 year. Therefore, AP was evaluated as an extraintestinal involvement of ulcerative colitis.
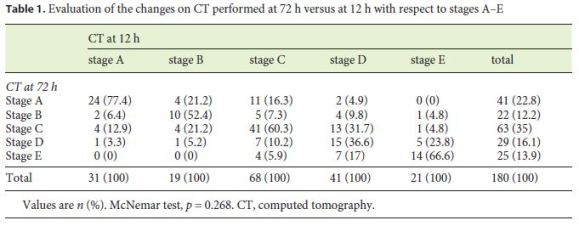
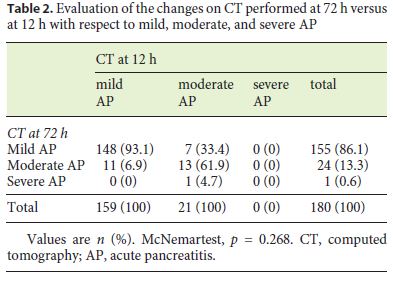
Based on the CT performed within the first 12 h, 31 (17.2%), 19 (10.6%), 68 (37.8%), 41 (22.8%), and 21 (11.7%) patients were classified as stages A, B, C, D, and E, respectively, while at 72 h, 41 (22.8%), 22 (12.2%), 63 (35%), 29 (16.1%), and 25 (13.9%) were classified as stages A, B, C, D, and E, respectively. While in the first examination 159 (88.3%) and 21 (11.7%) patients had mild and moderate pancreatitis, respectively, at 72 h, 155 (86.1%) patients had mild pancreatitis, 24 (13.3%) had moderate pancreatitis, and 1 (0.6%) had severe pancreatitis according to the Balthazar scoring system. No significant change was observed between the CT scans performed within the first 12 h and at 72 h (p = 0.268). The changes in the radiological severity of AP according to Balthazar are shown in Table 1 based on stage and in Table 2 based on the score. Although only 2 of our patients developed pancreatic necrosis, none of our patients developed organ failure.
The relationships between the stage of AP and biochemical parameters are shown in Tables 3 and 4. Considering the correlation between the CT scores and the day 1 and 3 serum amylase and day 3 CRP values, we next tried to define the optimal cutoff points to define CT stages D and E (Table 5). The optimal cutoff point for amylase on day 1 was determined as 1,360 U/L (sensitivity, 0.60; specificity, 0.7429; positive predictive value, 0.625; negative predictive value, 0.72). The cutoff point for amylase on day 3 was 135 U/L (sensitivity, 0.6933; specificity, 0.6667; positive predictive value, 0.5977; negative predictive value, 0.7527). The cutoff point detected for CRP on day 3 was 8.7 mg/dL (sensitivity, 0.6933; specificity, 0.7524; positive predictive value, 0.6667; negative predictive value, 0.7745). Finally, the optimal cutoff point detected for the CRP rate was found to be 6 (sensitivity, 0.6533; specificity, 0.7809; positive predictive value, 0.6805; negative predictive value, 0.7592) (Table 5).
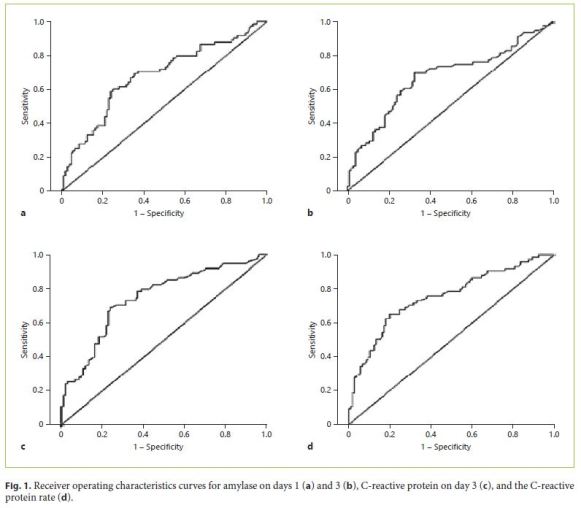
The areas under the curve, as calculated by receiver operating characteristics curve analysis, were found to be 0.682 (95% confidence interval [CI]: 0.601–0.762) for amylase on day 1, 0.670 (95% CI: 0.587–0.753) for amylase on day 3, 0.747 (95% CI: 0.673–0.821) for CRP on day 3, and 0.753 (95% CI: 0.679–0.826) for the CRP rate. The areas under the curve obtained for these parameters were all higher than the chance value of 0.5 (p < 0.01) (Fig. 1).
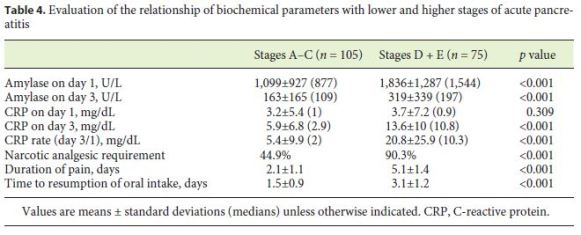
Lastly, we divided the patients into 2 groups according to the CT findings: patients whose highest CT score was A–C (without peripancreatic fluid collection, n = 105) and D + E (with peripancreatic fluid collection, n = 75). While a narcotic analgesic for relief of pain was needed by 44.9% in the first group, it was needed by 90.3% in the second group (p < 0.001). The duration of pain was 2.1 ± 1.1 and 5.1 ± 1.4 days, respectively (p < 0.001). Oral feeding was started on days 1.5 ± 0.9 in the first group and on days 3.1 ± 1.2 in the second group (p < 0.001) (Table 4).
Discussion
Although numerous scoring systems and markers have been suggested to predict the severity of AP, there is no consensus on which one to use in clinical practice. Large studies revealed that the Balthazar score is a helpful method in determining the severity of AP [20]. However, CT scan is recommended within 6–10 days of onset to obtain a high accuracy rate, as pancreatic necrosis does not develop within the first 48 h [9]. The study conducted by Khan and Talib [12] stated that CT does not reveal any abnormal findings in 10.8% of AP patients, whereas the rate has been reported to be as high as 14–30% in some studies. Thus, absence of abnormal findings on CT performed within the first few hours does not exclude the possibility of AP [12]. Nevertheless, some studies have stated that CT performed early can predict changes in the course of AP [21, 22]. The study conducted by Munoz-Bongrand et al. [13] found that, based on CT scans performed within the first hours of admission, no patient with stage A and B disease developed complications, and that the complication rate was significantly higher in patients with stage E compared to those with stage C and D disease.
Previous studies have also reported that the presence and degree of pancreas parenchyma morphology and peripancreatic inflammatory collections are closely correlated with the clinical course [13, 21, 22]. In our study, CT scans performed within 12 h of pain onset demonstrated that while patients with stage A and B disease showed no progression to stage E, a limited number of patients with stage C and D progressed. In this study, we showed that the most important determinant of the course of the pancreatitis was the presence of extrapancreatic fluid collection (stages D and E), which is easily diagnosed with plain CT without contrast administration. Ultrasonography may also be used for this purpose; however, because of poor bowel movements and resultant intestinal gas accumulation, the quality of examination is sometimes poor. We also showed that, in patients with mild pancreatitis according to the HAPS and Imrie scores at admission, there was virtually no change between the CT examinations at admission and 72 h later.
CRP is an acute-phase reactant that is released from hepatocytes in response to interleukin 1 and 6 in the circulation. It is a marker commonly used for determining the severity of AP [9, 18]. A CRP value > 15 mg/dL after 48 h was characterized as a prognostic factor in the Santorini consensus conference (1999), World Gastroenterology Congress Guideline (2002), United Kingdom Guideline (2005), and Japanese criteria [23]. In addition, Arvanitakis et al. [24] stated that there was a significant correlation between MR imaging performed within the first hours and the CRP level at 48 h. Although some studies have stated that the baseline CRP level has a predictive value for determining the severity of AP [25, 26], its accuracy is rather poor [1, 9]. The study conducted by Bhatia et al. [25] demonstrated that AP followed a milder course in patients with low baseline CRP values, with less accompanying complications. In our study, we found that baseline CRP was not satisfactory for predicting very mild pancreatitis.
In general, amylase and lipase levels are considered not to correlate with the severity of the disease [9]. However, some studies have demonstrated significant correlations [27, 28]. For example, the study conducted by Nordestgaard et al. [27] demonstrated significantly higher amylase levels in patients with stage A and B compared to those with stage C and D disease, and the study conducted by Chang et al. [10] demonstrated lower amylase and lipase levels in severe AP, although this was not significant. In contrast, the study conducted by Robert et al. [28] demonstrated significantly higher amylase and lipase levels in patients with severe AP. In our study, we found that the amylase levels at baseline and at 72 h correlated with the severity of AP in mild pancreatitis. In the studies by Nordestgaard et al. [27] and Chang et al. [10], alcohol was the culprit in the majority of patients with severe AP, and the amylase and lipase levels were inherently low in alcoholrelated AP. In our study, we included a select group of patients with mild pancreatitis mostly with biliary etiology, and alcohol was a very rare cause. Another study with more equal etiological distribution between mild and severe AP groups also showed that the serum lipase levels had a positive correlation with the severity of AP [18].
Pancreatic necrosis develops secondary to active pancreatic inflammation, with increased vascular permeability, vascular spasm, systemic hypotension with leakage of fluid, and increased viscosity and hemoconcentration [29]. Increased serum creatinine levels and a glomerular filtration rate < 90 mL/min are important predictors for severe AP [1, 29]. The study conducted by Bota et al. [20] showed that creatinine levels were significantly higher in patients with severe AP. Furthermore, a creatinine level > 1.8 mg/dL within the first 48 h was found to be correlated with the development of necrosis in the pancreas in one previous study [29]. In our study, the creatinine values did not reach the upper limit of normal; and there was no significant correlation detected between the creatinine level and severity of AP.
Severity of pain and its duration are well-established diagnostic and partly prognostic factors for AP, and studies have revealed that the severity of pain correlates with the severity of AP [30]. Management of pain includes administration of narcotic and nonnarcotic analgesic drugs. In our study, we noticed that the need for narcotic analgesic medications increased significantly in stage D and E pancreatitis which was classified using the Balthazar system. Moreover, we noticed that the duration of pain was significantly longer in these cases. Previously, it was recommended not to resume oral feeding until the pancreatic enzyme levels returned to normal and pain subsided. However, currently, it is encouraged to allow early initiation of oral re-feeding, with recent studies demonstrating that, when compared to enteral nutrition, parenteral nutrition causes more damage to the humoral and cellular immune system and increases the proinflammatory response, intestinal permeability, and rates of infection by increasing bacterial translocation [31]. Previous studies have revealed that approximately 21.9–42% of patients do not tolerate early initiation of oral intake, and it has been reported that this intolerance is especially high in severe AP [32]. In our study, time to resumption of oral intake was significantly longer in AP cases classified as class D and E. Furthermore, the clinical course was significantly different between the patients with and without fluid collection; while the patients in the latter group were free of pain within a few days, even without narcotic analgesics in approximately half the cases, the pain subsided slowly in the former group and narcotic analgesics were required in most patients.
There have been several studies evaluating the use of home health-care services for administering chemotherapy, following symptoms after chemotherapy, treatment with low-molecular-weight heparin therapy for proximal deep venous thrombosis, and treatment of pulmonary tuberculosis and acute cholecystitis [7]. In the study of cases with mild AP by Ince et al. [7], half of the patients were hospitalized and treated, while the other half was treated at home by being monitored. A comparison between the 2 groups revealed that there was no significant difference between clinical complaints and oral intake time, but the cost was found to be significantly lower in home care patients. In our study, we think that home care and treatment are more appropriate to reduce the cost of patients and protect them from multidrug-resistant nosocomial infections in hospitals due to the absence of organ failure in patients following mild AP.
Conclusions
While admission CT showed significant power in determining the course of the disease in mild pancreatitis patients, the predictive roles of the initial serum amylase and CRP levels are limited. Thus, in patients with mild pancreatitis according to the HAPS and Imrie scores at admission, in case imaging reveals no fluid collection, these patients may be considered for outpatient management. Widespread use of this practice may have significant cost-saving effects.
References
1 Phillip V, Steiner JM, Algül H. Early phase of acute pancreatitis: assessment and management. World J Gastrointest Pathophysiol. 2014 Aug;5(3):158–68. [ Links ]
2 Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006 May;354(20):2142–50. [ Links ]
3 Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013 Jun;144(6):1252–61. [ Links ]
4 Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012 Nov;143(5):1179–1187.e3. [ Links ]
5 Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006 Oct;101(10):2379–400. [ Links ]
6 Jain S, Gupta S, Chawla AS, Agarwal Y, Thukral BB. Comparative study of Balthazar Computed Tomography Severity Index and Modified Computed Tomography Severity Index in predicting the outcome of acute pancreatitis. Apollo Med. 2014;11(2):74–83. [ Links ]
7 Ince AT, Senturk H, Singh VK, Yildiz K, Danalioğlu A, Cinar A, et al. A randomized controlled trial of home monitoring versus hospitalization for mild non-alcoholic acute interstitial pancreatitis: a pilot study. Pancreatology. 2014 May-Jun;14(3):174–8. [ Links ]
8 Fisic E, Poropat G, Bilic-Zulle L, Licul V, Milic S, Stimac D. The role of IL–6, 8, and 10, sTNFr, CRP, and pancreatic elastase in the prediction of systemic complications in patients with acute pancreatitis. Gastroenterol Res Pract. 2013;2013:282645. [ Links ]
9 Mofidi R, Patil PV, Suttie SA, Parks RW. Risk assessment in acute pancreatitis. Br J Surg. 2009 Feb;96(2):137–50. [ Links ]
10 Chang KC, Changchien CS, Kuo CM, Chiu YC, Chuah SK, Chiu KW, et al. Clinical analysis of the efficacy in lipase/amylase ratio for acute pancreatitis. J Intern Med Taiwan. 2005;16:113–20. [ Links ]
11 Kuo DC, Rider AC, Estrada P, Kim D, Pillow MT. Acute Pancreatitis: whats the Score? J Emerg Med. 2015 Jun;48(6):762–70. [ Links ]
12 Khan AA, Talib A. Early computerized tomographic scan in mild pancreatitis may be deceiving: an experıence of tertiary care hospital. J Postgrad Med Inst. 2012;26(3):324–9. [ Links ]
13 Munoz-Bongrand N, Panis Y, Soyer P, Riché F, Laisné MJ, Boudiaf M, et al. Serial computed tomography is rarely necessary in patients with acute pancreatitis: a prospective study in 102 patients. Am J Surg. 2001 Aug;193(2):146–52. [ Links ]
14 Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013 Sep;108(9):1400–15. [ Links ]
15 Lankisch PG, Weber-Dany B, Hebel K, Maisonneuve P, Lowenfels AB. The harmless acute pancreatitis score: a clinical algorithm for rapid initial stratification of nonsevere disease. Clin Gastroenterol Hepatol. 2009 Jun;7(6):702–5. [ Links ]
16 Imrie CW, Benjamin IS, Ferguson JC, McKay AJ, Mackenzie I, ONeill J, et al. A single-centre double-blind trial of Trasylol therapy in primary acute pancreatitis. Br J Surg. 1978 May;65(5):337–41. [ Links ]
17 Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990 Feb;174(2):331–6. [ Links ]
18 Al-Qahtani HH, Alam MK, Waheed M. Comparison of Harmless Acute Pancreatitis Score with Ransons Score in Predicting the Severity of Acute Pancreatitis. J Coll Physicians Surg Pak. 2017 Feb;27(2):75–9. [ Links ]
19 Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1–15. [ Links ]
20 Bota S, Sporea I, Sirli R, Popescu A, Strain M, Focsa M, et al. Predictive factors for severe evolution in acute pancreatitis and a new score for predicting a severe outcome. Ann Gastroenterol. 2013;26(2):156–62. [ Links ]
21 Spitzer AL, Thoeni RF, Barcia AM, Schell MT, Harris HW. Early nonenhanced abdominal computed tomography can predict mortality in severe acute pancreatitis. J Gastrointest Surg. 2005 Sep-Oct;9(7):928–33. [ Links ]
22 De Waele JJ, Delrue L, Hoste EA, De Vos M, Duyck P, Colardyn FA. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: evaluation of a new scoring system. Pancreas. 2007 Mar;34(2):185–90. [ Links ]
23 Takeda K, Yokoe M, Takada T, Kataoka K, Yoshida M, Gabata T, et al. Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J Hepatobiliary Pancreat Sci. 2010 Jan;17(1):37–44. [ Links ]
24 Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, et al. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004 Mar;126(3):715–23. [ Links ]
25 Bhatia M, Nirhale DS, Athavale VS, Calcuttawala M, Kale A, Sankhe M. C-reactive protein with sequential organ failure assessment score: valuable parameters in managing acute pancreatitis. Trop J Med Res. 2014;17(2):86–90. [ Links ]
26 McMahon MJ, Bowen M, Mayer AD, Cooper EH. Relation of alpha 2-macroglobulin and other antiproteases to the clinical features of acute pancreatitis. Am J Surg. 1984 Jan;147(1):164–70. [ Links ]
27 Nordestgaard AG, Wilson SE, Williams RA. Correlation of serum amylase levels with pancreatic pathology and pancreatitis etiology. Pancreas. 1988;3(2):159–61. [ Links ]
28 Robert JH, Frossard JL, Mermillod B, Soravia C, Mensi N, Roth M, et al. Early prediction of acute pancreatitis: prospective study comparing computed tomography scans, Ranson, Glascow, Acute Physiology and Chronic Health Evaluation II scores, and various serum markers. World J Surg. 2002 May;26(5):612–9. [ Links ]
29 Muddana V, Whitcomb DC, Khalid A, Slivka A, Papachristou GI. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2009 Jan;104(1):164–70. [ Links ]
30 Schorn S, Ceyhan GO, Tieftrunk E, Friess H, Demir IE. Pain Management in Acute Pancreatitis. Pancreapedia. 2015. doi: 10.3998/panc.2015.15. [ Links ]
31 Spanier BW, Bruno MJ, Mathus-Vliegen EM. Enteral nutrition and acute pancreatitis: a review. Gastroenterol Res Pract. 2011;2011:857949. [ Links ]
32 Marik PE. What is the best way to feed patients with pancreatitis? Curr Opin Crit Care. 2009 Apr;15(2):131–8. [ Links ]
Statement of Ethics
The study was performed prospectively at our center after obtaining approval from the local ethics board. All study participants provided written consent prior to study enrollment.
Disclosure Statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
* Corresponding author.
Yusuf Kayar, MD
Gastroenterology Clinic of Bezmialem Vakıf University
Vatan Street, Fatih
TR–34093 Istanbul (Turkey)
E-Mail ykayar@yahoo.com
Received: May 15, 2018; Accepted after revision: September 14, 2018














