Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.26 no.3 Lisboa jun. 2019
https://doi.org/10.1159/000492202
ORIGINAL ARTICLE
Polidocanol Foam Injected at High Doses with Intravenous Needle: The (Almost) Perfect Treatment of Symptomatic Internal Hemorrhoids
Polidocanol espumoso em doses altas injetado com agulha endovenosa: O tratamento (quase) perfeito das hemorróidas internas sintomáticas
Vítor Fernandesa,b, Jorge Fonsecac,d
aClínica de Gastrenterologia de Almada, Almada, Portugal; bClínica CUF Almada, Monte da Caparica, Portugal; cHospital Garcia de Orta, Almada, Portugal; dCentro de Investigação Interdisciplinar Egas Moniz, Monte da Caparica, Portugal
* Corresponding author.
ABSTRACT
Background and Aims: Hemorrhoid disorders are common. This study aimed to assess the efficacy and safety of polidocanol foam injected at high doses with intravenous needle for the treatment of symptomatic internal hemorrhoids that prolapse or bleed. Methods: We evaluated 2,000 consecutive patients with prolapsed hemorrhoids (grades II/III/IV) recruited over 6 years. Foam injection was performed in one to four sessions with polidocanol 2%: 10 mL of the mixture (2 mL liquid plus air) or 20 mL (4 mL liquid plus air). The number of sessions and amount of foam injected depended on initial hemorrhoid size, compliance to receive foam, and clinical response. The mixture, prepared using a three-way tap connected to two 10/20-mL syringes, was injected immediately after preparation using an intravenous needle. The primary endpoint was self-reported satisfaction without major complications at 4 weeks. Results: Efficacy was very high, with 1,957 patients (98%) reporting satisfaction regarding blood loss control and prolapse reduction. The procedure was well tolerated: 1,838 patients (92%) presented mild/no pain. Objective reduction of prolapse volume was documented in 86% of reobserved cases. Complications were rare and usually minor: only 3 cases of clinically significant bleeding (0.15%), 2 of whom were on dual antiplatelet therapy plus oral anticoagulation, 2 cases of rectal abscess, 8 hemorrhoid thromboses, and 1 urinary retention requiring catheter (0.7% severe complications). Conclusions: Treatment of internal hemorrhoids with polidocanol foam injected in high doses is very effective and safe for the control of blood loss and prolapse, even for patients on anticoagulation/antiplatelet treatment.
Keywords: Polidocanol foam, Hemorrhoids, Prolapse, Hematochezia, Treatment
RESUMO
Introdução e objetivo: A doença hemorroidária é muito vulgar. O presente estudo pretende avaliar a eficácia e a segurança do polidocanol espumoso em doses altas injetado com agulha endovenosa no tratamento das hemorroidas internas que prolapsam e sangram. Métodos: Foram avaliados 2,000 doentes consecutivos, com hemorroidas prolapsadas (graus II/III/IV), ao longo de 6 anos. O polidocanol espumoso foi injetado em uma a quatro sessões: 10 ml de mistura (2 ml de polidocanol a 2% + ar) ou 20 ml de mistura (4 ml de polidocanol a 2% + ar). O número de sessões e a quantidade de espuma injetada dependeram do tamanho inicial das hemorróidas, da complacência durante a injeção e da resposta clínica. A mistura foi preparada usando uma torneira de três vias e duas seringas de 10/20 ml e injetada imediatamente após a preparação, usando uma agulha endovenosa. O objetivo primário foi a satisfação avaliada pelo doente, sem complicações graves, 4 semanas após o tratamento. Resultados: A eficácia foi muito elevada, com 1,957 doentes (98%) satisfeitos com a redução do prolapso e o controlo da hemorragia. O tratamento foi bem tolerado: 92% (1,838 doentes) não tiveram dor ou sofreram dor ligeira. A redução do prolapso foi documentada em 86% dos casos reobservados. As complicações foram raras e maioritariamente ligeiras: só 3 casos sofreram hemorragia significativa (0.15%), dois dos quais sob anticoagulação e dupla antiagregação; 2 casos de abcesso rectal; 8 tromboses hemorroidárias; e uma retenção urinária necessitando algaliação (complicações graves: 0.7%). Conclusões: O tratamento das hemorróidas internas com polidocanol espumoso injetado em dose elevada é eficaz, controlando a hemorragia e o prolapso, e seguro, mesmo em doentes sob anticoagulação/antiagregação.
Palavras-Chave: Polidocanol espumoso, Hemorróidas, Prolapso, Hematoquésia, Tratamento
Introduction
Internal and external hemorrhoids are normal vascular structures [1]. External hemorrhoids manifest as a painful perianal swelling associated with venous thrombosis and less commonly hematoma. When necessary, anti-inflammatory drugs, ice, and blood/thrombus/venous plexus drainage are recommended therapies, but these procedures are beyond the scope of the present study [2, 3]. Internal hemorrhoids are formed by arterioles, venules, and capillaries, supported by connective tissue. They act as vascular cushions or “cavernous bodies” that swell with blood and help to seal the anal canal [4]. They contribute with about 10% of the basal anal pressure, being important for the “fine continence” [5]. Internal hemorrhoids may present clinically as painless bleeding after defecation and, when becoming bulky and prolapsed (grades III/IV), can cause discomfort, burning sensation, pruritus, and presence of red blood in underwear [6, 7].
Treatment of internal hemorrhoids should only be performed in patients with bleeding or symptoms associated with prolapse. Treatment depends on hemorrhoid severity, classified in grades I–IV (position statement 2004). Internal hemorrhoids grade I are small, do not prolapse, and may be fulgurated (for example using photocoagulation or argon plasma coagulation) or sclerosed through injection [8]. Hemorrhoids grade II only prolapse with defecation effort. Elastic banding and/or sclerosis treatment are usually recommended. Meta-analyses of nonoperative therapy show that patients who undergo sclerotherapy have a relatively high relapse rate and that rubber band ligation is associated with the lowest recurrence rate of the nonoperative techniques [9]. Surgery is reserved for hemorrhoids grade III and IV that are exteriorized (prolapsed) [2, 10].
There are several complications of instrumental and surgical treatment of hemorrhoids, which may be serious in some patients [11–14].
Elastic banding can result in severe late bleeding, usually between days 7 and 22 after treatment in the authors’ experience. The more effective the banding (“pistols” with deep and wide cylinder, 1 × 1 cm), the bigger the ulcer after the band falls and the greater the bleeding risk. In our experience with more than 10,000 patients treated over a 22-year period, late bleeding occurred in almost 4.5% of patients when two to three bands were applied per session (unpublished data). Pain, local discomfort, and tenesmus sometimes develop even when elastic bands are placed > 1 cm above the dentate line. Urinary retention, infection, and sepsis are rare complications [3, 10].
Although classical hemorrhoidectomy (Milligan-Morgan and Ferguson) is highly effective, the postoperative period is very painful, mainly during the first 3 weeks. Anal deformity, discharge, and incontinence may be important sequela. Furthermore, late bleeding, urinary retention, and sepsis can also be potential adverse events [2, 10, 12, 14].
Longo hemorrhoidopexy is less painful but also less effective for prolapse treatment. The most striking complication is chronic persistent anal pain, which is not uncommon. The pain is thought to be associated with staple placement and venous plexus dissection near the dentate line [15–17].
Injection of a sclerosing agent is considered poorly effective and therefore used only for small hemorrhoids. Liquid polidocanol 1 or 2% is commonly injected through the anoscope in low doses using a 1.0-mL syringe (e.g., insulin syringe) and a 25- to 27-G needle (e.g., subcutaneous needle). Experts often recommend 2 mL per session.
More than 10 years ago, we begun using polidocanol 2% foam, which results from the mixture of liquid with air, changing drug characteristics with more sclerosing capacity and adherence to the endothelium with the same safety profile, but there is only one import study of its use and only on first-grade hemorrhoidal disease [18, 19]. We learned with vascular surgery that used it regularly. We started by gradually increasing the dose in patients with hemorrhoids grade II, III, and IV under oral anticoagulant or double antiplatelet therapy and active bleeding. In these patients, there were few therapeutic options given the high bleeding risk associated with surgery and elastic banding. We verified that it was an extremely safe and effective procedure. Furthermore, we increased the dose and used an intravenous needle (21-G green, intravenous needles) of greater caliber which made it possible to inject thicker and “creamy” polidocanol foam (Figure 1). When preparing the foam, we need a three-way tap adapted to two syringes. We obtained as a gold standard the mixture of 2 mL polidocanol liquid with air using two 10-mL syringes or 4 mL polidocanol using two 20-mL syringes. Thus, a mixture of 10 mL (2 mL of liquid polidocanol mixed with air) or 20 mL (4 mL liquid polidocanol mixed with air) is injected, depending on the hemorrhoid size and its compliance to receive the mixture. The foamy polidocanol that results from the mixing of the liquid polidocanol with air has greater adhesion to the endothelium, greater capacity of sclerosis, and greater efficacy, as documented in several phlebology studies, maintaining safety.
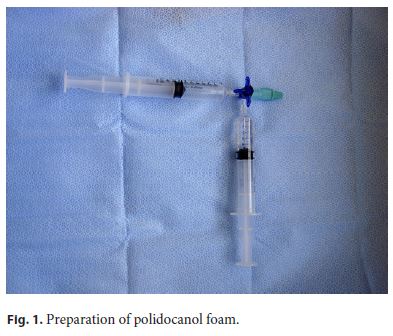
The present study intended to evaluate the use of polidocanol foam in high doses over an intravenous needle for the treatment of a large number of patients with symptomatic internal hemorrhoids. Since grade I hemorrhoids are easy to treat and do not prolapse, they were not included in this study.
Patients and Methods
We performed a prospective single-center study to evaluate the efficacy and safety of polidocanol foam for the treatment of symptomatic internal hemorrhoids. Patients were included at a proctology outpatient clinic of the gastroenterology department of a large hospital in the Lisbon area, a tertiary center for hemorrhoid therapy. The study aimed to evaluate 2,000 patients. The same gastroenterologist treated all the patients. Inclusion criteria were: (1) age ≥18 years; (2) internal hemorrhoids grades II, III, or IV presenting with hemorrhage, prolapse, or both; (3) written informed consent; (4) ability to fulfill the follow-up protocol. Exclusion criteria were: (1) age < 18 years; (2) anal fissure; (3) anal infection/suppuration; (4) anal pain other than caused by hemorrhoids; (5) contraindication to polidocanol use: known allergy (anaphylaxis) to polidocanol.
Patients were encouraged to defecate before the procedure but did not under go any bowel preparation, including cleaning enemas. Treatment was performed with the patient in the knee-chest position or lying on the left side. All treatments were performed in an outpatient basis and without any sedation. No prophylactic antibiotics were given. All large and symptomatic hemorrhoids were aimed to be treated at the same session, although a second session might be needed.
The following were used for each patient: (1) a large adult anoscope; (2) polidocanol 2%, 2 mL of liquid to mix with air in order to obtain 10 mL of polidocanol foam, or 4 mL to mix with air in order to obtain 20 mL of polidocanol foam; (3) three-way tap and two 10-mL syringes in order to obtain 10 mL of polidocanol foam, or two 20-mL syringes in order to obtain 20 mL of polidocanol foam; (4) a 21-G needle with an extension line.
To prepare the foam, a three-way tap adapted to two syringes was used. A mixture of either 2 mL polidocanol liquid with air using two 10-mL syringes, or 4 mL polidocanol with air using two 20-mL syringes was used. A mixture of 10 mL (2 mL of liquid polidocanol mixed with air) or 20 mL (4 mL liquid polidocanol mixed with air) was injected, depending on the size of the hemorrhoid and its compliance to receive the mixture. It was very easy to manage the two syringes. The liquid was placed in a syringe, in the other the amount of air we wanted. The three-way tap was used for mixing.
With the anoscope introduced, the hemorrhoid to be treated was clearly identified. The polidocanol foam was prepared in the treatment room and was injected immediately after being prepared. Delay may decrease foam volume, allow for liquid accumulation, and potentially reduced efficacy. The polidocanol foam was injected above the dentate line. Each hemorrhoid was injected once, in several directions and at variable depths, until the hemorrhoid turned larger and whitish. A small, self-limited bleed was common.
After the procedure, patients were allowed to use paracetamol as needed for pain or discomfort control. They received the physician’s direct phone number and were instructed to report any problem. In the first and fourth week after treatment, all patients were contacted by phone.
Success was defined as self-reported reduction or disappearance of bleeding and prolapse at 4 weeks, without major complications. Complications were defined as follows: (1) Minor complications: (a) postprocedure pain was defined as mild, moderate, or intense, treated with paracetamol/NSAIDs without need for hospital admission. (2) Major complications: (a) clinically significant bleeding defined as the need for any of the following: blood transfusion, in-hospital admission, or antiplatelet/anticoagulation suspension; (b) severe pain requiring hospital admission; (c) urinary retention with need for urinary catheter placement; (d) unplanned hemorrhoid surgery on follow-up.
Results
A total of 2,000 consecutive patients treated with polidocanol foam were included (Table 1) between January 2012 and August 2017, with ages ranging from 22 to 94 years (mean age 54 years); 1,162 of them were males (58%). The shortest follow-up was 4 months. Hemorrhoid grade had the following distribution: grade II, 1,222 patients (61%); grade III, 577 patients (29%); grade IV, 201 patients (10%).
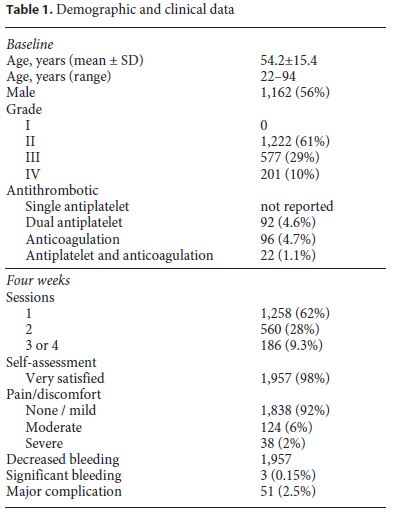
A total of 210 patients (10.5%) were taking anticoagulants or double antiplatelet therapy: 92 patients were under antiplatelet therapy with two antiplatelet drugs, 96 patients were under anticoagulation, and 22 patients were simultaneously under anticoagulation and antiplatelet therapy. For this study, patients taking only one antiplatelet agent were not considered as having an increased bleeding risk.
The number of treatment sessions needed to achieve satisfactory clinical outcome was the following: 1,258 patients (63%) underwent 1 session; 560 patients (28%) underwent 2 sessions; 182 patients (9%) underwent 3 or 4 sessions.
At telephone follow-up, 1,957 (98%) reported to be “very satisfied” with the result of the treatment. The reported discomfort was the following: 1,838 patients (92%) experienced no or slight pain controlled with paracetamol; 124 patients (6%) reported moderately intense pain; 38 patients (2%) suffered severe pain controlled with para- cetamol plus NSAIDs and not requiring hospital admission (Table 1). This was associated with either intra-anal hemorrhoid thrombosis or intrarectal suppuration (a marker of infection) with rectal tenesmus.
Surgery was performed in 10 cases (0.5%), in 8 cases to treat thrombosis and in 2 for suppuration. Anal suppuration was only observed after the second treatment session, when the hemorrhoid tissue had already suffered partial fibrosis. Most of the cases occurred in the early phase of this study.
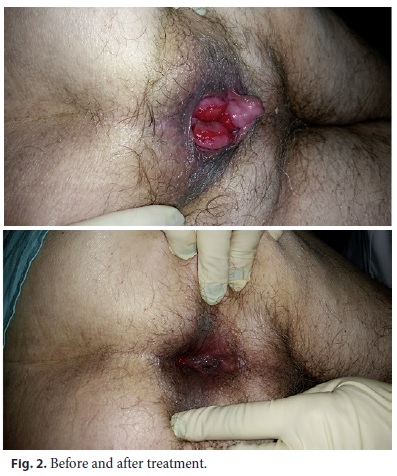
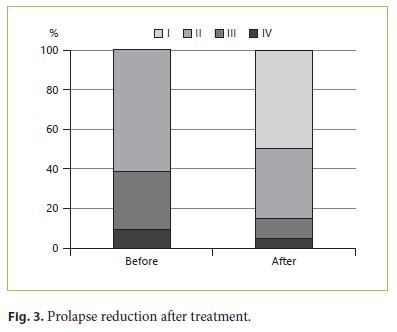
Although it was not part of the protocol, 1,112 patients were reexamined at their own request. Of these, 954 (86%) had reduction of the prolapse confirmed by directed observation by the investigator (Fig. 2, 3).
Twenty-two patients were simultaneously under anticoagulation and antiplatelet therapy. Of these, 2 (9%) (both on apixaban, acetylsalicylic acid, and clopidogrel) suffered clinically significant bleeding, requiring in-hospital admission, blood transfusion, and suspension of anticoagulant therapy. Despite the bleed, these patients presented adequate fibrosis after 3 weeks. Only 1 case of clinically significant bleeding occurred in patients without anticoagulation or antiplatelet therapy (1 out of 1,790 [0.05%]).
A few male patients complained from transient dysuria (at 24–48 h), but only 4 male patients (0.3%) suffered from transient urinary retention, and only 1 patient needed Foley catheter placement.
Discussion
Hemorrhoid disorders affect almost 5% of the western population and are a very frequent motive for attending a gastroenterology outpatient clinic [10, 20]. Besides causing pain or discomfort and bleeding, the symptoms often cause apprehension, fear of cancer, and psychological and social embarrassment. Furthermore, patients often described their complaints with shame or a sense of indignity.
A large number of drugs, instrumental procedures, and surgical interventions are used in hemorrhoid therapy, but their overall efficacy is only moderate, and they have frequent adverse side effects and complications, in particular with surgery [2, 10, 12, 14].
In our experience, polidocanol foam injected into the hemorrhoids is very effective, reducing bleeding and prolapse in 98% of patients, even in a complex population with 39% of patients having grade III and IV. Much larger amounts than usual (10 or 20 mL of the mixture) may be applied when polidocanol is used with liquid. Internal hemorrhoids (arterioles and veins, supported by connective tissue) function as a cushion/pillow (corpus cavernosum) with compliance to receive 10 mL, 20 mL, or more of foamy polidocanol, depending on the size/compliance. In our experience, we have documented greater effectiveness with high doses and safety. Also, it is a very safe therapy, with only 0.7% of serious complications, such as major bleeding, urinary retention, or infection/suppuration requiring surgery. The therapeutic thrombosis and fibrosis of internal hemorrhoids induced by polidocanol foam is very well tolerated, as it progresses without clinically significant symptoms in the large majority of patients – only 2% reported severe pain, which was controlled with paracetamol plus NSAIDs.
Polidocanol foam proved to be effective and safe even for patients under anticoagulation/antiplatelet therapy. For these patients, polidocanol foam is our preferred therapy, as it does not require any adjustment or inter ruption of the anticoagulation/antiplatelet therapy and was associated with acceptable bleeding rates. Only 3 out of 2,000 patients (0.15%) presented clinically significant bleeding, and all were easily managed. Two of them were simultaneously under anticoagulation and antiplatelet therapy. Eventually, the patients under this association may need a closer follow-up after the procedure. Clearly, polidocanol foam is the therapy of choice. For these patients, the liquid form of polidocanol is poorly effective. Ligation and surgery are at high risk for late-onset bleeding. In this group of patients, we recommend polidocanol foam because it is very effective and the risk of bleeding is acceptable (low), and there are no other options if we want to keep up antiplatelet therapy/anticoagulation.
Other complications, such as transient urinary retention, were very rare, reported in only 0.3% of males. There were no cases of embolism in our experience. Foamy polidocanol adheres to the endothelium, provoking inflammatory response and sclerosis. Furthermore, vascular surgeons inject the foamy polidocanol directly into the external saphenous vein, and this is considered a very safe procedure.
Serious complications arose after the second treatment session and seem to have been due to a too deep or forced injection of the foam. Most of them occurred at the beginning of the study, evidencing the need for a progressive learning curve with this technique, in particular when performing repeated therapeutic sessions. It is advisable to inject the foam only in soft hemorrhoids. The suppurations arose as a complication in an early phase of the study: they were due to the forced and deep injection. The injection has to be into the submucosa. Hemorrhoids have compliance. Polidocanol foam should not be injected deeply or forced if fibrosis already exists (previously treated hemorrhoids). Any deep or forced injection should be avoided, in particular in previously treated hemorrhoids, which may lead to foam injection into the deep muscular layers, into the prostate, or into the seminal vesicles. Carefully used, polidocanol foam is very safe. It should be stressed that complete patient information is mandatory and that, after the procedure, the physician must be easily available for the patient’s doubts or procedure complications.
Conclusions
Polidocanol foam therapy of symptomatic hemorrhoid diseases is very safe. It does not need any special bowel preparation, sedation, or pain control, and it can be performed on an outpatient basis. Polidocanol foam should be used as first-line treatment of most hemorrhoid patient, including those under anticoagulation and antiplatelet therapy. Gastroenterologists, surgeons, and proctologists who usually perform therapeutic treatment of benign proctologic lesions should become proficient with this technique.
References
1 Jacobs D: Clinical practice. Hemorrhoids. N Engl J Med 2014;371:944–951. [ Links ]
2 Bleday R, Breen E: Home and office treatment of symptomatic hemorrhoids. UpToDate 2017. http://www.uptodate.com/contents/treatment-of-hemorrhoids. [ Links ]
3 Rivadeneira DE, Steele SR, Ternent C, et al: Practice parameters for the management of hemorrhoids (revised 2010). Dis Colon Rectum 2011;54:1059–1064. [ Links ]
4 Singer M: Hemorrhoids; in Beck DE, Robert PL, Saclarides TJ, et al (eds): The ASCRS Textbook of Colon and Rectal Surgery, ed 2. New York, Springer, 2011, pp 175–202. [ Links ]
5 Schubert MC, Sridhar S, Schade RR, Wexner SD: What every gastroenterologist needs to know about common anorectal disorders. World J Gastroenterol 2009;15:3201–3209. [ Links ]
6 Riss S, Weiser FA, Schwameis K, et al: The prevalence of hemorrhoids in adults. Int J Colorectal Dis 2012;27:215–220. [ Links ]
7 Bleday R, Breen E: Hemorrhoids: clinical manifestations and diagnosis. UpToDate 2017. http://www.uptodate.com/contents/hemorrhoids-clinical-manifestations-anddiagnosis. [ Links ]
8 Linares Santiago E, Gómez Parra M, Mendoza Olivares FJ, et al: Effectiveness of hemorrhoidal treatment by rubber band ligation and infrared photocoagulation. Rev Esp Enferm Dig 2001;93:238–247. [ Links ]
9 MacRae HM, McLeod RS: Comparison of hemorrhoidal treatments: a meta-analysis. Can J Surg 1997;40:14–17. [ Links ]
10 Rivadeneira DE, Steele SR: Surgical treatment of hemorrhoidal disease. UpToDate 2017. http://www.uptodate.com/contents/surgicaltreatment-of-hemorrhoidal-disease. [ Links ]
11 Sneider EB, Maykel JA: Diagnosis and management of symptomatic hemorrhoids. Surg Clin North Am 2010;90:17–32. [ Links ]
12 Chand M, Nash GF, Dabbas N: The management of haemorrhoids. Br J Hosp Med (Lond) 2008;69:35–40. [ Links ]
13 MacRae HM, McLeod RS: Comparison of hemorrhoidal treatment modalities. A metaanalysis. Dis Colon Rectum 1995;38:687–694. [ Links ]
14 Lohsiriwat V: Treatment of hemorrhoids: a coloproctologist’s view. World J Gastroenterol 2015;21:9245–9252.
15 Jayaraman S, Colquhoun PH, Malthaner RA: Stapled versus conventional surgery for hemorrhoids. Cochrane Database Syst Rev 2006;4:CD005393. [ Links ]
16 Watson AJ, Hudson J, Wood J, et al: Comparison of stapled haemorrhoidopexy with traditional excisional surgery for haemorrhoidal disease (eTHoS): a pragmatic, multicentre, randomised controlled trial. Lancet 2016;388:2375–2385. [ Links ]
17 Giordano P, Gravante G, Sorge R, et al: Longterm outcomes of stapled hemorrhoidopexy vs conventional hemorrhoidectomy: a metaanalysis of randomized controlled trials. Arch Surg 2009;144:266–272. [ Links ]
18 Moser KH, Mosch C, Walgenbach M, Bussen DG, Kirsch J, Joos AK, Gliem P, Sauerland S: Efficacy and safety of sclerotherapy with polidocanol foam in comparison with fluid sclerosant in the treatment of first-grade haemorrhoidal disease: a randomised, controlled, single-blind, multicentre trial. Int J Colorectal Dis 2013;28:1439–1447. [ Links ]
19 Ouvry P, Allaert FA, Desnos P, et al: Efficacy of polidocanol foam versus liquid in sclerotherapy of the great saphenous vein: a multicenter randomized controlled trial with a 2-year follow-up. Eur J Vasc Endovasc Surg 2008;36:366–370. [ Links ]
20 Johanson JF, Sonnenberg A: The prevalence of hemorrhoids and chronic constipation: an epidemiologic study. Gastroenterology 1990;98:380–386. [ Links ]
Statement of Ethics
A written consent form, which included reference to the risk of posttreatment pain, bleeding, infection, urinary retention, and soiling was required.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Prof. Jorge Fonseca
Department of Gastroenterology, Hospital Garcia de Orta
Avenida Torrado da Silva
PT–2800 Almada (Portugal)
E-Mail jorgedafonseca@hotmail.com
Received: May 12, 2018; Accepted after revision: July 16, 2018














