Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.23 no.2 Lisboa abr. 2016
https://doi.org/10.1016/j.jpge.2016.02.005
EDITORIAL
Sprue-Like Enteropathy Associated With Olmesartan: A New Kid on the Enteropathy Block
Enteropatia Tipo Sprue Induzida Por Olmesartan: Uma Nova Entidade no Campo Das Enteropatias
Isabel A. Hujoela, Alberto Rubio-Tapiaa,b, *
a Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA
b Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
* Corresponding author.
Sprue-like enteropathy associated with olmesartan, first identified by our group in 2012,1 is characterized by chronic diarrhea (often severe) and weight loss that is unresponsive to a gluten-free diet. Laboratory work-up commonly reveals non-specific anemia, hypoalbuminemia, electrolyte imbalance, and vitamin deficiencies, consistent with a severe malabsorption process. Histopathological findings include a combination of duodenal villous atrophy, increased intraepithelial lymphocytes, and a thickened subepithelial collagen layer (collagenous sprue). Histologic changes can be limited to the small bowel, or may include the entire gastrointestinal tract, with findings such as lymphocytic/collagenous gastritis and colitis. Individuals with sprue-like enteropathy associated with olmesartan have negative celiac serology. The majority may have either HLA-DQ2 or DQ8 haplotypes (61–81%).1 Diagnosis of olmesartan associated enteropathy should therefore be considered in cases of villous atrophy with negative celiac serology (so-called seronegative villous atrophy). Confirmation of diagnosis requires clinical resolution of symptoms after olmesartan withdrawal. Mucosal recovery is also expected within 3–6 months of olmesartan withdrawal and a follow-up duodenal biopsy is reasonable.
Severe sprue-like enteropathy associated with olmesartan appears to be rare although a spectrum of disease severity may be possible.2 The annual incidence rate of enteropathy in a French population-based study among patients treated with olmesartan for at least 6 months was calculated at 1.3 cases per 1000 individuals per year (95% confidence interval (CI) of 0.5–2.6).3 This rate is not significantly different from the rate of 0.63 cases of incident celiac disease per 1000 reported by the Mini-Sentinel (95% CI: .38–.99) (p = 0.16).4 The Mini-Sentinel reported that rates of incident celiac disease were of similar magnitude for all angiotensin receptor blockers with, for instance, a rate of 0.43 cases per 1000 (95% CI: 0.33–0.55) for losartan. Mini-Sentinel data therefore suggest that enteropathy may be a class-related drug effect. Such a hypothesis is supported by sporadic case-reports of enteropathy possibly associated with irbesartan,5 losartan,6 and valsartan.7 However, a nation-wide case-control Swedish study failed to show any association between the use of either angiotensin converting enzyme blockers or non-olmesartan angiotensin receptor blockers and subsequent villous atrophy.8 Thus, there is clear predominance of published data relating olmesartan to enteropathy (Table 1).
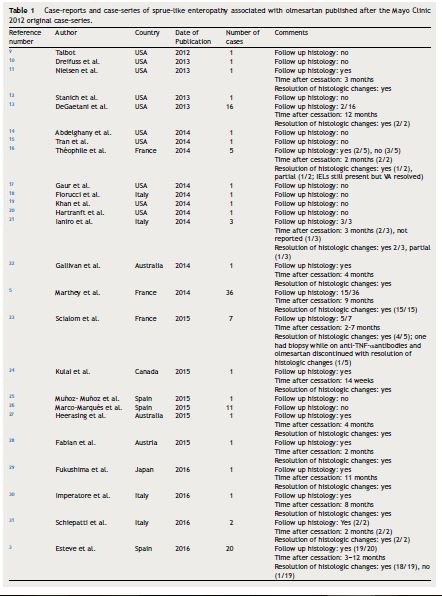
Sprue-like enteropathy associated with olmesartan should be ruled out early in the investigation of patients with seronegative villous atrophy. Indeed, a case-series on 72 patients with seronegative villous atrophy found the most frequent etiologies to be seronegative CD (28%), medication-related (26%), unclassified sprue (14%), autoimmune enteropathy (4%), and giardia (4%).13 Of the medication-related seronegative villous atrophy, roughly 84% were attributed to olmesartan.13 Early identification of individuals with sprue-like enteropathy associated with olmesartan is clinically relevant as symptoms can be severe and/or life-threatening with expected clinical response within days of olmesartan withdrawal.1
In this issue of GE Portuguese Journal of Gastroenterology, case reports by da Silva et al.32, Carneiro et al.33 and Eusébio et al.34, highlight the importance of considering sprue-like enteropathy associated with olmesartan when approaching a patient with seronegative villous atrophy and provide further information to aid in diagnosis of this emergent disease.
In their case-report, da Silva et al. outline a practical algorithm to approach diagnosis of seronegative villous atrophy that does not respond to a gluten free diet.32 The first proposed step following testing for celiac serology is a review of the patient's medication list. In the four cases reported in this issue, symptoms resolved within 48 h to one week of discontinuation of olmesartan.32–34 Trialing a patient off of a medication early on in the evaluation of seronegative villous atrophy could therefore provide both a rapid and cost-effective diagnosis. It is our practice that following olmesartan withdrawal, we try to understand the primary indication for olmesartan therapy and reassess the need for alternative medications together with the patient's primary care physician. It has been our experience that a considerable number of patients do not need any medications after suspension of olmesartan.
Eusébio et al.34 identify a unique finding of elevated transaminases in a case of olmesartan associated enteropathy. They propose that this may be due to the same mechanism behind the hypertransaminasemia seen in CD.
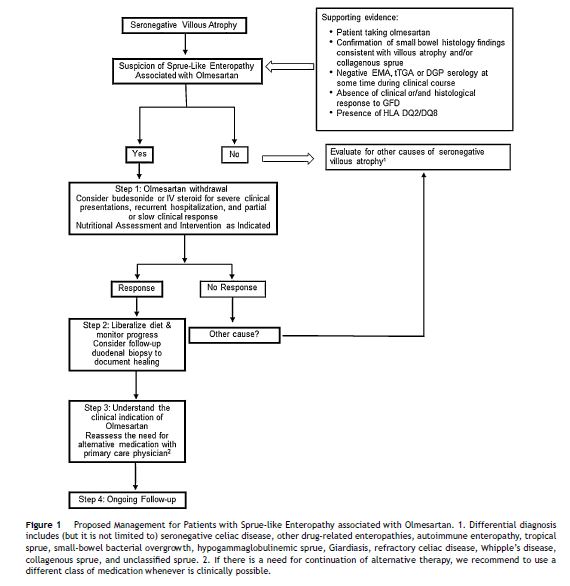
Carneiro et al.33 report on a case diagnosed with systemic sclerosis during the work-up for sprue-like enteropathy associated with olmesartan. The association with autoimmune diseases has been reported in several other studies.3,5,23 In line with this observation, there are reports of individuals with sprue-like enteropathy associated with olmesartan responding to immunosuppressive treatment.5,23 Our open-label experience suggests that some patients with severe symptoms, recurrent hospitalizations due to dehydration or both slow and/or partial response to olmesartan withdrawal may have some benefit from a short course of steroids such as budesonide (Fig. 1).
The association between autoimmune diseases and olmesartan associated enteropathy is consistent with the emerging evidence supporting an immune-based pathophysiology. One recent study by our group looking at duodenal biopsies of those taking olmesartan versus those who had discontinued the medication showed an increased CD8+ cells, FoxP3+ cells, and IL15R in biopsies of those taking olmesartan, similar to what is seen in CD.35 In addition, we demonstrated an increased IL15 expression and disruption of tight junction proteins (ZO-1) in olmesartan-treated Caco-2 cells.35 This suggests that olmesartan may trigger a similar change in intestinal epithelial cells as gluten does in those with CD although further study of the underlying mechanisms would be needed to fully understand the pathophysiology of sprue-like enteropathy associated with olmesartan, the new kid on the enteropathy block.
References
1. Rubio-Tapia A, Herman ML, Ludvigsson JF, Kelly DG, Mangan TF, Wuet T, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87:732-8. [ Links ]
2. Greywoode R, Braunstein ED, Arguelles-Grande C, Green PH, Lebwohl B. Olemsartan, other anti-hypertensives, and chronic diarrhea among patients undergoing endoscopic procedures: a case-control study. Mayo Clin Proc. 2014;89:1239-43. [ Links ]
3. Esteve M, Temiño R, Carrasco A, Batista L, Del Val A, Blé M, et al. Potential coeliac disease markers and autoimmunity in olmesartan induced enteropathy: a population-based study. Dig Liver Dis. 2016;48:154-61. [ Links ]
4. Sentinel M. Angiotensin II Receptor Blockers and Celiac Disease. Available from: http://www.mini-sentinel.org/work_products/Assessments/Mini-Sentinel_Angiotensin-II-Receptor-Blockers-and-Celiac-Disease.pdf (cited 6.02.16). [ Links ]
5. Marthey L, Cadiot G, Seksik P, Pouderoux P, Lacroute J, Skinazi F, et al. Olmesartan-associated enteropathy: results of a national survey. Aliment Pharmacol Ther. 2014;40:1103-9. [ Links ]
6. Negro A, Rossi GM, Santi R, Iori V, De Marco L. A case of severe sprue-like enteropathy associated with losartan. J Clin Gastroenterol. 2015;49:794. [ Links ]
7. Herman ML, Rubio-Tapia A, Wu TT, Murray JA. A case of severe sprue-like enteropathy associated with valsartan. ACG Case Rep J. 2015;2:92-4. [ Links ]
8. Mårild K, Lebwohl B, Green PH, Murray JA, Ludvigsson JF. Blockers of angiotensin other than olmesartan in patients with villous atrophy: a nationwide case-control study. Mayo Clin Proc. 2015;90:730-7. [ Links ]
9. Talbot GH. Small bowel histopathologic findings suggestive of celiac disease in an asymptomatic patient receiving olmesartan. Mayo Clin Proc. 2012;87:1231-2. [ Links ]
10. Dreifuss SE, Tomizawa Y, Farber NJ, Davison JM, Sohnen AE. Spruelike enteropathy associated with olmesartan: an unusual case of severe diarrhea. Case Rep Gastrointest Med. 2013;2013:618071. [ Links ]
11. Nielsen JA, Steephen A, Lewin M. Angiotensin- II. inhibitor (olmesartan)-induced collagenous sprue with resolution following discontinuation of drug. World J Gastroenterol. 2013;19:6928-30. [ Links ]
12. Stanich PP, Yearsley M, Meyer MM. Olmesartan-associated sprue-like enteropathy. J Clin Gastroenterol. 2013;47:894-5. [ Links ]
13. DeGaetani M, Tennyson CA, Lebwohl B, Lewis SK, Abu H, Arguelles-Grande C, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108:647-53. [ Links ]
14. Abdelghany M, Gonzalez L, Slater J, Begley C. Olmesartan associated sprue-like enteropathy and colon perforation. Case Rep Gastrointest Med. 2014;2014:494098. [ Links ]
15. Tran TH, Li H. Olmesartan and drug-induced enteropathy. Pharm Ther. 2014;39:47-50. [ Links ]
16. Théophile H, David XR, Miremont-Salamé G, Haramburu F. Five cases of sprue-like enteropathy in patients treated by olmesartan. Dig Liver Dis. 2014;46:465-9. [ Links ]
17. Gaur V, Albeldawi M, Weber L. Chronic diarrhea and weight loss. Gastroenterology. 2014;146(347):591. [ Links ]
18. Fiorucci G, Puxeddu E, Colella R, Paolo G, Villanacci V, Bassotti G. Severe spruelike enteropathy due to olmesartan. Rev Esp Enferm Dig. 2014;106:142-4. [ Links ]
19. Khan AS, Peter S, Wilcox CM. Olmesartan-induced enteropathy resembling celiac disease. Endoscopy. 2014;46(Suppl. 1):E97-8. [ Links ]
20. Hartranft ME, Regal RE. “Triple phase” budesonide capsules for the treatment of olmesartan-induced enteropathy. Ann Pharmacother. 2014;48:1234-7.
21. Ianiro G, Bibbò S, Montalto M, Ricci R, Gasbarrini A, Cammarota G. Systematic review: sprue-like enteropathy associated with olmesartan. Aliment Pharmacol Ther. 2014;40:16-23. [ Links ]
22. Gallivan C, Brown I. Olmesartan induced enterocolitis. Pathology. 2014;46:360-1. [ Links ]
23. Scialom S, Malamut G, Meresse B, Guegan N, Brousse N, Verkarre V, et al. Gastrointesitnal disorder associated with olmesartan mimics autoimmune enteropathy. PLOS ONE. 2015;10:e0125024. [ Links ]
24. Kulai T, Arnason T, MacIntosh D, Igoe J. Severe sprue-like enteropathy associated with olmesartan. Can J Gastroenterol Hepatol. 2015:16873 (Epub ahead of print).
25. Muñoz-Muñoz C, López-Vivancos J, Huaman W, García-Cors M. Sprue-like enteropathy due to olmesartan. Rev Esp Enferm Dig. 2015;107:647-8. [ Links ]
26. Marco-Marqués A, Sanahuja-Martinez A, Bosca-Watts MM, Tosca-Cuquerella J, Mas-Mercader P, Andrade-Gamarra V, et al. Could HLA-DQ suggest why some patients have olmesartan-related diarrhea and others don’t? Am J Gastroenterol. 2015;110:1507-8.
27. Heerasing N, Hair C, Wallace S. Olmesartan-induced enteropathy. Intern Med J. 2015;45:117-8. [ Links ]
28. Fabian E, Schiller D, Wenzl H, Lackner C, Donnerer J, Ziachehabi A, et al. Case No 156: 82-year-old woman with chronic diarrhea and weight loss of 20 kilograms. Wien Klin Wochenschr. 2015;127:974-80. [ Links ]
29. Fukushima M, Kitamoto H, Inokuma T, Imai Y. Severe spruelike enteropathy associated wtih olmesartan observed by double-balloon enteroscopy. Gastrointest Endosc. 2016;83:269-70. [ Links ]
30. Imperatore N, Tortora R, Capone P, Caporaso N, Rispo A. An emerging issue in differential diagnosis of diarrhea: sprue-like enteropathy associated with olmesartan. Scand J Gastroenterol. 2016;51:378-80. [ Links ]
31. Schiepatti A, Biagi F, Cumetti D, Luinetti O, Sonzogni A, Mugellini A, et al. Olmesartan-associated enteropathy: new insights on the natural history? Report of two cases. Scand J Gastroenterol. 2016;51:152-6. [ Links ]
32. da Silva BM, Neves SJ, Martínez AG, de Jesus Geneux K, García JL, Antolín SM, et al. Enteropathy associated with olmesartan. GE Port J Gastroenterol. 2016;23:96-100. [ Links ]
33. Carneiro L, Moreira A, Pereira A, Andrade A, Soares J, Silva A, et al. Olmesartan-induced sprue like enteropathy. GE Port J Gastroenterol. 2016;23:101-5. [ Links ]
34. Eusébio M, Caldeira P, Gião A, Ramos A, Velasco F, Cadillá J, et al. Olmesartan-induced enteropathy – an unusual cause of villous atrophy. GE Port J Gastroenterol. 2016;23:91-5.
35. Marietta EV, Nadeau AM, Cartee AK, Singh I, Rishi A, Choung RS, et al. Immunopathogenesis of olmesartan-associated enteropathy. Aliment Pharmacol Ther. 2015;42:1303-14. [ Links ]
Conflict of interest
The authors declare no conflicts of interest.
* Corresponding author.
E-mail address: rubiotapia.alberto@mayo.edu (A. Rubio-Tapia).














