Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.4 Lisboa ago. 2019
https://doi.org/10.1159/000492637
ORIGINAL ARTICLE
Fistula Recurrence: A Clinical Reality after Successful Endoscopic Closure of Laparoscopic Sleeve Gastrectomy Fistulas
Recorrência de Fístulas: Uma Realidade Clínica após Encerramento Eficaz de Fístulas pós Gastrectomia Vertical (Sleeve)
Patricia Sousaa, Carlos Noronha Ferreiraa, João Coutinhob, Fátima Carepab, Rosário Rosab, Andreia Barãob, Carlos Marques Ferreirab, José Giraob, António Ruivob, Henrique Bicha Castelob, João Lopesa, Amélia Almeidac, Luís Carrilho Ribeiroa, José Velosaa
aServiço de Gastrenterologia e Hepatologia, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte, Lisbon, Portugal; bServiço de Cirurgia, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte, Lisbon, Portugal; cServiço de Anestesiologia, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte, Lisbon, Portugal
* Corresponding author.
ABSTRACT
Background and Aims: Laparoscopic sleeve gastrectomy (LSG)-related fistulas are important and potentially fatal complications. We aimed at determining the incidence, predictive factors, and management of recurrence of post-LSG fistulas. Methods: This is a retrospective cohort study of 12 consecutive patients with LSG fistulas managed endoscopically between 2008 and 2013. We analyzed factors associated with recurrence of post-LSG fistulas and the efficacy of a primarily endoscopic approach to manage fistula recurrence. Results: The average age at fistula detection after LSG was 43.3 ± 10.9 years, and 10 (83%) patients were female. The median interval between surgery and initial fistula detection was 14 (4–145) days. Fistulas were located at the gastric cardia in 9/12 patients. A median of 4 (1–10) endoscopies were performed per patient until all fistulas were successfully closed. The median follow-up was 30.5 (15–72) months. Fistula recurrence was detected in 3 (25%) female patients with an average age of 31.7 ± 7.9 years after a median of 119 (50–205) days of the initial fistula closure. Fistulas in all 3 patients recurred at the gastric cardia and were successfully managed endoscopically. There was a second recurrence in 1 patient after 6 months, and she was re-operated with anastomosis of a jejunal loop at the site of the fistula orifice at the gastric cardia. We did not find any factors at initial fistula detection that were significantly associated with fistula recurrence. There were no deaths related to initial fistula after LSG and fistula recurrence. Conclusions: A primarily endoscopic approach is an effective and safe method for the management of fistulas after LSG. Fistula recurrence occurred in 25% of patients and was managed endoscopically. Key Messages: Although we could not define predictive factors of post-LSG fistula recurrence, it is a clinical reality and can be managed endoscopically.
Keywords: Laparoscopic sleeve gastrectomy, Fistula, Endoscopy, Fistula recurrence
RESUMO
Objectivos: As fistulas pós-gastrectomia vertical (sleeve) laparoscópica (LSG) são complicações importantes e potencialmente fatais. O objectivo do estudo foi determinar a incidência, factores preditivos e manejo da recorrência de fistulas pós LSG. Métodos: Estudo retrospectivo de 12 doentes com fistulas pós LSG manejados endoscopicamente entre 2008 e 2013. Analisámos factores associados à recorrência de fistulas pós LSG e a eficácia da abordagem endoscópica. Resultados: Idade média na detecção das fistulas pós LSG foi de 43.3 ± 10.9 anos e 10 (83%) doentes eram mulheres. O intervalo mediano entre a cirurgia e a detecção da fistula inicial foi de 14 (4–145) dias. As fistulas localizaram-se no cárdia em 9/12 doentes. Foram realizadas em mediana 4 (1–10) endoscopias por doente até ao encerramento eficaz das fistulas. O tempo mediano de seguimento foi de 30.5 (15–72) meses. A recorrência das fistulas foi detectada em 3 (25%) doentes, todas mulheres, com idade média de 31.7 ± 7.9 anos, após um tempo mediano de 119 (50–205) dias após encerramento da fistula inicial. As recorrências das fistulas nas três doentes ocorreram no cárdia e foram manejados endoscopicamente. Houve uma segunda recorrência de fistula numa doente após 6 meses que foi reoperada com anastomose de ansa jejunal no local do orifício de fistula no cárdia. Não conseguimos determinar factores na altura da detecção da fistula inicial pós LSG significativamente associados com recorrência de fistulas. Não houve mortalidade associada às fistulas pós LSG (inicial ou recorrência). Conclusões: A abordagem primariamente endoscópica das fistulas pós LSG é um método eficaz e seguro. A recorrência de fistulas ocorreu em 25% dos doentes. As recorrências de fistulas pós LSG são manejáveis endoscopicamente. Mensagens chave: Embora não tenhamos conseguido definir factores preditivos de recorrência de fistulas pós LSG, a recorrência de fistulas é uma realidade clínica e é manejável endoscopicamente.
Palavras-Chave: Gastrectomia vertical (sleeve) laparoscópica, Fistula, Endoscopia, Recorrência de fistula
Introduction
Globally, there are an estimated 1 billion people who are overweight and nearly 300 million adults who are obese [1]. Surgical options are increasingly used to induce weight loss in morbidly obese patients, especially those with associated comorbidities. The most popular surgical techniques are gastric bypass, sleeve gastrectomy, and adjustable gastric band [2]. Mortality rates in weight loss surgeries in obese patients range from 0.1 to 5%, and morbidity ranges from 4 to 22% [3, 4]. The creation of dedicated surgical teams and the use of a laparoscopic approach have resulted in a decrease in morbidity and mortality rates [3, 4]. Sleeve gastrectomy, also known as vertical gastrectomy, involves the removal of two-thirds of the gastric volume along the greater curvature, resulting in a tubular morphology of the gastric remnant. This alteration of the stomach results in early satiety with progressive and consistent weight loss after surgery [2, 5]. This surgical intervention is especially advocated in young morbidly obese patients given that it tends to be more physiological than gastric bypass surgery and is associated with comparable weight loss and resolution of comorbidities, including diabetes mellitus and metabolic syndrome [6, 7].
The incidence of fistulas after laparoscopic sleeve gastrectomy (LSG) ranges from 1.7 to 4% [3, 6, 8]. Fistulas after LSG result in acute and severe peritonitis, with mortality ranging from 8 to 37.5% [3]. The management of these fistulas is usually difficult and results in prolonged hospitalization. In the past, patients were re-operated with surgical attempts at closing the fistulas often ending in failure and total gastrectomy [2, 9]. Several studies have shown the importance and efficacy of an endoscopic approach which is minimally invasive for the management of these complications, thus avoiding the risks of surgical re-intervention [3, 10]. There have been case reports of recurrence of postsurgical fistulas in the upper digestive tract after endoscopic management [11, 12], but a recent comprehensive review on endoscopic management of post-LSG fistulas has not dealt with fistula recurrence [8].
At our hospital, patients with fistulas after LSG have been managed with a primarily endoscopic approach since 2008. We analyzed the incidence and factors influencing the recurrence of fistulas and its management after effective closure of LSG-related fistulas with a primarily endoscopic approach.
Materials and Methods
We performed a retrospective analysis of the medical charts of 12 consecutive patients who were treated for fistulas after LSG between 2008 and 2013 at a referral center in the south of Portugal. Due to the retrospective nature of the study, individual informed consent was not obtained, and patients were managed as per conventional standards of care. Institutional Review Board approval was obtained for the study. The bariatric surgery was laparoscopic and performed by experienced teams according to standard surgical techniques [13]. All patients with fistulas related to LSG were managed with a primarily endoscopic approach.
The occurrence of clinical symptoms and signs of sepsis after LSG was suggestive of the development of a fistula. The clinical suspicion of fistula was confirmed by an esophageal contrast study or abdominal CT scan before endoscopy and was performed in the majority of patients. Esophageal contrast studies were also performed after endoscopic management to demonstrate fistula closure, confirming the success of endoscopic treatment within 48 hin case of placement of over-the-scope clips (OTSC) or throughthe-scope clips (TTSC) and after at least 5 days in patients in whom a covered or partially covered stent was placed. CT scans were performed in the majority of patients to confirm the diagnosis and to detect and monitor the evolution of intra-abdominal collections and, when clinically indicated, to confirm successful closure of fistula orifices with oral contrast. Successful fistula closure was defined by absence of extraluminal leakage of oral contrast in esophageal contrast studies and/or CT scan.
All endoscopic procedures were performed by experienced endoscopists under deep sedation with propofol or general anesthesia under the care of an experienced anesthesiologist. In most cases, the endoscopies were performed with fluoroscopic control. Although we do not follow a specific management protocol for fistulas after bariatric surgery, in patients with a large fistula orifice (> 1 cm) or a long duration between surgery and fistula detection (> 4 weeks) or evidence of fibrosis making an effective closure of the fistula with clips difficult, we prefer to use covered metallic stents. The strategy used for the management of the fistulas was adapted to the clinical situation of each patient and the type of fistula. Therapeutic options used, alone or in combination, were TTSC and OTSC, metallic and plastic stents, and injection of cyanoacrylate and application of argon plasma at the fistula orifice. At our endoscopy unit, argon plasma is routinely applied empirically on the borders of chronic (> 4 weeks) and recurrent fistulas as we believe that this may stimulate angiogenesis and mucosal healing.
Recurrence of fistulas was associated with a need for hospital re-admission in all 3 patients in whom this occurred. We analyzed the influence of age, gender, pre- and postoperative body mass index (BMI), interval between surgery and fistula detection, as well as time to fistula closure, location and size of the fistula orifice, and number of endoscopies and hospitalizations as potential factors for fistula recurrence. Statistical analysis with descriptive statistics, Mann-Whitney test for independent samples, and Fishers exact test were performed using SPSS IBM®, version 20.
Results
Of the 12 patients with fistulas after LSG included in this study, 10 were operated at our center and 2 patients were referred for management to our endoscopy unit from other hospitals as shown in Figure 1. There were 371 patients who underwent LSG at our hospital during the study period, and, therefore, the incidence of fistulas after LSG at our hospital was 2.7%.
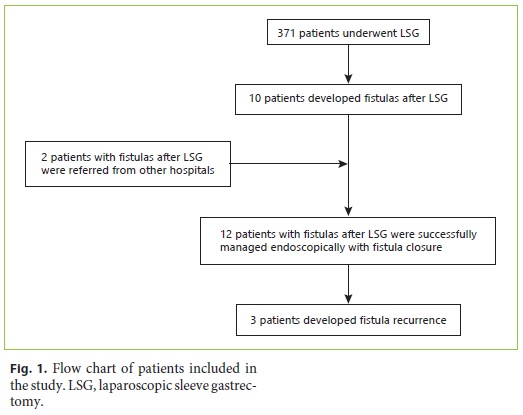
The demographic and clinical characteristics of the patients developing fistulas after LSG are shown in Table 1. The mean age at the time of development of fistulas after LSG was 43.3 ± 10.9 years, and 10 (83%) patients were female. The average BMI was 45.4 ± 7.2. Nine patients were morbidly obese (BMI 35–49.9), and 3 were severely obese (BMI > 50). The median follow-up time was 30.5 (15–72) months. The median time to detection of fistulas after LSG was 14 (4–145) days after surgery. According to the Rosenthal classification, 3 patients had acute, 8 patients early, and 1 patient chronic fistulas at the time of diagnosis [13].
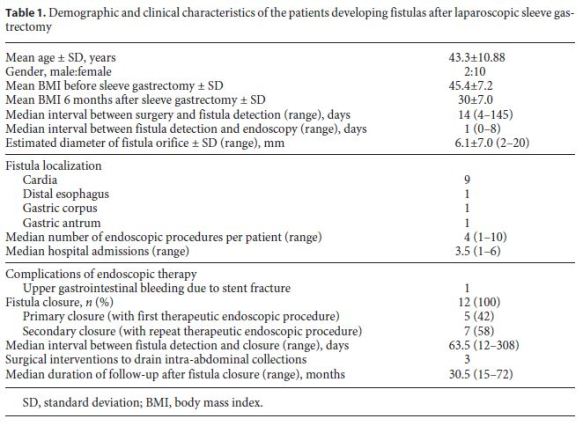
All patients were admitted to the surgical department or intensive care unit and were treated with broad-spectrum antibiotics. Abdominal CT scans were performed in all patients, and when required, intra-abdominal collections were drained either by percutaneous ultrasoundguided catheters or by laparoscopic peritoneal lavage. The median time between fistula detection and endoscopy was 1 (0–8) day, and in most cases (9/12, 75%), endoscopy was performed in less than 24 h after the diagnosis.
In 9 patients, the fistulas were located at the cardia, while the fistula orifice was located in the distal esophagus (n = 1), gastric corpus (n = 1), and at the transition of the gastric corpus and antrum (n = 1) in the remaining 3 patients. The mean diameter of fistula orifices as estimated endoscopically was 6.1 ± 7.0 (2–20) mm. In 7/12 (58.3%) patients, the fistulas had a diameter of ≤5 mm.
Covered metallic stents were used in 7 of the 12 patients. In 4 patients, a fully covered metallic stent (4 Hanarostent® 20/26 × 140, 30/36 × 240 mm, 1 Taewoong Niti-S® 24/32 × 230 mm) was used, and in 3 patients, a partially covered metallic stent (2 Ultraflex® 23/28 × 70/100 and 1 Hanarostent® 22/28 × 80/120 mm) was chosen. The stents were removed 6–8 weeks later, and at the time of stent removal, the fistula orifices had effectively closed in all but one patient. This patient had a chronic fistula orifice at the cardia which closed after several sessions of argon plasma application at the fistula orifice over a 6-month period during which nutrition was maintained with a nasoenteric tube.
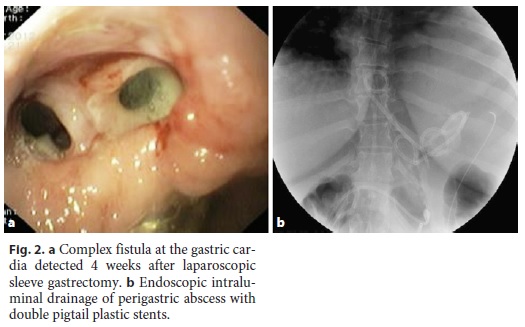
In 1 patient (patient 11) who developed sepsis 4 weeks after LSG, a large perigastric abscess was identified and drained with 2 double pigtail plastic stents placed endoscopically through the fistula orifice (Fig. 2). Broad-spectrum antibiotics were administered, and after 10 days, the patient had normalization of acute phase reactants and resolution of sepsis. The plastic stents were removed, and argon plasma was applied to fistula orifice margins, followed by placement of a partially covered metallic stent during the same procedure. Other combined endoscopic therapies done in the patients were the placement of clips (n = 8), argon plasma coagulation application on the borders of the fistula orifices (n = 4), and cyanoacrylate glue injection (n = 2). These endoscopic interventions are summarized in Table 2.
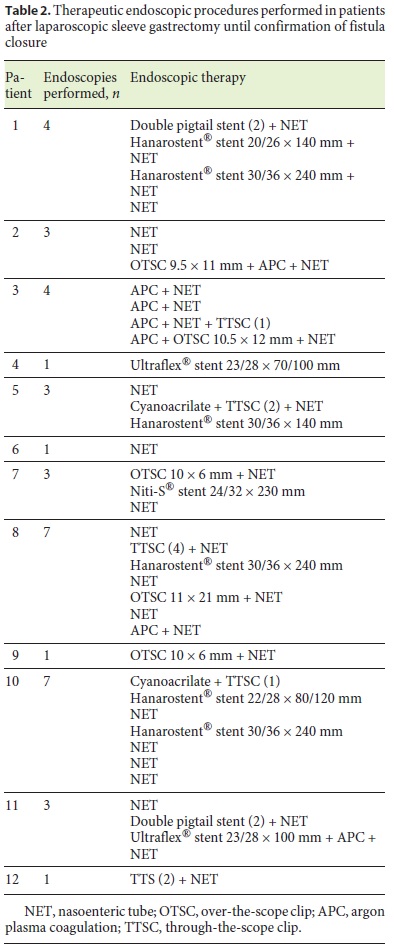
A nasoenteric tube was placed in 11 of 12 patients in order to allow for immediate enteral feeding even when the primary closure of the fistula orifice was unsuccessful. Three patients also underwent additional surgical intervention with laparoscopic peritoneal lavage and placement of abdominal drains. In 1 patient, suturing of the fistula orifice was attempted without clinical success.
The endoscopic approach to fistula management after LSG was successful in closing fistula orifices in all patients, with the first endoscopic therapeutic procedure being successful in closing the fistula in 5 (41%) patients.
The median interval between fistula detection and confirmation of closure of the fistula was 63.5 (12–308) days. There were no major complications of endoscopic therapy with only 1 case of gastrointestinal bleeding caused by fracture of a metallic stent in a patient on anticoagulant medication. The stent was removed without difficulty with resolution of the gastrointestinal bleeding episode.
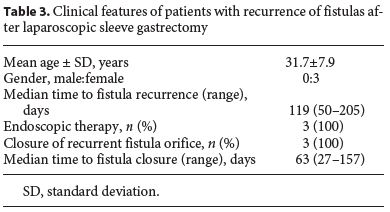
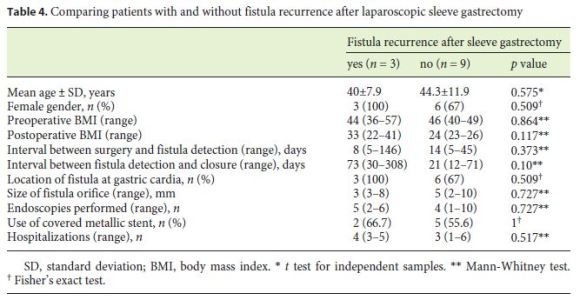
The median follow-up of the patients was 30.5 (15–72) months. In 3 (25%) patients, there was fistula recurrence after a median of 119 (50–205) days following the closure of the initial fistula orifice. All patients were female and were managed endoscopically. The endoscopic procedures used in these patients were as follows: covered metallic stent (n = 1), nasoenteric tube (n = 1), and TTSC to close the fistula orifice after application of argon plasma coagulation on the margins of the fistula orifice (n = 1) (Fig. 3, 4). The median time to confirmation of successful closure of the recurrent fistula was 63 (27–157) days. Table 3 summarizes this group of patients. We could not detect any factors associated with fistula recurrence, as can be seen in Table 4. One of the patients had a second recurrence of the fistula at the gastric cardia 6 months after closure of fistula recurrence and was managed by reoperation and anastomosis of a jejunal loop to the fistula orifice with no further incidents.
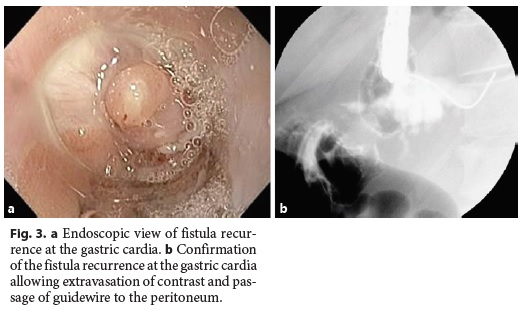
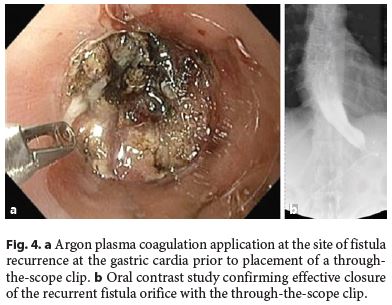
Discussion
The incidence of fistulas after LSG at our hospital was 2.7% (10/371), which is similar to what has been reported in various studies (1.7–4%) [3, 6, 8]. The postoperative course in patients who develop fistulas after LSG is usually challenging with prolonged hospitalizations and morbidity rates as well as increased risk of mortality ranging from 8 to 37.5% [3, 14]. Fortunately, there was no mortality related to fistula development after LSG in our study cohort.
The free communication of the fistula with the peritoneum results in acute peritonitis and intra-abdominal abscesses that may be associated with acute respiratory distress syndrome, sepsis, and multi-organ failure often requiring management in an intensive care unit setting [4, 14]. For this reason, all patients in our study were managed in a critical care unit after fistula detection.
The majority of fistulas are known to be located in the proximal third of the stomach, usually near the gastric cardia [8], and this was also observed in 9 (75%) patients in our study. Following the development of a fistula, the tissue surrounding the fistula orifice is friable and edematous due to inflammation with consequent ineffectiveness of surgical sutures at that location to close the fistula orifice [3, 9], ultimately requiring total gastrectomy in some patients [4]. As these patients are often seriously ill with sepsis, a second major surgery often results in severe stress with a consequent higher risk of mortality [14]. The endoscopic approach has several potential advantages, including its minimally invasive nature, lack of influence of BMI, and the minimal inflammation produced by the procedure, which does not interfere with the healing process [3]. Surgery, however, continues to have a role in the management of patients with large intra-abdominal abscesses which cannot be drained by a percutaneous approach [4]. Drainage of intra-abdominal abscesses and fluid collections is performed as part of the management strategy either prior, during, or immediately after the endoscopic closure of the fistula orifice.
The endoscopic interventions at our center were guided by 3 principles which have been previously mentioned by other authors: (1) identification of the fistula orifice and drainage with debridement of perigastric collections when possible; (2) closure or exclusion of the fistula orifice with clips or covered metallic stents; and (3) nasoenteric tube placement for early introduction of enteral nutrition [3, 8].
The average size of the initial fistula was 6.1 ± 7.0 mm in the study cohort. Fistula orifices ≥10 mm in size have been reported to have more difficulty healing with stents compared to smaller fistulas [15]. In a recent review on endoscopic management of complications of bariatric surgery by Eisendrath and Deviere [8], the endoscopic approach to managing fistulas after bariatric surgery was associated with a success rate of over 80%. In 4 patients, a fully covered metallic stent (4 Hanarostent® 20/26 × 140, 30/36 × 240 mm, 1 Taewoong Niti-S® 24/32 × 230 mm) was used, and in 3 patients, a partially covered metallic stent (2 Ultraflex® 23/28 × 70/100 and 1 Hanarostent® 22/28 × 80/120 mm) was chosen. We prefer to use the specific stents for managing complications after bariatric surgery developed by Hanarostent® and Taewoong®, metallic stents with a larger diameter and partially covered metallic stents in order to decrease the risk of migration. In a recent meta-analysis, stent placement was effective in managing post-LSG fistulas in 73% of patients with a low number of stents per patient and migration rates of 27.1 and 31.5% according to the type of esophageal stent utilized [16].
In patients in whom the fistula orifice is small (< 1 cm) and when the fistula is detected within 4 weeks after LSG, we prefer to use the Ovesco® OTSC or the conventional TTSC ResolutionTM (Boston Scientific, USA). The initial fistulas after LSG were successfully managed with a primarily endoscopic approach in all 12 patients. The median interval between fistula detection and confirmation of closure of the fistula was 63.5 (12–308) days.
After a median follow-up of 30.5 (15–72) months, recurrence of fistulas was detected in 3/12 (25%) patients after a median of 119 (50–205) days. Recurrences of fistulas were located in the gastric cardia in all 3 patients. In 1 patient, fistula recurrence was attributed to a perigastric abscess which had been left undrained and which subsequently opened into the gastric lumen via the previously healed fistula orifice. In the other 2 patients, no contributing factor could be attributed to the recurrence of the fistula.
The fistulas healed in all 3 patients with the therapeutic endoscopic techniques used, and 2 patients have been asymptomatic without further recurrence of the fistulas after more than 2 years of follow-up. One patient had a second recurrence of the fistula 6 months after fistula closure and was re-operated; a Roux-en-Y fistulo-jejunostomy at the fistula orifice site at the gastric cardia was performed as has been successfully done in other patients [17].
We could speculate that the pathophysiological mechanisms postulated to explain the development of fistulas following LSG could also explain fistula recurrence. Some authors suggest that the gastric cardia is a region of high pressure, and episodes of retching may result in a massive increase in pressure at the cardia, resulting in the development of a fistula. Other authors have suggested that the gastric cardia in patients who develop fistulas has abnormal vasculature with poor blood supply, and the resultant ischemia and peristaltic dysfunction facilitate the development of fistulas [3, 18]. Although the time to detection of fistulas after LSG was longer in patients with fistula recurrence (35 [7–146] vs. 14 [4–45] days in patients without fistula recurrence) and the time to fistula closure observed in patients with fistula recurrence was shorter (21 [12–71] days) compared to that in patients without fistula recurrence (73 [30–308] days), these diferences were not significant. We did not find any factors at initial fistula detection significantly associated with fistula recurrence during follow-up, probably due to the small number of patients included in our study.
Our study has some limitations due to its retrospective nature and the consequent risk of selection bias. Additionally, the cohort of consecutive patients with post-LSG fistulas is relatively small, which may explain the lack of power in detecting differences in patient who did and did not develop fistula recurrence after successful initial closure of post-LSG fistula. However, we believe that the results of this study are clinically relevant and may justify a long-term follow-up of these patients.
Conclusion
A primarily endoscopic therapeutic approach was a highly effective and safe management strategy for fistulas after LSG in appropriately selected patients. Despite the retrospective nature and potential selection bias, up to 25% of the patients in this retrospective cohort study had a recurrence of the fistulas, highlighting the need for a close follow-up of these patients and for prospective randomized studies to confirm these findings. Endoscopic therapeutic techniques are effective in managing fistula recurrence in appropriately selected patients.
References
1 Quigley S, Colledge J, Mukherjee S, Patel K: Bariatric surgery: a review of normal postoperative anatomy and complications. Clin Radiol 2011;66:903–914. [ Links ]
2 Fridman A, Moon R, Cozacov Y, Ampudia C, Lo Menzo E, Szomstein S, et al: Procedurerelated morbidity in bariatric surgery: a retrospective short- and mid-term follow-up of a single institution of the American College of Surgeons Bariatric Surgery Centers of Excellence. J Am Coll Surg 2013;217:614–620. [ Links ]
3 Bège T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noël P, et al: An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc 2011;73:238–244. [ Links ]
4 Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, et al: Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc 2012;27:240–245. [ Links ]
5 Nienhuijs SW, de Zoete JP, Berende CAS, de Hingh IHJT, Smulders JF: Evaluation of laparoscopic sleeve gastrectomy on weight loss and co-morbidity. Int J Surg 2010;8:302–304. [ Links ]
6 Aurora AR, Khaitan L, Saber AA: Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc 2012;26:1509–1515. [ Links ]
7 Pirolla EH, Jureidini R, Barbosa ML, Ishikawa LC, Camargo PR: A modified laparoscopic sleeve gastrectomy for the treatment of diabetes mellitus type 2 and metabolic syndrome in obesity. Am J Surg 2012;203:785–792. [ Links ]
8 Eisendrath P, Deviere J: Major complications of bariatric surgery: endoscopy as first-line treatment. Nat Rev Gastroenterol Hepatol 2015;12:701–710. [ Links ]
9 Jurowich C, Thalheimer A, Seyfried F, Fein M, Bender G, Germer C-T, et al: Gastric leakage after sleeve gastrectomy – clinical presentation and therapeutic options. Langenbecks Arch Surg 2011;396:981–987. [ Links ]
10 Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, Fantini A, et al: Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg 2009;19:821–826. [ Links ]
11 Zanotti D, Elkalaawy M, Mohammadi B, Hashemi M, Jenkinson A, Adamo M: Gastrocutaneous fistula 4 years after a fully resolved staple line leak in sleeve gastrectomy. J Surg Case Reports 2015;2015:1–3. [ Links ]
12 Truong S, Bohm G, Klinge U, Stumpf M, Schumpelick V: Results after endoscopic treatment of postoperative upper gastrointestinal fistulas and leaks using combined Vicryl plug and fibrin glue. Surg Endosc 2004;18:1105–1108. [ Links ]
13 Rosenthal RJ: International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of > 12,000 cases. Surg Obes Relat Dis 2012;8:8–19. [ Links ]
14 Puli SR, Spofford IS, Thompson CC: Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc 2012;75:287–293. [ Links ]
15 Baretta G, Campos J, Correia S, Alhinho H, Marchesini JB, Lima JH, et al: Bariatric postoperative fistula: a life-saving endoscopic procedure. Surg Endosc 2015;29:1714–1720. [ Links ]
16 Okazaki O, Bernardo WM, Brunaldi VO, Junior CCC, Minata MK, de Moura DTH, et al: Efficacy and safety of stents in the treatment of fistula after bariatric surgery: a systematic review and meta-analysis. Obes Surg 2018;28:1788–1796. [ Links ]
17 Elie C, Antoine Y, Mubarak A, Ronald D, Bernard D, Salman A, et al: Roux-en-Y fistulojejunostomy as a salvage procedure in patients with post-sleeve gastrectomy fistula: midterm results. Surg Endosc 2016; 30:4200–4204. [ Links ]
18 Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, Fantini A, et al: Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg 2009;19:821–826. [ Links ]
Statement of Ethics
Due to the retrospective nature of the study, individual informed consent was not obtained. Institutional Review Board approval was obtained for the study.
Disclosure Statement
None of the authors have any conflicts of interest to declare.
Funding Sources
There was no funding for the study.
* Corresponding author.
Dr. Carlos Noronha Ferreira
Serviço de Gastrenterologia e Hepatologia
Hospital de Santa Maria, Centro Hospitalar Lisboa Norte
Avenida Egas Moniz, PT–1649-035 Lisbon (Portugal)
E-Mail carlosnferreira@homail.com
Received: April 23, 2018; Accepted after revision: July 31, 2018
Acknowledgements
We are grateful to the nursing staff of the endoscopy unit and the surgery department for their care of patients involved in the study.
Author Contributions
Patricia Sousa: data collection, preparation and critical review of the manuscript. Carlos Noronha Ferreira: data analysis, preparation and critical review of the manuscript. João Coutinho, Fátima Carepa, Rosário Rosa, Andreia Barão, Carlos Marques Ferreira, José Girao, António Ruivo, Henrique Bicha Castelo, João Lopes, Amélia Almeida, Luís Carrilho Ribeiro, and José Velosa: critical review of the manuscript.














