Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.25 no.5 Lisboa out. 2018
https://doi.org/10.1159/000487154
REVIEW ARTICLE
Proton Pump Inhibitors: Are They a Real Threat to the Patient?
Inibidores da Bomba de Protões: Serão Eles uma Ameaça à Segurança do Doente?
Sofia Xavier, Joana Magalhães, José Cotter
Gastroenterology Department, Hospital da Senhora da Oliveira, Guimarães, Portugal; CVS/3Bs Associate Laboratory, University of Minho, Campus de Gualtar, Braga, Portugal; Life and Health Sciences Research Institute, School of Medicine, University of Minho, Campus de Gualtar, Braga, Portugal
* Corresponding author.
ABSTRACT
Background: Proton pump inhibitors are among the most frequently prescribed drugs in the world and are generally considered safe. However, there is growing concern regarding their safety. Summary: A nonsystematic review of the current literature was performed regarding proton pump inhibitors and their adverse effects. Proton pump inhibitors seem to be associated with fundic gland polyp development (without clinical relevance) and Clostridium difficile infection. Also, in cirrhotic patients, their prescription should be carefully reviewed. Regarding their association with other enteric infections, micronutrient deficiency, dementia, and chronic kidney disease, current evidence is still of low quality, and further studies are needed. Key Messages: Considering the current evidence, most patients with a clear clinical indication for proton pump inhibitor treatment should probably benefit from the maintenance of their treatment without significant adverse effects. However, higher-quality studies are needed to confirm or dismiss most of the proposed adverse effects.
Keywords: Proton pump inhibitors, Safety, Adverse effects
RESUMO
Introdução: Os inibidores da bomba de protões estão entre dos fármacos mais utilizados a nível mundial e globalmente considerados seguros. Contudo, evidência recente tem levantado dúvidas sobre o seu perfil de segurança. Sumário: Efetuada uma revisão não-sistemática da literatura relativamente aos inibidores da bomba de protões e seus efeitos adversos. Os inibidores da bomba de protões parecem associar-se significativamente com o desenvolvimento de pólipos das glândulas fûndicas (sem significado clínico) e com a infeção por Clostridium difficile. Além disso, em doentes cirróticos a sua prescrição deve ser cuidadosamente revista. A sua associação com outras infeções entéricas, défice de micronutrientes, demência e doença renal crónica provêm de evidência de baixa qualidade e mais estudos são necessários. Mensagens chave: Tendo em conta a evidência atual, a maioria dos doentes com indicação para terapêutica com inibidores da bomba de protões podem beneficar da sua manutenção sem efeitos adversos significativos. Contudo, estudos de melhor qualidade são necessários para confirmar ou desmentir a maioria dos efeitos secundários propostos.
Palavras-Chave: Inibidores da bomba de protões, Segurança, Efeitos adversos
Introduction
Proton pump inhibitors (PPIs) have been available since 1989, when the first drug of this class, omeprazole, was released. They are currently one of the most frequently prescribed drugs [1] and are available for over-thecounter acquisition in several countries.
They decrease acid production by irreversible blockage of H+/K+-adenosine triphosphatase that is present on gastric parietal cells and are currently the treatment of choice in several clinical conditions, such as symptomatic and complicated gastroesophageal reflux disease (GERD), Zollinger-Ellison syndrome, prevention of ulcers in nonsteroidal anti-inflammatory drug (NSAID) users, induction of peptic ulcer healing, and even in the eradication of Helicobacter pylori.
In symptomatic GERD, PPIs are capable of controlling symptoms in a higher percentage of patients than histamine 2 receptor (H2R) antagonists, and in patients with erosive GERD, this class of drugs is superior to placebo, H2R, and sucralfate in inducing healing [2]. PPI use in patients with Barrett esophagus is also associated with a decreased risk of progression to neoplastic Barrett esophagus, compared to H2R antagonists or no acid suppressive therapy, and is currently recommended as chemoprophylaxis in this group of patients [3].
There are several conditions for which NSAIDs are the mainstay of treatment. However, these drugs are associated with morbidity and even mortality, mainly due to gastrointestinal (GI) side effects that can range from a simple erosion to an ulcer complicated by bleeding or perforation [4]. In patients under NSAIDs, PPIs are effective in the prevention of gastric and duodenal ulcers, are superior to placebo and H2R antagonists, and are not associated with the GI side effects reported in misoprostolusers [4].
PPIs are also effective in the induction of peptic ulcer healing and in patients with ulcers with a high risk of bleeding; PPI infusion therapy is associated with a reduced risk of rebleeding, surgery, and mortality [5]. Also, PPIs are included in every treatment scheme for H. Pylori eradication, since they have themselves a weak antibacterial effect and are capable of stabilizing and raising the antibacterial effects of the antibiotics [6].
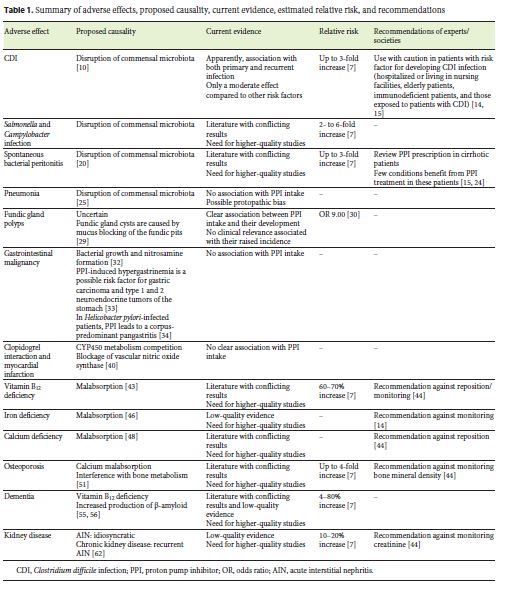
They are generally considered safe and are associated with mild side effects; however, there is growing concern regarding their safety. In this review, we will discuss the proposed mechanisms by which PPIs may induce adverse effects, evaluate the current evidence, and summarize current recommendations (Table 1). To help in the interpretation of the current evidence, we will also report, when available, the application of Hill criteria [7]. These include 9 parameters (strength of association, consistency, specificity, temporality, biological gradient, biological plausibility, coherence, experiment, and analogy) and try to differentiate between causality and association [8].
Methods
A nonsystematic review of the current literature was performed regarding PPIs and their adverse effects. We performed a bibliographic search on PubMed/Medline (http://www.ncbi.nlm.nih.gov/pubmed/) using the following keywords: proton pump inhibitors; risks; and adverse effects. Only articles written in English were reviewed. Data collected from systematic reviews, meta-analyses, and guidelines/position statements published in the last 10 years were preferred; however, when there was a lack of information in this time period, we used older publications.
Proposed Side Effects of PPIs
Infections
Several works have published articles regarding PPI use and their association with increased infection risk. Gastric acid secretion is part of the local defense system against ingested pathogens and is also determinant of the composition of the GI flora. PPI-induced hypochlorhydria seems capable of altering GI microbiota and is, therefore, predisposing patients to GI infections [9].
Clostridium difficile Infection
Clostridium difficile is a gram-positive spore-forming bacterium, and intestinal colonization by this agent is facilitated by disruption of commensal microbiota, as described in patients treated with PPIs. In fact, studies performed in healthy volunteers showed that after only 4–8 weeks of high-dose PPI, there were increased bacterial taxa associated with C. difficile in stools [10]. A metaanalysis of 50 controlled observational studies showed a significant association between acid suppressant therapy use and risk of developing C. difficile infection (CDI) (odds ratio [OR] 1.26) [11]. Also, a systematic review and meta-analysis of 16 observational studies showed that patients under PPI therapy had an increased risk of recurrent CDI with an OR of 1.52, even after adjustment for age and other potential confounders [12]. Even though the current evidence seems consensual in establishing an association between PPI use and CDI, the risk associated with PPIs is only modest when compared to other drugs, like antibiotics [13]. Evaluating Hill criteria, the current evidence has a moderate strength, and both temporality and plausibility are present; however, other criteria have not been established yet [7].
Both experts and national gastroenterology societies reinforce the need to review PPI dose and treatment duration in patients with risk factors for CDI, including those hospitalized or living in nursing facilities, elderly patients, immunodeficient patients, and those exposed to patients with CDI [14, 15].
Campylobacter and Salmonella Infection
GI microbiota alterations induced by PPI use may predispose patients to infections with pathogens other than C. difficile, particularly Salmonella and Campylobacter. Regarding this topic, the literature is less consensual. Garcia Rodriguez et al. [16] found a significant association between PPI use and increased risk of bacterial gastroenteritis compared to nonuse, regardless of the treatment duration (relative risk [RR] 2.9), and this risk was further increased with the double dose (RR 5.0). Also, a systematic review including 6 studies assessing enteric infection risk in PPI users identified an increased risk of such infections in patients under acid suppressant agents (OR 2.55) [17]. On the other hand, a retrospective analysis of almost 2 million patients, of which over 350,000 were under PPI treatment, found that PPI users had 3.1- to 6.9-fold higher rates of Campylobacter and Salmonella infections even before PPI prescription [18]. Another study assessing the safety of PPI treatment, including data from 2 controlled randomized clinical trials, with 12- and 5-year follow-up, was not able to find significant differences between users and nonusers regarding enteric infections [19]. Evaluating Hill criteria, the current evidence has a moderate strength, and temporality, consistency, biological gradient, plausibility, and analogy are present; however, other criteria have not been established yet [7].
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis (SBP) is one possible complication of cirrhosis, and an alteration in intestinal wall permeability seems to play a role in the pathogenesis of this condition [20]. As described before, PPIs can induce GI microbiota alterations and promote the overgrowth of pathogenic agents. A recently published metaanalysis found an increased risk of SBP in PPI users when compared to nonusers (hazard ratio [HR] 1.72) [21], and these findings were similar to those reported previously by Xu et al. [22] who also found an increased risk of SBP in this population (OR 2.13). However, not all authors report an increased risk of SBP in PPI users, and a metaanalysis including 10 case-control and 6 cohort studies found that the association of PPIs with SBP was only observed in case-control studies (OR 2.97) and did not find an association between PPI intake and in-stay and 30-day mortality [23]. Evaluating Hill criteria, the current evidence has a weak strength, and only temporality and plausibility are present [7].
Despite conflicting reports in the literature, several national gastroenterology societies reinforce the need to review PPI prescription in cirrhotic patients, particularly because few conditions showed evidence of benefit with these drugs [15, 24].
Pneumonia
Community-acquired pneumonia (CAP) is another infectious complication associated with PPI use in some studies. The proposed mechanism is that PPI-induced upper GI bacterial overgrowth can predispose to respiratory infections through potential micro-aspirations or translocation to the lung [25].
A large meta-analysis including 26 studies and 200,000 patients found an increased risk of 1.49 for CAP with PPI therapy, regardless of PPI dose or patient age, and these patients also had an increased risk for hospitalization due to CAP (OR 1.61) [26]. Interestingly, the authors found that treatment with a PPI for less than 1 month was associated with the highest risk of CAP (OR 2.10), and such risk decreased and lost statistical significance as the duration of PPI therapy increased. Actually, another study found that the risk for CAP was limited to patients starting PPI within the last 30 days and that this risk increased progressively with a shorter duration of treatment, reaching an OR of 6.53 when it was started in the 2 days before CAP diagnosis [25]. These results led some authors to propose that the association between PPI use and CAP is a result of a protopathic bias, which occurs when a drug is used to treat an early sign of the outcome, creating the appearance that it is actually associated with the outcome [7].
Also, other studies have not been able to find such an association between PPI and CAP, including a randomized, double-blinded, placebo-controlled trial designed to assess esomeprazole efficacy in the prophylaxis of peptic ulcers in patients under low-dose acetylsalicylic acid (the OBERON study), which found similar rates of pneumonia in patients under PPI and placebo [27]. Evaluating Hill criteria, the current evidence has a weak strength, and only plausibility is present [7].
GI Malignancy
Fundic Gland Polyps
Fundic gland polyp (FGP) development has long been associated with PPI use. They are found in up to 23% of endoscopies, and dysplasia is found in < 1% of these polyps [28]. The mechanism involved in the increased prevalence of FGP in PPI users is still uncertain, but one hypothesis is that fundic gland cysts are caused by mucus blocking of the fundic pits [29].
A prospective study assessing 1,780 patients undergoing upper gastroduodenal endoscopy concluded that PPI use for over 12 months was a risk factor for FGP development with an OR of 9.00, and none of the polyps was found to have dysplasia [30]. This raised incidence of FGPs is not associated with clinical relevance, as a study including over 100,000 patients found that these polyps seemed to inversely correlate with gastric neoplasia [31]. According to Hill criteria, this is the only adverse side effect regarding which the current evidence has a high strength, and temporality, consistency, specificity, plausibility, and experiment are present [7].
Gastric Malignancy
Other authors have raised concern regarding a possible association between PPI intake and gastric cancer. This potential adverse effect has several proposed mechanisms [32]. PPIs suppress gastric acid secretion and may interfere with bacterial growth and nitrosamine formation. Also, the reduction of gastric acid secretion can lead to hypergastrinemia, which has been identified as a possible risk factor for gastric carcinoma and type 1 and 2 neuroendocrine tumors of the stomach [33]. In addition, in patients with H. pylori infection, PPI-induced hypochlorhydria leads to a shift from a gastritis confined to the antrum to a corpus-predominant pangastritis [34].
A meta-analysis of 11 observational studies concluded that both PPI treatment and H2R antagonists were associated with an increased risk of gastric cancer, even though the authors were not able to assess the effect of underlying gastric conditions, such as H. pylori infection [32]. However, an FDA-mandated study was not able to find an increased incidence of either gastric or any other GI malignancy in patients under PPI treatment [35], and a Cochrane Database systematic review concluded that, currently, there is no clear evidence that long-term use of PPIs can cause or accelerate the progression of corpus gastric atrophy or intestinal metaplasia, and no participant in the included studies showed any dysplastic or neoplastic changes [36]. Also, 2 studies aimed to assess the long-term safety of PPI under controlled randomized clinical trial conditions (LOTUS and SOPRAN studies) found no gastric carcinoids or adenocarcinomas in patientsduring their course [19].
Despite the fact that the current evidence does not support a clear association between PPI treatment and gastric malignancy, patients with a clinical indication for longterm PPI treatment should be tested and, when positive, treated for H. Pylori infection in order to prevent a progression of gastritis [34].
Clopidogrel Interaction and Myocardial Infarction
Clopidogrel, an antiplatelet drug, is an inactive prodrug that needs to be activated by cytochrome P450. Since PPIs are also primarily metabolized by this cytochrome, there has been concern that PPIs may decrease clopidogrel efficacy through a competitive metabolism effect.
A retrospective cohort study of 8,205 patients taking clopidogrel after hospitalization for an acute coronary syndrome found that use of clopidogrel plus PPI was associated with an increased risk of death or rehospitalisation compared to use of clopidogrel alone (OR 1.25) [37]. However, the COGENT study, a randomized controlled trial in which patients were given either clopidogrel + PPI or clopidogrel + placebo, found similar rates of cardiovascular events in the 2 groups (HR 0.99) [38]. A recently published systematic review and meta-analysis including 30 observational studies and 4 randomized controlled trials reported very interesting findings [39]. The study found higher rates of all-cause mortality, nonfatal myocardial infarction, stroke, revascularization, and stent thrombosis in patients receiving PPIs plus clopidogrel when compared to patients receiving clopidogrel alone. However, when assessing only the data from randomized controlled trials, no differences were found regarding ischemic outcomes. Considering this sub-analysis, the authors concluded that observational studies may include several biases responsible for the differences in the results.
Besides an interaction with clopidogrel, other authors proposed a different mechanism through which PPIs could increase the risk of myocardial infarction. This is based on the ex vivo finding that PPIs can directly block vascular nitric oxide synthase, therefore promoting vascular contraction [40]. A recent publication assessing a general population found GERD patients exposed to PPIs to have a 1.16-fold increased association with myocardial infarction, and this association existed regardless of clopidogrel use [41]. This association had previously been proposed during the SOPRAN trial, in which the omeprazole group had more reports of myocardial infarction [19]. However, the FDA assessed the available evidence and concluded that the differences reported do probably not indicate the presence of a true effect; therefore, the long-term use of these drugs is not likely to be associated with an increased risk of heart problems [42].
According to Hill criteria, the current evidence regarding both clopidogrel interaction and myocardial infarction risk has a weak strength, and only temporality and plausibility criteria are present [7].
Micronutrient Deficiencies
Vitamin B12
Vitamin B12 requires the presence of gastric acid and pepsin to be released from dietary proteins and become able to proceed with the complex process that leads to its absorption in the GI tract. Consequently, PPIs can theoretically lead to vitamin B12 malabsorption; however, conflicting results have been published. A case-control study performed in patients with an incident diagnosis of B12 deficiency found both PPI and H2R antagonists use for over 2 years to be associated with an increased risk of vitamin B12 deficiency (OR 1.65 and 1.25, respectively) [43]. However, another case-control study performed in patients over 65 years of age was not able to find a difference in vitamin B12 levels between users and nonusers of PPIs, nor between their mean corpuscular volume or homocysteine levels [44].
Considering the current evidence, a Best Practice Advice issued in 2017 by the American Gastroenterology Association recommends against routine monitoring or raised intake of vitamin B12 in patients under PPIs [45].
Iron
As for vitamin B12, dietary iron absorption seems to be facilitated by gastric acid, and therefore, PPIs can theoretically lead to iron malabsorption. A retrospective study trying to assess the effect of PPI use on iron deficiency anemia concluded that the use of PPIs for over 1 year was associated with lower hemoglobin, hematocrit, and mean corpuscular volume compared to nonuse [46]. Another study performed in patients with hemochromatosis concluded that PPI use reduced the need for phlebotomies and that PPI intake could reduce the absorption of iron from a regular meal [47]. However, a previous study including over 100 patients with Zollinger-Ellison syndrome under PPI therapy for a mean of 5.7 years was not able to find a decrease in iron stores in these patients [48].
Even though these results should raise our attention, current evidence is provided by small-sampled studies providing low-quality evidence, and experts recommend against the routine investigation of anemia in patients on PPIs [14].
Calcium
Calcium absorption seems to be influenced by gastric pH, and in vitro studies show that increased pH reduces calcium absorption, leading to the assumption that PPIs can reduce calcium absorption [49]. A randomized placebo-controlled study reported that with only a 1-week course of PPI, elderly women had a significant decrease in fractional calcium absorption under fasting conditions [49]. However, another study performed in postmenopausal women was not able to find differences in fractional calcium absorption before and after 30 days of PPI use [50], and also other authors have reported that gastric pH alterations are not enough to impair GI calcium absorption [51].
Considering the current evidence, a Best Practice Advice issued in 2017 by the American Gastroenterology Association recommends against routine raised intake of calcium in patients under PPIs [45].
Bone Fracture and Osteoporosis
An association between PPI use and bone fractures was also suggested. The mechanisms included not only the malabsorption of calcium, but also an interference with bone metabolism caused by the hyperparathyroidism seen in patients with hypergastrinemia.
A systematic review and meta-analysis of 18 observational studies concluded that PPI intake modestly increased the risk of hip (RR 1.26), spine (RR 1.58), and any-site fracture (RR 1.33), with similar risks for patients using PPI for under or over 1 year [52]. The authors, however, admitted that their results could have been influenced by cofounders and bias associated with the observational studies included in the meta-analysis.
Studies assessing bone mass showed conflicting results. Maggio et al. [53] assessed cortical and trabecular bone mineral density (BMD) and cross-sectional area and concluded that PPI users showed lower trabecular BMD than nonusers, even after age and gender adjustments. However, another study assessing not only areal BMD but also changes in bone structure which would predispose to fractures in the absence of changes in BMD concluded that long-term PPI use was not associated with changes in BMD or bone structure that would predispose to bone fractures [54]. Reviewing Hill criteria, the current evidence regarding bone fracture risk in PPI users has a weak strength, and only temporality and plausibility are present [7].
Considering the current evidence, a Best Practice Advice issued in 2017 by the American Gastroenterology Association recommends against routine monitoring of BMD in patients under PPIs [45], and the FDA also determined that an osteoporosis and fracture warning on PPI treatment was not indicated [55].
Dementia
PPIs have 2 proposed mechanisms contributing to dementia. The first is the proposed association between PPIs and a reduction in vitamin B12, which can contribute to a decreased cognitive function. The second is based on the observation that PPI treatment enhances the production of β-amyloid, a key event in the pathogenesis of Alzheimer disease. PPIs lead to increased production of several isoforms of β-amyloid in mouse brains [56], and these may be due to a direct PPI modulation of 2 protease enzymes responsible for cleavage of amyloid precursor protein [56] or through blockage of the vacuolar-type adenosine triphosphatase proton pumps, which increases the pH of microglial lysosomes, leading to decreased degradation of β-amyloid [57].
An observational study using primary care patients over 75 years of age found that PPI use was significantly associated with an increased risk of any dementia (HR1.38) and Alzheimer disease (HR 1.44) compared to nonuse [58]. Another observational study also concluded that patients under regular PPI medication had a significantly increased risk of dementia compared to nonusers (HR 1.44) [59]. In contrast, a recently published case-control study was not able to find an association between PPI use and risk of Alzheimer disease, and higher doses or a longer duration of use was also not associated with an increased risk [60]. Reviewing Hill criteria, the current evidence has a weak strength, and only temporality is present [7]; therefore, more studies are needed to conclude about the effect of PPIs on dementia.
Kidney Diseases
Early since the clinical use of PPIs, isolated cases of acute interstitial nephritis have been attributable to PPI use [61], and the largest case series included only 18 cases [62]. The exact mechanism is still unknown, but it seems to be triggered by a hypersensitivity immune reaction to the drug or one of its metabolites [62]. This class effect is more commonly seen in the elderly [62]. Reviewing Hill criteria, the current evidence has a weak strength, and only temporality and experimental criteria are present [7].
Until recently, little was known about the impact of PPI use on the development of chronic kidney disease (CKD), and some authors proposed that PPIs can induce CKD due to recurrent episodes of acute interstitial nephritis [63]. One large observational study found that patients under PPI had a 3.3% absolute risk increase of CKD (number needed to harm = 30), but it also reported a higher incidence of hypertension, a known risk factor for CKD, in the PPI user group [63]. Another observational study based on the Healthcare database found that patients using PPIs had a higher risk of developing CKD (OR 1.29) [64], and yet another study found that patients under PPI had a significantly elevated risk of doubling of serum creatinine level (HR 1.53) and of having a decline > 30% in estimated glomerular filtration rate (HR 1.32) [65]. The latter study also found that patients who used PPIs for longer durations had higher rates of renal adverse outcomes when compared to ≤30-day use; however, uses over 720 days seemed to protect patients from CKD. This variability in the effect of PPI use duration on renal function raises questions regarding whether a confounding factor may have influenced the results.
Even though these findings should draw the attention of the scientific community to this issue and lead to the development of higher-quality studies designed to assess the impact of PPIs on renal function, the current evidence is still lacking strength. The results obtained are based on a retrospective analysis and may have been influenced by unidentified confounders. Reviewing Hill criteria, the current evidence regarding renal failure risk in PPI users has a weak strength, and only temporality criteria are present [7].
A Best Practice Advice issued in 2017 by the American Gastroenterology Association recommends against routine screening/monitoring of serum creatinine in patients under PPIs [45].
Conclusion
PPIs are widely used and available drugs. They were developed over 30 years ago, and their clinical efficiency made them become the first-line therapy in several clinical conditions, so that they are one of the most frequently prescribed pharmacological groups all over the world.
Recently, many investigations have been published regarding their safety, drawing attention to previously unsuspected adverse effects. This new evidence has alarmed not only the scientific community but also the general population. However, after reviewing the evidence produced, we understand that most studies suggesting adverse effects of PPIs are of low quality, subject to many confounders, and lacking reproducibility, and higherquality studies are needed to confirm or dismiss most of the proposed adverse effects.
Considering the current evidence, patients with a clear clinical indication for PPI treatment should probably benefit from the maintenance of their treatment. Nevertheless, we should not forget that some patients may be under PPI treatment without a clear indication and even that patients may be self-medicating with this over-the-counter drug. Therefore, an effort should be made to withdraw the drug in those who do not require it and to reduce PPI use to the lowest needed dose in those requiring long-term treatments.
References
1 Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL: Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 2015;314:1818–1831. [ Links ]
2 Katz PO, Gerson LB, Vela MF: Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–328;quiz 329. [ Links ]
3 Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology: ACG clinical guideline: diagnosis and management of Barretts esophagus. Am J Gastroenterol 2016;111:30–50;quiz 51. [ Links ]
4 Lanza FL, Chan FK, Quigley EM; Practice Parameters Committee of the American College of Gastroenterology: Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 2009;104:728–738. [ Links ]
5 Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R, Racz I, Arezzo A, Hoffmann RT, Lesur G, de Franchis R, Aabakken L, Veitch A, Radaelli F, Salgueiro P, Cardoso R, Maia L, Zullo A, Cipolletta L, Hassan C: Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015;47:a1–a46. [ Links ]
6 Romano M, Cuomo A: Eradication of Helicobacter pylori: a clinical update. MedGenMed 2004;6:19. [ Links ]
7 Vaezi MF, Yang YX, Howden CW: Complications of proton pump inhibitor therapy. Gastroenterology 2017;153:35–48. [ Links ]
8 Hill AB: The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [ Links ]
9 Tleyjeh IM, Bin Abdulhak AA, Riaz M, Alasmari FA, Garbati MA, AlGhamdi M, Khan AR, Al Tannir M, Erwin PJ, Ibrahim T, Allehibi A, Baddour LM, Sutton AJ: Association between proton pump inhibitor therapy and Clostridium difficile infection: a contemporary systematic review and meta-analysis. PLoS One 2012;7:e50836. [ Links ]
10 Freedberg DE, Toussaint NC, Chen SP, Ratner AJ, Whittier S, Wang TC, Wang HH, Abrams JA: Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology 2015;149:883–885.e889. [ Links ]
11 Cao F, Chen CX, Wang M, Liao HR, Wang MX, Hua SZ, Huang B, Xiong Y, Zhang JY, Xu YL: Updated meta-analysis of controlled observational studies: proton-pump inhibitors and risk of Clostridium difficile infection. J Hosp Infect 2018;98:4–13. [ Links ]
12 Tariq R, Singh S, Gupta A, Pardi DS, Khanna S: Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med 2017;177:784–791. [ Links ]
13 Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ: Clostridium difficile-associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis 2007;45:1543–1549. [ Links ]
14 Eusebi LH, Rabitti S, Artesiani ML, Gelli D, Montagnani M, Zagari RM, Bazzoli F: Proton pump inhibitors: risks of long-term use. J Gastroenterol Hepatol 2017;32:1295–1302. [ Links ]
15 de la Coba Ortiz C, Arguelles Arias F, Martin de Argila de Prados C, Judez Gutierrez J, Linares Rodriguez A, Ortega Alonso A, Rodriguez de Santiago E, Rodriguez-Tellez M, Vera Mendoza MI, Aguilera Castro L, Alvarez Sanchez A, Andrade Bellido RJ, Bao Perez F, Castro Fernandez M, Giganto Tome F: Proton-pump inhibitors adverse effects: a review of the evidence and position statement by the Sociedad Espanola de Patologia Digestiva. Rev Esp Enferm Dig 2016;108:207–224. [ Links ]
16 Garcia Rodriguez LA, Ruigomez A, Panes J: Use of acid-suppressing drugs and the risk of bacterial gastroenteritis. Clin Gastroenterol Hepatol 2007;5:1418–1423. [ Links ]
17 Leonard J, Marshall JK, Moayyedi P: Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007;102:2047–2056;quiz 2057. [ Links ]
18 Brophy S, Jones KH, Rahman MA, Zhou SM, John A, Atkinson MD, Francis N, Lyons RA, Dunstan F: Incidence of Campylobacter and Salmonella infections following first prescription for PPI: a cohort study using routine data. Am J Gastroenterol 2013;108:1094–1100. [ Links ]
19 Attwood SE, Ell C, Galmiche JP, Fiocca R, Hatlebakk JG, Hasselgren B, Langstrom G, Jahreskog M, Eklund S, Lind T, Lundell L: Long-term safety of proton pump inhibitor therapy assessed under controlled, randomised clinical trial conditions: data from the SOPRAN and LOTUS studies. Aliment Pharmacol Ther 2015;41:1162–1174. [ Links ]
20 Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M, Ghirlanda G, Gasbarrini A: Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol 2010;105:323–327. [ Links ]
21 Dam G, Vilstrup H, Watson H, Jepsen P: Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology 2016;64:1265–1272. [ Links ]
22 Xu HB, Wang HD, Li CH, Ye S, Dong MS, Xia QJ, Zhang AQ, Pan K, Ge XL, Dong JH: Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res 2015;14:7490–7501. [ Links ]
23 Yu T, Tang Y, Jiang L, Zheng Y, Xiong W, Lin L: Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: a meta-analysis. Dig Liver Dis 2016;48:353–359. [ Links ]
24 Scarpignato C, Gatta L, Zullo A, Blandizzi C; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology; the Italian Association of Hospital Gastroenterologists; the Italian Federation of General Practitioners: Effective and safe proton pump inhibitor therapy in acidrelated diseases – a position paper addressing benefits and potential harms of acid suppression. BMC Med 2016;14:179. [ Links ]
25 Sarkar M, Hennessy S, Yang YX: Protonpump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med 2008;149:391–398. [ Links ]
26 Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA: Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One 2015;10:e0128004. [ Links ]
27 Scheiman JM, Devereaux PJ, Herlitz J, Katelaris PH, Lanas A, Veldhuyzen van Zanten S, Naucler E, Svedberg LE: Prevention of peptic ulcers with esomeprazole in patients at risk of ulcer development treated with low-dose acetylsalicylic acid: a randomised, controlled trial (OBERON). Heart 2011;97:797–802. [ Links ]
28 Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B; British Society of Gastroenterology: The management of gastric polyps. Gut 2010;59:1270–1276. [ Links ]
29 Martin FC, Chenevix-Trench G, Yeomans ND: Systematic review with meta-analysis: fundic gland polyps and proton pump inhibitors. Aliment Pharmacol Ther 2016;44:915–925. [ Links ]
30 Zelter A, Fernandez JL, Bilder C, Rodriguez P, Wonaga A, Dorado F, Galich M, Viola LA: Fundic gland polyps and association with proton pump inhibitor intake: a prospective study in 1,780 endoscopies. Dig Dis Sci 2011;56:1743–1748. [ Links ]
31 Genta RM, Schuler CM, Robiou CI, Lash RH: No association between gastric fundic gland polyps and gastrointestinal neoplasia in a study of over 100,000 patients. Clin Gastroenterol Hepatol 2009;7:849–854. [ Links ]
32 Ahn JS, Eom CS, Jeon CY, Park SM: Acid suppressive drugs and gastric cancer: a metaanalysis of observational studies. World J Gastroenterol 2013;19:2560–2568. [ Links ]
33 Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC, Jensen RT; North American Neuroendocrine Tumor Society (NANETS): NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010;39:735–752. [ Links ]
34 Malfertheiner P, Megraud F, OMorain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel: Management of Helicobacter pylori infection – the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30. [ Links ]
35 Schneider JL, Kolitsopoulos F, Corley DA: Risk of gastric cancer, gastrointestinal cancers and other cancers: a comparison of treatment with pantoprazole and other proton pump inhibitors. Aliment Pharmacol Ther 2016;43:73–82. [ Links ]
36 Song H, Zhu J, Lu D: Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst Rev 2014;12:CD010623. [ Links ]
37 Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS: Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 2008;301:937–944. [ Links ]
38 Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, Shook TL, Lapuerta P, Goldsmith MA, Laine L, Scirica BM, Murphy SA, Cannon CP; COGENT Investigators: Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010;363:1909–1917. [ Links ]
39 Melloni C, Washam JB, Jones WS, Halim SA, Hasselblad V, Mayer SB, Heidenfelder BL, Dolor RJ: Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes 2015;8:47–55. [ Links ]
40 Ghebremariam YT, LePendu P, Lee JC, Erlanson DA, Slaviero A, Shah NH, Leiper J, Cooke JP: Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 2013;128:845–853. [ Links ]
41 Shah NH, LePendu P, Bauer-Mehren A, Ghebremariam YT, Iyer SV, Marcus J, Nead KT, Cooke JP, Leeper NJ: Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One 2015;10:e0124653. [ Links ]
42 Update of safety review – follow-up to the August 9, 2007, communication about the ongoing safety review of omeprazole and esomeprazole. US Food and Drug Administration, 2007. https://www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/ucm123229.htm. [ Links ]
43 Lam JR, Schneider JL, Zhao W, Corley DA: Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013;310:2435–2442. [ Links ]
44 den Elzen WP, Groeneveld Y, de Ruijter W, Souverijn JH, le Cessie S, Assendelft WJ, Gussekloo J: Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment Pharmacol Ther 2008;27:491–497. [ Links ]
45 Freedberg DE, Kim LS, Yang YX: The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 2017;152:706–715. [ Links ]
46 Sarzynski E, Puttarajappa C, Xie Y, Grover M, Laird-Fick H: Association between proton pump inhibitor use and anemia: a retrospective cohort study. Dig Dis Sci 2011;56:2349–2353. [ Links ]
47 Hutchinson C, Geissler CA, Powell JJ, Bomford A: Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut 2007;56:1291–1295. [ Links ]
48 Stewart CA, Termanini B, Sutliff VE, Serrano J, Yu F, Gibril F, Jensen RT: Iron absorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment Pharmacol Ther 1998;12:83–98. [ Links ]
49 OConnell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ: Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 2005;118:778–781. [ Links ]
50 Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Ziegler TE, Penniston KL, Alvig AL, Shafer MM: Do proton pump inhibitors decrease calcium absorption? J Bone Miner Res 2010;25:2786–2795. [ Links ]
51 Serfaty-Lacrosniere C, Wood RJ, Voytko D, Saltzman JR, Pedrosa M, Sepe TE, Russell RR: Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr 1995;14:364–368. [ Links ]
52 Zhou B, Huang Y, Li H, Sun W, Liu J: Protonpump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int 2016;27:339–347. [ Links ]
53 Maggio M, Lauretani F, Ceda GP, De Vita F, Bondi G, Corsonello A, Cattabiani C, Lattanzio F, Ruggiero C, Nouvenne A, Meschi T, Bandinelli S, Ferrucci L: Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone 2013;57:437–442. [ Links ]
54 Targownik LE, Goertzen AL, Luo Y, Leslie WD: Long-term proton pump inhibitor use is not associated with changes in bone strength and structure. Am J Gastroenterol 2017;112:95–101. [ Links ]
55 FDA Drug Safety Communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. US Food and Drug Administration, 2011. https://www.fda.gov/Drugs/DrugSafety/ucm213206.htm. [ Links ]
56 Badiola N, Alcalde V, Pujol A, Munter LM, Multhaup G, Lleo A, Coma M, Soler-Lopez M, Aloy P: The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS One 2013;8:e58837. [ Links ]
57 Fallahzadeh MK, Borhani Haghighi A, Namazi MR: Proton pump inhibitors: predisposers to Alzheimer disease? J Clin Pharm Ther 2010;35:125–126. [ Links ]
58 Haenisch B, von Holt K, Wiese B, Prokein J, Lange C, Ernst A, Brettschneider C, Konig HH, Werle J, Weyerer S, Luppa M, Riedel-Heller SG, Fuchs A, Pentzek M, Weeg D, Bickel H, Broich K, Jessen F, Maier W, Scherer M: Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015;265:419–428. [ Links ]
59 Gomm W, von Holt K, Thome F, Broich K, Maier W, Fink A, Doblhammer G, Haenisch B: Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016;73:410–416. [ Links ]
60 Taipale H, Tolppanen AM, Tiihonen M, Tanskanen A, Tiihonen J, Hartikainen S: No association between proton pump inhibitor use and risk of Alzheimers disease. Am J Gastroenterol 2017;112:1802–1808. [ Links ]
61 Ruffenach SJ, Siskind MS, Lien YH: Acute interstitial nephritis due to omeprazole. Am J Med 1992;93:472–473. [ Links ]
62 Geevasinga N, Coleman PL, Webster AC, Roger SD: Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol 2006;4:597–604. [ Links ]
63 Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Grams ME: Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016;176:238–246. [ Links ]
64 Arora P, Gupta A, Golzy M, Patel N, Carter RL, Jalal K, Lohr JW: Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol 2016;17:112. [ Links ]
65 Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z: Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2016;27:3153–3163. [ Links ]
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Dr. Sofia Xavier
Gastroenterology Department, Hospital da Senhora da Oliveira
Rua dos Cutileiros, Creixomil
PT–4835-044 Guimarães (Portugal)
E-Mail smaxavier@gmail.com
Received: November 8, 2017; Accepted after revision: January 18, 2018
Funding Sources
No funding was used for the development of this work.
Author Contributions
Sofia Xavier performed the literature search, analyzed clinical data, designed the text structure, and wrote the text. Joana Magalhães collaborated in the text writing and made several critical corrections and revisions. Jose Cotter suggested the theme to be reviewed, contributed to the literature revision and analysis, and made several critical corrections and revisions. All authors approved the final version of the article.














