Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.25 no.2 Lisboa abr. 2018
https://doi.org/10.1159/000480707
CLINICAL CASE STUDY
Endometriosis: A Rare Cause of Large Bowel Obstruction
Endometriose: uma causa rara de oclusão colorretal
Gonçalo Alexandrinoa, Luís Carvalho Lourençoa, Rita Carvalhoa, Cisaltina Sobrinhob, David Valadas Hortaa, Jorge Reisa
aGastroenterology Department, and bGeneral Surgery Department, Hospital Prof. Doutor Fernando Fonseca, Amadora, Portugal
* Corresponding author.
ABSTRACT
Large bowel obstruction can result in significant morbidity and mortality, especially in cases of acute complete obstruction. There are many possible causes, the most common in adults being colorectal cancer. Endometriosis is a benign disease, and the most affected extragenital location is the bowel, especially the rectosigmoid junction. However, transmural involvement and acute occlusion are very rare events. We report an exceptional case of acute large bowel obstruction as the initial presentation of endometriosis. The differential diagnosis of colorectal carcinoma may be challenging, and this case emphasizes the need to consider intestinal endometriosis in females at a fertile age presenting with gastrointestinal symptoms and an intestinal mass causing complete large bowel obstruction.
Keywords: Large bowel obstruction, Stenosis, Endometriosis,·Endoscopy
RESUMO
A obstrução do cólon pode causar morbilidade e mortalidade significativas, especialmente em casos de obstrução aguda completa. Existem diversas causas, sendo a mais comum em adultos o cancro colorretal. A endometriose é uma doença benigna e o intestino é a localização extragenital mais afectada pela doença, sobretudo ao nível da transição rectossigmoideia. Contudo, o envolvimento transmural e oclusão aguda são situações extretamente raras. O diagnóstico diferencial com cancro colorretal pode ser desafiante e este caso enfatiza a necessidade de considerar a endometriose intestinal em mulheres em idade fértil com sintomas gastrointestinais e a presença de uma massa intestinal a causar obstrução completa do cólon.
Palavras-Chave: Oclusão colorretal, Estenose, Endometriose, Endoscopia
Introduction
Large bowel obstruction (LBO) can result in significant morbidity and mortality, especially in cases of acute complete obstruction. The development of colonic ischemia in the upstream dilated bowel may cause perforation with subsequent peritonitis and sepsis. LBO often presents as an emergency that requires early and accurate diagnosis and management [1]. LBO is 4–5 times less common than small bowel obstruction, but it accounts for nearly 2–4% of all surgical admissions [2]. LBO occurs mainly in the elderly [1–3].
There are many causes of acute LBO, which can be divided into neoplastic and nonneoplastic pathologies. The most frequent etiology in adults is colorectal cancer, accounting for just over 50% of cases. Around 10% of colon malignancies initially present with LBO. Colorectal cancer, diverticulitis, and volvulus account for approximately 80– 85% of all LBO cases. Nonneoplastic causes are relatively uncommon. Rare causes of obstruction include intussusception, Crohn disease, extrinsic tumors, fecal impaction, foreign body, infection, acute (toxic) or chronic megacolon, adhesion-related obstruction, and endometriosis [1–3].
Endometriosis is a benign gynecological disease, estimated to affect 7–10% of women [4]. The bowel is the most affected extragenital location, with a prevalence of 3–12%, especially in the rectosigmoid junction (50–90%) [5]. However, transmural involvement and acute occlusion are very rare events [6].
The authors report a rare case of a young female who presented with acute LBO. The histological analysis of the surgical specimen revealed the unexpected diagnosis of intestinal endometriosis. To the best of our knowledge and after a review of the literature, only few similar reports have been found.
Clinical Case
A 30-year-old female was admitted to the emergency department presenting with a 4-day history of sudden onset of abdominal distention, diffuse and moderate colicky abdominal pain, vomiting, and no gas or stool passing. She had no past medical history and was not taking any drugs. On physical examination, she had a distended and tympanic abdomen, slightly painful to palpation, without signs of peritoneal irritation. The vital signs and laboratory tests were within normal values. The abdominal X-ray showed exuberant colonic distention and a stop sign in the rectosigmoid junction (Fig. 1). An abdominal and pelvic computed tomography (CT) identified a concentric parietal thickening in the rectosigmoid junction with no permeable lumen and dilatation of the upstream intestinal loops (Fig. 2).
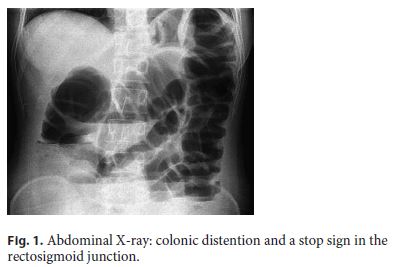
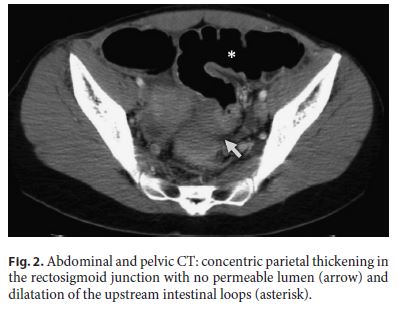
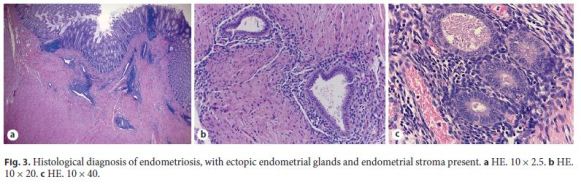
The diagnosis of intestinal occlusion was assumed, so the patient underwent an exploratory laparotomy. There was a periuterine inflammatory mass involving the anterior rectal wall and an anterior resection of the rectum was performed with primary anastomosis and protective ileostomy. Histological analysis of the operative specimen revealed rectal and nodal endometriosis, with free surgical margins (Fig. 3).
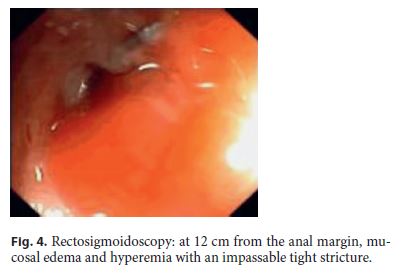
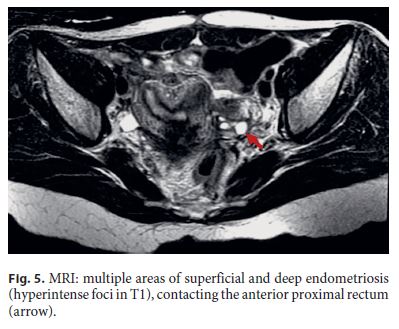
Three months after surgery, the patient was asymptomatic. For endoscopic reassessment before intestinal transit restoration, a rectosigmoidoscopy was performed up to 12 cm from the anal margin. At this level, edema was detected and the mucosa was hyperemic with a tight stricture (Fig. 4). Pelvic magnetic resonance imaging (MRI) revealed several hyperintense foci in T1, corresponding to multiple areas of superficial and deep endometriosis, one of them in contact with the anterior proximal rectum, with no large nodules in the anastomotic area (Fig. 5).
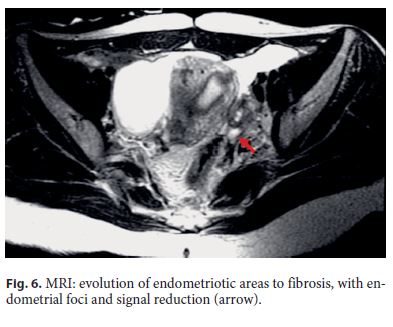
The patient was then medicated with Goserelin, a luteinizing hormone-releasing hormone analogue (3 administrations of 3.6 mg, with a 28-day interval between each dose, given subcutaneously). After medical therapy, a pelvic MRI documented favorable evolution, with reduction of the foci of endometriosis and frankreduction of the signal, translating evolution to fibrosis (Fig. 6). The endoscopic reassessment revealed a larger stenotic caliber, but still impassable with the adult colonoscope. Endoscopic dilatation (5 sessions with through-the-scope balloon up to 15 mm) was successfully performed (Fig. 7).
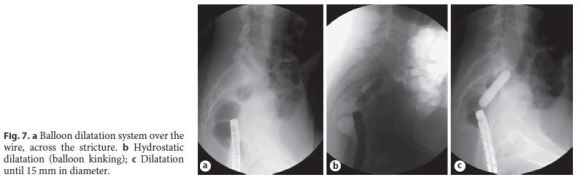
After endoscopic dilatation sessions, the patient underwent an intestinal transit restoration surgery. She is now under close follow-up and 1 year after surgery she still is asymptomatic.
Discussion
Endometriosis is a benign gynecological disease defined as the presence of endometrial tissue outside the uterine cavity, predominantly in the pelvic compartment. It is an estrogen-dependent chronic inflammatory condition affecting women in the reproductive period, and it is associated with infertility. The disease has a peak between25 and 35 years of age and is estimated to affect 7–10% of women. The percentage of patients experiencing severe symptoms or complications is about 3% of women at a fertile age [4, 7].
The pathogenesis of endometriosis is not fully understood, but the most accepted theory suggests retrograde menstruation as the etiology of this disease. Menstrual blood transports cells from the lining of the uterus which come to lie on the surfaces of the pelvis where they attach, grow, and develop into endometriosis. Endometrial implants on the peritoneum and pelvic viscera adhere to the intestinal serosal surface and may invade the submucosa [7, 8].
The most frequent location of the disease is the ovary, followed by the Douglas cul-de-sac and the uterosacral ligaments. Bowel is the most affected extragenital location (3–12%), mostly in the rectosigmoid junction (50– 90%). There may also be involvement of the small bowel (2–16%), appendix (3–18%), and cecum (2–5%). The ileum is affected in 4.1% of patients [5].
The usual symptoms include heavy or irregular periods, cyclic pelvic pain, dyspareunia, and dysmenorrhea [7]. Patients with bowel endometriosis may experience rectal pain, which worsens with defecation, sitting and menstruation, fecal tenesmus, constipation, diarrhea, rectal bleeding, nonspecific abdominal pain, and subocclusion symptoms. The clinical features of intestinal endometriosis vary depending on the site and extent of involvement. Transmural involvement is uncommon, but may cause severe complications such as occlusion, hemorrhage, or perforation. Presentation as acute occlusion is extremely rare [5, 6, 9].
Colonic endometriosis diagnosis requires a high index of suspicion. Presentation as complete LBO may simulate colon carcinoma and can be a clinical challenge due to absence of pathognomonic symptoms and similar colonoscopic and radiologic findings. Colonoscopy and barium enema of intestinal endometriosis typically show a stenotic lesion with intact mucosa. CT may only reveal an extrinsic bowel compression. Colonoscopy is of limited value as the mucosal biopsy from the narrowed site is normal and the stricture is due to pressure effect from the muscular coat and serosal involvement [10]. As mucosal involvement may be absent and conventional biopsy forceps take only superficial samples, an exact diagnosis may fail [10, 11]. Distinguishing colonic endometriosis from other gastrointestinal pathologies, such as inflammatory bowel disease, ischemic colitis, malignancy, and postanastomotic strictures, may be very difficult [6, 8, 9].
The preoperative differential diagnosis in this setting is often impossible, many times depending on postoperative histological confirmation [6, 8, 9]. In our patient, all the evidence was for a bowel obstruction secondary to a lower gastrointestinal malignancy, until the histology result revealed the diagnosis of bowel endometriosis. The gold standard for diagnosis is then laparoscopy, which allows the operator to evaluate both the genital and intestinal tracts. In cases of total obstruction, it is recommended to perform full surgical extirpation of all visible colorectal endometriosis and resection of the bowel. This approach seems to have very satisfactory long-term outcomes [6, 8, 9].
Although hormone therapy is primarily the treatment of choice for endometriosis, it cannot reverse stricturing in the bowel, so in acute or subacute intestinal obstruction, the mechanical blockage requires urgent surgical intervention [12]. Chronic symptoms generally subside promptly during GnRH agonist treatment, owing to induced hypoestrogenism [7]. Luteinizing hormone-releasing hormone analogues play an important role in the treatment and are indicated for pain relief and reduction of the size and number of endometriotic lesions [13], aswe observed in our patient, with an extremely favorable evolution documented in MRI.
As an alternative to surgery, endoscopic balloon dilatation has also been described as a possible treatment when the histological diagnosis is already known [14], which was not the case in our patient.
Colonic resections in this setting improve quality of life but can result in different complications, including anastomotic strictures (AS) [15], as seen in this case. The incidence of AS after colorectal surgery for endometriosis has been found to range between 2 and 5% [16–18], but in a recent series of 113 colonic resections there was a rate of 12%, a higher incidence than for other indications than endometriosis. The etiology of AS is rarely known and seems to be multifactorial [19]. Several endoscopic dilatation techniques can be used [20] to treat AS. Generally, pneumatic dilatations are preferred due to its lower risk of mucosal laceration and perforation. With balloon dilatations, 2–4 procedures are often needed. The procedure is usually well tolerated and has an efficacy of 90% [19]. Our patient experienced AS after surgery, which was successfully treated with 5 sessions of balloon dilatation.
In cases where dilatations fail with persistence of long and fibrous stenosis, surgery or placement of a metal stent are possible alternatives. However, the use of metal stents in this context is controversial and they are generally reserved for patients with colonic carcinoma in subocclusion before elective surgery or as palliative treatment for inoperable patients [19]. There are very few cases of colorectal endometriosis reported in the literature where self-expandable metallic stents were used as a bridge for further surgery [12, 21].
In conclusion, endometriosis may present as a severe rectosigmoid stricture leading to obstructive symptoms. Although an initial presentation as complete LBO, as seen in this case, is extremely rare, intestinal endometriosis should be considered in the differential diagnosis of females at a fertile age presenting with gastrointestinal symptoms and an intestinal mass causing complete LBO.
References
1 Baron TH: Acute colonic obstruction. Gastrointest Endosc Clin N Am 2007;17:323– 339. [ Links ]
2 Ramanathan S, Ojili V, Vassa R, Nagar A: Large bowel obstruction in the emergency department: imaging spectrum of common and uncommon causes. J Clin Imaging Sci 2017;7:15. [ Links ]
3 Yeo HL, Lee SW: Colorectal emergencies: review and controversies in the management of large bowel obstruction. J Gastrointest Surg 2013;17. [ Links ]
4 Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S: Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol 2017;209:3–7. [ Links ]
5 López Carrasco A, Hernández Gutiérrez A, Hidalgo Gutiérrez PA, Rodríguez González R, Marijuán Martín JL, Zapardiel I, et al: Ileocecal endometriosis: diagnosis and management. Taiwan J Obstet Gynecol 2017;56:243– 246. [ Links ]
6 Doh K, Thiam I, Ka S, Dial C, Woto-Gaye G: Rectal endometriosis: an exceptional etiology of acute intestinal occlusion. Ann Pathol 2016;36:412–414. [ Links ]
7 Vercellini P, Viganò P, Somigliana E, Fedele L: Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 2014;10:261–275. [ Links ]
8 Bidarmaghz B, Shekhar A, Hendahewa R: Sigmoid endometriosis in a post-menopausal woman leading to acute large bowel obstruction: a case report. Int J Surg Case Rep 2016;28:65–67. [ Links ]
9 Gupta RK, Agrawal CS, Yadav RP, Uprety D, Sah PL: Rectosigmoid endometriosis causing an acute large bowel obstruction: a report of a case and a review of the literature. JNMA J Nepal Med Assoc 2011;51:83–86. [ Links ]
10 Paksoy M, Karabiçak I, Ayan F, Aydoğan F: Intestinal obstruction due to rectal endometriosis. Mt Sinai J Med 2005;72:405–408. [ Links ]
11 Bozdech JM: Endoscopic diagnosis of colonic endometriosis. Gastrointest Endosc 1992;38:568–570. [ Links ]
12 Whelton C, Bhowmick A: Acute endometrial bowel obstruction – A rare indication for colonic stenting. Int J Surg Case Rep 2013;4:160–163. [ Links ]
13 Abu Hashim H: Gonadotrophin-releasing hormone analogues and endometriosis: current strategies and new insights. Gynecol Endocrinol 2012;28:314–321. [ Links ]
14 Miller ES, Barnett RM, Williams RB: Sigmoid endometirotic stricture treated with endoscopic balloon dilatation: case report and literature review. Md Med J 1990;39:1081–1084. [ Links ]
15 Dubernard G, Piketty M, Rouzier R, Houry S, Bazot M, Darai E: Quality of life after laparoscopic colorectal resection for endometriosis. Hum Reprod 2006;21:1243–1247. [ Links ]
16 Delaunay-Tardy K, Bartheélémy C, Dumas O, Balique JG, Audigier JC: Endoscopic therapy of benign colonic postoperative strictures: report of 27 cases. Gastroenterol Clin Biol 2003;27:610. [ Links ]
17 Kwon YH, Jeon SW, Lee YK: Endoscopic management of refractory benign colorectal strictures. Clin Endosc 2013;46:472. [ Links ]
18 Davis B, Rivardeneira DE: Complications of colorectal anastomoses: leaks, strictures and bleeding. Surg Clin North Am 2013;93:61. [ Links ]
19 Anaf V, Gocevska S, Lemoine O, El Nakadi I, Buggenhout A, Zalcman M, et al: The problem of anastomotic stricture after rectosigmoid resection in deep infiltrating endometriosis. J Gynecol Surg 2016;32:35–42. [ Links ]
20 Venkanesh KS, Ramanujam PS, McGee S: Hydrostatic balloon dilatation of benign colonic anastomotic strictures. Dis Colon Rectum 1992;35:789. [ Links ]
21 Forshaw MJ, Sankararajah D, Stewart M, Parker MC: Self-expanding metallic stents in the treatment of benign colorectal disease: indications and outcomes. Colorectal Dis 2006;8:102–111. [ Links ]
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
* Corresponding author.
Dr. Gonçalo Alexandrino
Gastroenterology Department, Hospital Prof. Doutor Fernando Fonseca E.P.E.
IC 19 PT–2720-276 Amadora (Portugal)
E-Mail goncaloalexandrino@gmail.com
Received: June 29, 2017; Accepted after revision: August 9, 2017














