Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
GE-Portuguese Journal of Gastroenterology
Print version ISSN 2341-4545
GE Port J Gastroenterol vol.24 no.3 Lisboa June 2017
https://doi.org/10.1159/000452691
ORIGINAL ARTICLE
CA 19-9 as a Marker of Survival and a Predictor of Metastization in Cholangiocarcinoma
CA 19-9 Marcador de Sobrevida e Preditor de Metastização no Colangiocarcinoma
Rosa Coelhoa, Marco Silvaa, Eduardo Rodrigues-Pintoa, Helder Cardosoa, Susana Lopesa, Pedro Pereiraa, Filipe Vilas-Boasa, João Santos-Antunesa, José Costa-Maiab, Guilherme Macedoa
Departments of aGastroenterology and bSurgery, Faculty of Medicine, Centro Hospitalar de São João, Porto, Portugal
* Corresponding author.
ABSTRACT
Background: Cholangiocarcinoma is the second most frequent primitive liver malignancy and is responsible for 3% of the malignant gastrointestinal neoplasms. The aims of this study were to determine the association of serum levels of CA 19-9 at diagnosis with other clinical data and serum liver function tests and to identify possible factors that influence the survival rates during follow-up. Methods: Retrospective observational study of 89 patients with a diagnosis of cholangiocarcinoma followed at the Department of Gastroenterology during 5 years. Statistical analyses were performed using SPSS version 20.0. Results: Patients were followed up for a median time of 127 days (IQR: 48–564), and the median age at diagnosis was 71.0 years (IQR: 62.0–77.5). The median survival rate was 14.0 months (IQR: 4.3–23.7), and the mortality rate was 79%. Patients with CA 19-9 levels ≥ 103 U/L had lower albumin levels and higher levels of alanine aminotransferase and γ-glutamyltransferase. CA 19-9 levels ≥ 103 U/L were associated with a higher probability of metastization ( p = 0.001) and lower rates of treatment with curative intent ( p = 0.024). In a multivariate analysis, CA 19-9 levels <103 U/L and surgery were independent predictors of survival. Conclusion: Predictive factors for overall survival were identified, namely presence of metastasis, surgery, and chemotherapy. CA 19-9 levels ≥ 103 U/L were predictive factors for survival and metastization.
Keywords: CA 19-9 antigen; Cholangiocarcinoma; Prognosis; Tumor biomarkers
RESUMO
Introdução: O colangiocarcinoma é o segundo tumor maligno do fígado primitivo mais frequente sendo responsável por 3% das neoplasias malignas gastrointestinais. Os objetivos do presente estudo foram determinar a associação entre os níveis séricos de CA 19-9 ao diagnóstico com dados clínicos, testes de função hepática e identificar possíveis fatores preditivos de sobrevida. Métodos: Estudo retrospetivo de 89 pacientes com diagnóstico de colangiocarcinoma com seguimento em centro único durante 5 anos. A análise estatística foi realizada utilizando o programa SPSS versão 20.0. Resultados: A média de idades ao diagnóstico dos doentes incluídos foi de 71.0 anos (IQR: 62.0–77.5) com seguimento durante 127 dias (IQR: 48–564). A taxa de sobrevida mediana foi de 14.0 meses (IQR: 4.3–23.7) e a taxa de mortalidade de 79%. Os doentes com níveis sérios de CA 19-9 ≥ 103 U/L apresentavam níveis inferiores de albumina e níveis superiores de alanina aminotransferase e gama-glutamil transferase. Valores de CA 19-9 ≥ 103 U/L associaram-se a maior probabilidade de metastização ( p = 0.001) e as taxas inferiores de tratamento com intenção curativa ( p = 0.024). Em análise multivariada, valores de sérios de CA 19-9 <103 U/L e realização de cirurgia foram preditores independentes de sobrevida. Conclusão: A presença de metastização, a realização de cirurgia ou quimioterapia constituíram-se como fatores preditivos de sobrevida global. Valores séricos de CA 19-9 ≥ 103 U/L foram preditores de menor sobrevivência e de metastização.
Palavras-Chave: Antigénio CA 19-9; Biomarcadores tumorais; Colangiocarcinoma; Prognóstico
Introduction
Cholangiocarcinoma (CCC) is the second most frequent primitive liver malignancy after hepatocellular carcinoma [1], and it is responsible for 3% of the malignant gastrointestinal neoplasms nowadays [2]. The peak of its incidence occurs in the seventh decade of life [3]. Recent epidemiological data describe an increased incidence of CCC independently of the increased incidence of cirrhosis [1].
CCCs arise from the epithelial cells of the intrahepatic and extrahepatic bile ducts and are classified according to their localization into intrahepatic, perihilar, or distal (extrahepatic). Perihilar tumors, also called Klatskin tumors, occur at the bifurcation of the right and left hepatic ducts and are the most common type. The intrahepatic CCCs (IH-CCCs) can originate in small intrahepatic ductules or large intrahepatic ducts proximal to the bifurcation of the right and left hepatic ducts. They are the least common CCCs, even though, for unknown reasons, the incidence of IH-CCC has increased in the last 2 decades [4–9].
The TNM staging system for CCC from the American Joint Committee on Cancer is mainly based on the extent of ductal involvement of the tumor [10]. There are, nowadays, separate staging systems for cancers of the perihilar and distal bile ducts, which differ in their definitions of the T stage and of the group stages. However, all of them are characterized in 3 stages: T (primary tumor), N (regional lymph nodes), and M (distant metastases). More than 90% of CCCs are histologically compatible with adenocarcinomas, the remainder being squamous cell tumors. The etiology remains undetermined in most of them [11], despite the identification of several risk factors [11–15], like primary sclerosing cholangitis (PSC), chronic parasitic infection, and fibropolycystic liver disease (e.g., Caroli syndrome, congenital hepatic fibrosis, and choledochal cysts). In these diseases, the underlying process is related to long-standing inflammation that induces hyperplasia, cellular proliferation, and ultimately malignant transformation. There is also a clear and strong association between chronic intrahepatic stone disease (hepatolithiasis) and CCC. There is still no consensus concerning cholelithiasis as a risk factor; in fact, the association between gallstones and gallbladder cancer is well established; however, the association between CCC and cholelithiasis is of low magnitude compared to gallbladder cancer [14, 15].
Most patients with CCC have unresectable disease at diagnosis with an overall survival (OS) rate of 6 months to 1 year [16]. In fact, despite progress in anticancer therapy, more aggressive surgical resection, advances in liver transplantation, and interventional supportive care, the median survival rate remains low [17]. At diagnosis, only 10% of the patients present early-stage disease and can be considered for curative resection [18].
The diagnosis of CCC is based on laboratory studies, such as elevated levels of alkaline phosphatase (ALP) and of total bilirubin (TB), and imaging techniques, such as abdominal computed tomography, magnetic resonance imaging, echo-endoscopy, and/or liver biopsy. The tumor markers carbohydrate antigen 19-9 (CA 19-9) and carcinoembryonic antigen are the most studied tumor markers in CCC and are elevated in 85 and 40% of the cases, respectively [19]. The efficacy of these tumor markers in screening tests has been widely analyzed, mainly in PSC [20–22]; nevertheless, their usefulness as a diagnostic tool has not been established yet [20]. According to Hultcrantz et al. [21], serum levels of carcinoembryonic antigen and CA 19-9 have limited specificity; however, in a paper by Levy et al. [22], a sensitivity and specificity of 78 and 98%, respectively, were reported if an absolute CA 19-9 level of 129 U/mL was considered in patients with PSC and a diagnosis of CCC.
There are few studies evaluating the role of serum levels of CA 19-9 as a predictor of treatment response and OS. Harder et al. [23] determined a cutoff of 300 U/mL as a predictor of survival in patients with advanced biliary tract cancer, including gallbladder and intra- and extrahepatic cancers. However, the role of CA 19-9 serum levels in terms of prognosis and survival rate in patients with CCC is still not known. Predictive survival factors for CCC and its relationship to CA 19-9 serum levels also remain under debate.
The aims of this study were (1) to determine the association of serum levels of CA 19-9 at diagnosis with clinical and serum liver function tests, such as markers of hepatocellular damage (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]), cholestasis (TB and ALP), and synthetic function with albumin levels, and (2) to identify possible factors that influence survival rates during follow-up.
Materials and Methods
A retrospective observational study was carried out based on the medical records of 91 patients with a new diagnosis of CCC followed at the Departments of Gastroenterology and Surgery of a university hospital during a period of 5 years. It is important to emphasize that all decisions were taken in a multidisciplinary context with the participation of gastroenterologists, surgeons, radiologists, and oncologists. The inclusion criteria were a definite diagnosis of CCC (histologic and/or radiologic confirmation), regular follow-up (at least 2 medical visits), and age >18 years.
Demographic information was collected, including the dates of birth and death, the date of symptom onset, and the date of CCC diagnosis. Analytical data at diagnosis, including ALP, TB, γ-glutamyltransferase (G-GT), ALT, AST, and albumin levels, were collected, as well as CA 19-9 and carcinoembryonic antigen serum values at diagnosis. A cutoff, based on the median value of the sample, was established for CA 19-9 levels, and its relationship with other clinical and analytical variables was determined. Predictive survival factors for patients with CCC were determined using Kaplan-Meier survival curves according to clinical and therapeutic options.
Statistical Analysis
Categorical data were reported as absolute frequencies ( n ) and relative frequencies (%). Continuous data were presented as medians and percentiles. To test for a nonnormal distribution of continuous variables for 2 independent groups, nonparametric tests were used: the Mann-Whitney test for 2 independent groups and the ANOVA test for more than 2 independent groups. The χ 2 test (Fisher exact test) was used to compare quantitative variables between groups.
The median value of CA 19-9, determined at the time of diagnosis of all the patients enrolled in the study, was considered by the authors as a new cutoff. The χ 2 test and the Mann-Whitney test were used to evaluate the association between the tumor marker and analytical and clinical factors.
OS was defined as the estimated time from diagnosis until death or until the last follow-up. Median survival rates with interquartile ranges (IQR) were reported. The Kaplan-Meier survival curves were used with an α value of 0.05, and survival curves were compared with the log-rank test.
All the described p values were 2 sided, and p values of <0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 20.0.
Results
Baseline Patients Characteristics
The baseline characteristics of the 89 patients enrolled in the cohort (55% men) with a new diagnosis of CCC are presented in Table 1. Patients were followed up for a median time of 127 days (IQR: 48–564), and the median age at diagnosis was 71.0 years (IQR: 62.0–77.5).
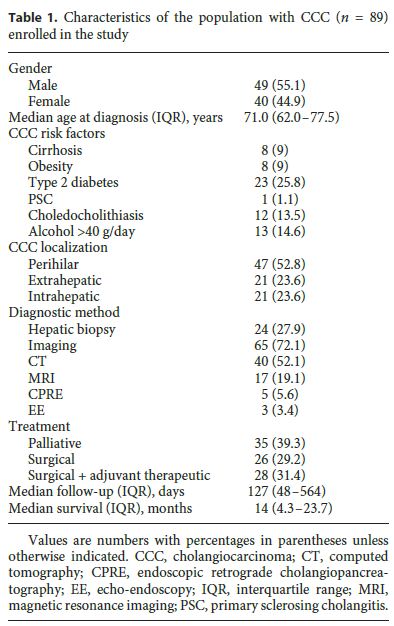
The number of CCC diagnoses progressively increased over the years, since the beginning of the cohort study, with an annual incidence of 14 CCCs in the period from 2008 to 2009, 31 CCCs in the period from 2010 to 2011, and 44 CCCs in the period from 2012 to 2013. The mortality rate at 6 months was 40.5%, with a median survival rate of 14.0 months (IQR: 4.3–23.7). Most patients (53%) had perihilar CCC, 24% had extrahepatic CCC, and 24% had IH-CCC, with the age at diagnosis being lower in IHCCC (median: 68.0 years [IQR: 51.0–72.5] vs. 74.0 years [IQR: 61.0–77.0], p = 0.014) than in patients with extrahepatic and hilar tumors.
The majority of the patients (30.3%) presented with jaundice and pruritus, and 20.2% presented with jaundice only. Weight loss and abdominal pain were reported by 9.0 and 13.5% of the patients, respectively. The diagnosis was made by imaging studies in 73% of cases, abdominal computed tomography being most frequently used (45%). Liver biopsy guided by ultrasound was performed in 15 (16.5%) patients, and IH-CCC was statistically significantly associated with a greater need for histology to make a diagnosis ( p < 0.001). Serum levels of G-GT, ALP, and TB were lower in IH-CCC than in patients with extrahepatic and perihilar tumors ( p < 0.001) (Table 1).
Thirty-two patients (36.0%) had distant metastasis (M1 according to the TNM classification), with liver metastasis being most common (12%). Twenty-six patients (29.2%) were submitted to surgery, and 28 patients (31%) underwent resection surgery plus adjuvant therapy. Hepatectomy was the most common surgical procedure (20%). Thirty-five patients (39.3%) underwent only palliative treatment with endoscopic transpapillary ( n = 23) and/or percutaneous transhepatic biliary drainage ( n = 21).
CA 19-9 Serum Levels
The median baseline CA 19-9 level was 103.0 U/mL (IQR: 15.0–576.0), with no differences found between genders and types of CCC. Markers of hepatocellular damage (AST and ALT), cholestasis (TB and ALP), and synthetic function with albumin levels at the time of diagnosis were analyzed in relation to CA 19-9 levels, considering the new cutoff established by the authors. Patients with serum levels of CA 19-9 ≥ 103 U/L had higher levels of ALP (460.0 U/L [IQR: 284.0–849.0] vs. 314.5 U/L [IQR: 101.9–503.3], p = 0.009) and G-GT (616.0 U/L [IQR: 120.2–1,044.0] vs. 236.0 U/L [IQR: 49.8–812.0], p = 0.021). Albumin serum levels were lower in patients with CA 19-9 levels ≥ 103 U/L (33.8 g/L [IQR: 29.7–35.7] vs. 36.5 g/L [IQR: 32.4–40.7], p = 0.002) (Table 2).
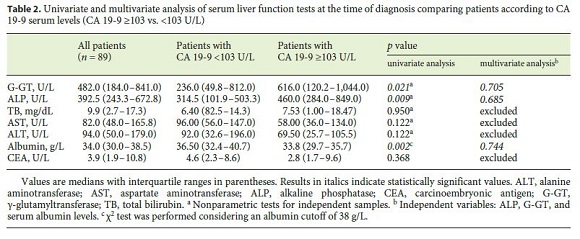
CA 19-9 levels ≥ 103 U/mL were associated with a lower OS of 7.5 months (IQR 0.0–16.3) versus 29.3 months (IQR: 18.1–40.4) ( p = 0.015). CA 19-9 levels <103 U/mL were associated with treatment with curative intent (surgery vs. palliative chemotherapy, p = 0.024). The probability of metastization at diagnosis was higher in patients with CA 19-9 levels ≥ 103 U/mL (odds ratio [OR]: 8.751 [95% confidence interval (CI): 2.270–32.264], p = 0.001).
Predictive Factors of Survival
The mortality rate during the follow-up period was 79% (37% at 3 months, 42% at 6 months, and 51% at 12 months), with a median survival of 14.0 months (IQR: 4.3–23.7). OS was not different between the 3 types of CCC. Higher survival rates at 3, 6, and 12 months were associated with prescription of chemotherapy ( p = 0.002, p = 0.001, and p = 0.003, respectively), surgery ( p < 0.001), and absence of metastization at diagnosis ( p < 0.001, p = 0.006, and p = 0.002) (Table 3). Survival rates at 6 and 12 months were associated with higher levels of albumin ( p = 0.012 and p = 0.032, respectively) and younger age ( p = 0.045 and p = 0.038, respectively).
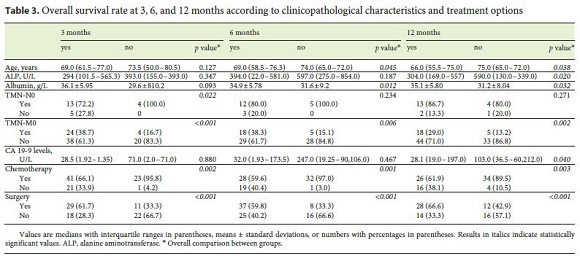
Survival rate at 3 months was associated with TNM-N0 ( p = 0.022). Survival at 12 months was associated with lower levels of CA 19-9 ( p = 0.040), lower levels of ALP ( p = 0.020), and a shorter period between the beginning of symptoms and diagnosis ( p = 0.029) (Table 3). Surgery and the presence of metastasis were independently associated with survival at 3, 6, and 12 months in the logistic regression.
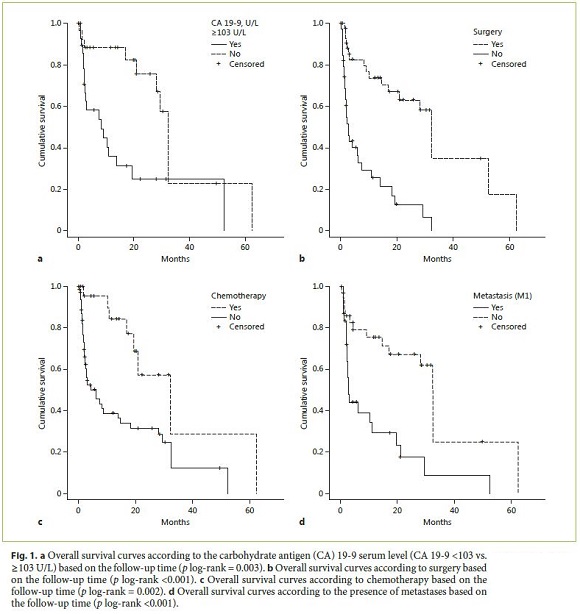
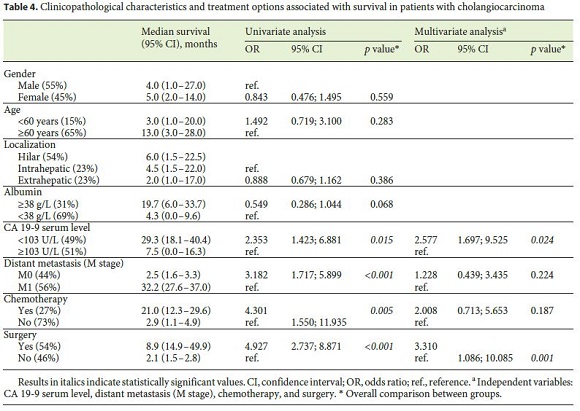
In a univariate analysis, OS was associated with CA 19-9 serum levels <103 U/L (29.3 months [95% CI: 18.1–40.4] vs. 7.5 months [95% CI: 0.0–16.3], p = 0.015), chemotherapy (21.0 months [95% CI: 12.3–29.6] vs. 2.9 months [95% CI: 1.1–4.9], p = 0.005), surgery (8.9 months [95% CI: 14.9–49.9] vs. 2.1 months [95% CI: 1.5–2.8], p < 0.001), and absence of metastases at diagnosis (2.5 months [95% CI: 1.6–3.3] vs. 32.2 months [95% CI: 27.6–37.0], p < 0.001) (Fig. 1). In a multivariate analysis, CA 19-9 serum levels <103 U/L and surgery were independent predictors of survival (Table 4).
Discussion
CCC is the second most common primary liver malignancy, and its incidence, for unknown reasons, is increasing globally [1]. This increase is probably related to the increase in the incidence of IH-CCC in Europe, North America, Asia, and Australia, since the incidence of extrahepatic CCC is declining internationally [4, 8, 24]. CA 19-9 was described as an important tool for the diagnosis of CCC in populations at risk, the most studied being patients with PSC.
In our cohort, the diagnosis of new cases of CCC increased during the 5 years of follow-up in a similar manner as in the most recent studies related to CCC incidence. However, these results should be considered with caution, as they might be overestimated since the hospital where the study was performed is a tertiary reference center. We also cannot exclude that more diagnoses are being made due to new and better available diagnostic methods for obstructive jaundice, which can identify biliary malignancies that might have gone undiagnosed previously. Another important factor could be the screening for hepatocellular carcinoma performed nowadays in patients with liver cirrhosis, which may have led to an increase in the diagnosis of CCC.
Concerning risk factors for CCC, almost 25% of the patients did not have any identified risk factor, as previously described in the literature, where a specific risk factor cannot be identified in many patients [21]. It is also interesting to note that only 1 patient had PSC and that choledocholithiasis and liver cirrhosis were only present in 13.5 and 9% of the patients, respectively. Twenty-six percent of the patients had diabetes, which has been described in previous case-control and cohort studies as a risk factor for biliary tract cancer [25]. However, it is not clear whether diabetes itself or other associated conditions (obesity or hyperlipidemia) represents a true risk factor for CCC.
The OS rate of 14.0 months (IQR: 4.3–23.7) was higher than previously described (between 6 months and 1 year), despite the fact that 36% of the patients had metastatic disease at the time of diagnosis, 29.2% of the patients were submitted to surgery, and 31% underwent resection surgery plus adjuvant therapy.
Using the median value of CA 19-9 serum levels at diagnosis (103 U/L), a new cutoff was established. CA 19-9 levels may reflect disease severity, since higher levels ( ≥ 103 U/L) are associated with increased levels of G-GT and ALP and lower levels of albumin. They may also be a prognostic marker, since levels ≥ 103 U/L were associated with metastasis and lower survival at 3, 6, and 12 months, and thus are an independent prognostic marker of survival. Lower values of this tumor marker were also associated with treatment with curative intent. The OR for survival was significantly higher in patients with CA 19-9 levels <103 U/L (OR: 2.353, p = 0.015) and in patients submitted to chemotherapy (OR: 4.301, p = 0.005), and it was even higher in patients submitted to surgery (OR: 4.927, p < 0.001) in the Cox regression analysis. In the multivariate analysis, the only 2 independent predictive factors of survival were surgery (OR: 3.310 [95% CI: 1.086–10.085], p = 0.001) and lower levels of CA 19-9 (OR: 2.577 [95% CI: 1.697–9.525], p = 0.024).
To the authors knowledge, no other studies have shown this association as clearly as the current study, and thus, these results should be taken into account when a diagnosis of CCC is made. The new cutoff established seems to be an important tool in detecting patients with a poor prognosis (lower survival rates and higher rates of metastasis), and, perhaps, it could be included in a future multifactorial analysis of survival. Predictive factors for OS were identified, namely presence of metastasis, surgery, and chemotherapy. In conclusion, this study provides important insights into the relevant role of CA 19-9 levels in the prognosis of CCC, as well as into other prognostic factors.
The retrospective nature and the small number of patients enrolled in the study may be limitations. Also, the authors analyzed all 3 different types of CCC together; however, as it is known from the literature, the biology, clinical behavior, and treatment approach are not the same for the 3 types of CCC. Yet, in our cohort, CA 19-9 serum levels and OS were not different between the 3 groups analyzed.
Nonetheless, one of the strengths of this study that can be pointed out is that all clinical decisions were taken in a multidisciplinary context with the participation of gastroenterologists, surgeons, radiologists, and oncologists, turning clinical decisions more homogeneous. Our results report data from a tertiary and single center and need to be supported by new population-based cohort studies.
References
1 Guedj N, Bedossa P, Paradis V: Pathology of cholangiocarcinoma. Ann Pathol 2010;30:455–463. [ Links ]
2 West J, Wood H, Logan RF, Quinn M, Aithal GP: Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer 2006;94:1751. [ Links ]
3 Anderson CD, Pinson CW, Berlin J, Chari RS: Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9: 43–57. [ Links ]
4 Patel T: Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353. [ Links ]
5 Shaib YH, Davila JA, McGlynn K, El-Serag HB: Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472. [ Links ]
6 Jepsen P, Vilstrup H, Tarone RE, Friis S, Sorensen HT: Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst 2007;99:895. [ Links ]
7 Rajagopalan V, Daines WP, Grossbard ML, Kozuch P: Gallbladder and biliary tract carcinoma: a comprehensive update, part 1. Oncology (Williston Park) 2004;18:889. [ Links ]
8 Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC: Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 2002;37:806. [ Links ]
9 Patel T: Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [ Links ]
10 Edge SB, Byrd DR, Compton CC, et al (eds): AJCC Cancer Staging Manual, ed 7. Springer, New York, 2010, p 219. [ Links ]
11 Chapman RW: Risk factors for biliary tract carcinogenesis. Ann Oncol 1999;10(suppl 4):308. [ Links ]
12 Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Loof L, Danielsson A, Hultcrantz R, et al: Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol 2002;36:321. [ Links ]
13 Lee YM, Kaplan MM: Primary sclerosing cholangitis. N Engl J Med 1995;332:924. [ Links ]
14 Watanapa P: Cholangiocarcinoma in patients with opisthorchiasis. Br J Surg 1996;83:1062. [ Links ]
15 Ahrens W, Timmer A, Vyberg M, Fletcher T, Guenel P, Merler E, Merletti F, et al: Risk factors for extrahepatic biliary tract carcinoma in men: medical conditions and lifestyle: results from a European multicentre case-control study. Eur J Gastroenterol Hepatol 2007;19:623. [ Links ]
16 Lubezky N, Facciuto M, Harimoto N, Schwartz ME, Florman SS: Surgical treatment of intrahepatic cholangiocarcinoma in the USA. J Hepatobiliary Pancreat Sci 2015;22:124–130. [ Links ]
17 Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, et al: Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer 2007;120:638. [ Links ]
18 Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Ilstrup DM: Outcomes after curative resections of cholangiocarcinoma. Arch Surg 1993;128:871–877; discussion 877–879. [ Links ]
19 Vauthey JN, Blumgart LH: Recent advances in the management of cholangiocarcinomas. Semin Liver Dis 1994;14:109. [ Links ]
20 Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, et al: Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut 2002;51(suppl 61):vi1–vi9. [ Links ]
21 Hultcrantz R, Olsson R, Danielsson A, Jarnerot G, Loof L, Ryden BO, Wahren B, et al: A 3-year prospective study on serum tumor markers used for detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol 1999;30:669. [ Links ]
22 Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD: The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci 2005;50:1734. [ Links ]
23 Harder J, Kummer O, Olschewski M, Otto F, Blum HE, Opitz O: Prognostic relevance of carbohydrate antigen 19-9 levels in patients with advanced biliary tract cancer. Cancer Epidemiol Biomarkers Prev 2007;16:2097–2100. [ Links ]
24 Welzel TM, McGlynn KA, Hsing AW, OBrien TR, Pfeiffer RM: Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 2006;98:873. [ Links ]
25 Jing W, Jin G, Zhou X, Zhou Y, Zhang Y, Shao C, et al: Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev 2012;21:24. [ Links ]
Statement of Ethics
All rules of the local ethics committee were followed, preserving patient identity and confidentiality.
Disclosure Statement
The authors declare that there are no conflicts of interest associated with this publication and there has been no financial support for this work.
* Corresponding author.
Dr. Rosa Coelho
Department of Gastroenterology, Centro Hospitalar de São João
Alameda Prof. Hernâni Monteiro
PO–4200-319 Porto (Portugal)
E-Mail rosacoelhoabrantes@hotmail.com
Received: May 30, 2016; Accepted after revision: September 26, 2016














