Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.22 no.5 Lisboa out. 2015
https://doi.org/10.1016/j.jpge.2015.05.001
ORIGINAL ARTICLE
Endoscopic Submucosal Dissection for Gastrointestinal Superficial Lesions: Initial Experience in a Single Portuguese Center
Dissecção Endoscópica da Submucosa para Lesões Gastrointestinais Superficiais: Experiência Inicial de um Único Centro Português
José Rodrigues, Joana Carmo, Liliana Carvalho, Pedro Barreiro∗, Cristina Chagas
Gastroenterology Department, Centro Hospitalar de Lisboa Ocidental, Lisbon, Portugal
* Corresponding author.
ABSTRACT
Introduction: Endoscopic submucosal dissection (ESD) is a minimally invasive organ-sparing endoscopic technique which allows en bloc resection of premalignant and early malignant lesions of the gastrointestinal tract regardless of size. In spite of the promising results, mainly from Japanese series, ESD is still not being widely used in western countries. This study aims to report the feasibility, safety and effectiveness of ESD technique for treating premalignant and early malignant gastrointestinal (GI) lesions (esophagus, gastric and rectum) in a Portuguese center.
Patient and Methods: From December 2011 to November 2014, 34 GI lesions were treated by ESD. The location, en bloc and pathological complete resection (R0) rates, procedure time, complications and local recurrence were retrospectively evaluated.
Results: From 34 resected lesions, 18 were gastric (GL), 15 were rectal (RL) and one esophageal (EL). En bloc resection for each location was 17/18 (94%), 11/15 (73%) and 1/1 respectively. R0 was achieved in 16/18 (89%) GL, 9/15 (60%) RL and 1/1 EL. Mean resection time was 67 min for GL, 142 min for RL and 40 min for EL. Complications included immediate (6%) and delayed (3%) minor bleeding but no perforation. One local residual lesion from a RL was reported in the follow-up, effectively treated with an endoscopic technique. Disease-specific survival was 100% over a mean follow-up period of 14 months.
Conclusion: ESD has shown to be a safe and feasible resection method, achieving high R0, low recurrence and complication rates. Our results are similar to those reported in other international series.
Keywords: Dissection; Gastrointestinal Endoscopy; Gastrointestinal Neoplasms; Precancerous Conditions
RESUMO
Introdução: A dissecção endoscópica da submucosa (DES) é uma técnica minimamente invasiva que permite a ressecção em bloco de lesões gastrointestinais pré-malignas e malignas precoces independentemente do seu tamanho. Apesar dos resultados promissores, principalmente em séries Japonesas, a DES ainda não é executada de forma generalizada no mundo Ocidental. O objetivo do estudo é reportar a exequibilidade, segurança e eficácia da técnica de DES no tratamento de lesões pré-malignas e malignas precoces do tubo digestivo (esófago, estômago e reto) num centro Português.
Doentes e Métodos: Entre dezembro de 2011 e novembro de 2014, 34 lesões gastrointestinais foram excisadas por DES. A sua localização, taxas de ressecção em bloco e ressecção histológica completa (R0), tempo do procedimento e recidiva local foram avaliados.
Resultados: De 34 lesões ressecadas, 18 foram gástricas (LG), 15 foram rectais (LR) e uma esofágica (LE). A ressecção em bloco em cada localização foi de 17/18 (94%), 11/15 (73%) e 1/1 respetivamente. A ressecção foi considerada R0 em 16/18 (89%) LG, 9/15 (60%) LR e 1/1 LE. Os tempos médios de ressecção foram de 67 min para LG, 142 min para LR e 40 min para LE. As complicações registadas incluíram hemorragia imediata (6%) e tardia (3%), sem casos de perfuração. Durante o período de seguimento é reportada uma lesão residual de uma LR, tratada eficazmente por técnica endoscópica. Verificou-se uma sobrevida específica da doenc¸a de 100% durante um período médio de seguimento de 14 meses.
Conclusão: A técnica de DES revelou ser segura e exequível, atingindo uma elevada taxa de R0 e baixas taxas de recidiva e complicações. Os resultados apresentados são semelhantes aos reportados em outras séries internacionais.
Palavras-Chave: Dissecção; Endoscopia Gastrointestinal; Lesões Pré-Cancerosas; Neoplasias Gastrointestinais
1. Introduction
Endoscopic submucosal dissection (ESD) was first described by Hirao et al. in the 80s and is characterized by three basic steps: fluid injection into the submucosa to separate the lesion from the muscle layer, circumferential cutting of the surrounding mucosa and dissection of the connective tissue of the submucosa beneath the lesion.1,2
This minimally invasive organ-sparing endoscopic technique allows en bloc resection of premalignant and early malignant lesions of the gastrointestinal tract, regardless of size, avoiding surgical morbidity. Comparing to endoscopic mucosal resection (EMR), ESD contributes to a better histological analysis, lower local recurrence rate and more curative resections.3,4
In spite of the promising results from Japanese and recent western series, ESD is still not being widely used in western countries.5-8
The aim of this study is to report the feasibility, safety and effectiveness of ESD technique for treating premalignant and early malignant gastrointestinal (GI) lesions (esophagus, gastric and rectum) in a European center.
2. Patients and Methods
2.1. Pre-procedure evaluation
Before the procedure a careful endoscopic staging was performed to all lesions. Morphological endoscopic classification was made according to Paris classification,9 and every sign suggestive of invasive lesion (e.g. Kudo VN) was looked for.10 When there were doubts about possible deep invasion, complementary endoscopic ultrasonography (EUS) staging was made.
All lesions had previous endoscopic biopsies confirming their neoplastic nature (pre-malignant or malignant lesions).
We proposed ESD for gastric and esophageal premalignant or early malignant lesions, with more than 10mm, with possibility of endoscopic curability based on Japanese criteria (Japanese Gastric Cancer Association and Japan Esophageal Society Guidelines).11,12
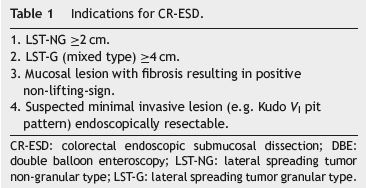
ESD indications for rectal neoplastic lesions treatment were adapted from those proposed by the Colorectal ESD Standardization Implementation Working Group and are shown in Table 1.13 Subepithelial lesions with more than 10mm were also included.
The procedures were performed after a multidisciplinary group decision and with the consent of the patients, after explaining the risks and benefits of the method and other therapeutic options.
2.2. Technical aspects of ESD procedures
Gastric and esophageal ESD were performed under general anesthesia with orotracheal intubation for airway protection while in the rectum just superficial sedation with midazolam was done. The patients were monitored with continuous electrocardiographic registration, pulse oximetry and noninvasive blood pressure measurement.
All procedures were done with conventional gastroscopes or colonoscopes (Olympus GIF-Q165) with soft straight distal attachments (Olympus) using Dual-Knife and/or IT-Knives (Olympus). For the different steps of the resection procedure, the ERBE ICC 200 electrosurgical generator was used: endocut mode for mucosal incision; forced coagulation for submucosal dissection; soft coagulation for hemostasis. Carbon dioxide was used for insufflation.
The following main steps were performed: (1) careful inspection of the lesion to determine the lateral margins with white light endoscopy and usually chromoendoscopy for gastric and esophageal lesions; (2) marking the borders of the lesion using soft coagulation current with the Dual Knife (not performed in rectal lesions); (3) submucosal solution injection with Voluven®, indigo carmine and epinephrine (1:250,000) to create a submucosal cushion underneath the lesion; (4) mucosal incision was then made using Dual Knife and/or It Knife; for gastric lesions, complete circumferential incision was always made before starting submucosal dissection (not always performed in the others locations); (5) complete submucosal dissection under the base of the lesion using Dual Knife and/or It Knife; during this phase repeated submucosal injection whenever needed and coagulation of visible vessels were performed; (6) at the end, careful inspection of the ulcer looking for complications; coagulation of visible vessels was routinely performed.
2.3. Histopathological assessment
Resection specimens were minimally stretched and fixed onto cork with needles. In cases of piecemeal resections, if possible, the specimen was reconstructed by appropriate fixation onto cork. Specimen size was measured and then it was sent for histopathological assessment fixed in formalin. Histopathological work-up provided information about lesion diameter, invasion depth, grading, presence or absence of lymphovascular invasion and lateral and vertical margins.
2.4. Post-procedure protocol and follow-up
All patients were admitted for surveillance and those who underwent upper GI ESD proton pump inhibitors were administered (40 mg two times per day) for 4-8 weeks. Oral liquid diet was started in the following day with discharge at 24-72H after the procedure, in the absence of complications.
All patients had a follow up endoscopy with scar biopsies at 3, 6 and 12 months after the procedure. Then, and if no residual lesion was identified, annual endoscopic surveillance was indicated for upper GI resections and every 2-3 years for rectal lesions.
2.5. Endoscopic outcomes and definitions
Resection was defined as en bloc when the lesion was resected in one piece, and piecemeal if in two or more pieces. A complete resection (R0) was considered when the resection was en bloc and both lateral and vertical margins were free of lesion; incomplete resection (R1) when the lesion invaded at least one of the margins (vertical or lateral). When an adequate evaluation of the margins was not possible, by fragmentation or coagulation effects, and complete resection was not ensured it was defined as Rx.
In endoscopic surveillance, a residual disease was defined as the presence of lesion at the same place where ESD was performed following a non-R0 resection, found in the first or second endoscopic controls; after this period or if resection was R0 it was considered local recurrence. A lesion found in another place during follow-up was defined as metachronous lesion.
Bleeding and perforation are the most common complications. Immediate bleeding, observed during the procedure, was considered major when it led to a decrease in hemoglobin level >2 g/dL, hemodynamic instability and/or surgery was necessary. As minimal immediate bleeding occurs in almost all procedures we have just considered minor immediate bleeding as a complication when it changed the procedure plan (e.g. use of hemoclips) or took ≥5 min to be controlled by endoscopy (without meeting criteria for major bleeding). Delayed bleeding, was defined when there was clinical evidence of bleeding (melena, hematemesis or haematochezia) that occurred until 30 days after the ESD. Another potentially dangerous complication of ESD is perforation. It was considered major or minor if it was radiologically evident with or without a visible wall defect, respectively.
3. Results
From December 2011 to November 2014, thirty-three patients, corresponding to thirty-four lesions, underwent ESD at our institution for treatment of gastrointestinal lesions: 18 gastric (n = 18, 53%), 15 in the rectum (n = 15, 44%) and one in the esophagus (n = 1, 3%). In five lesions EUS was performed previously to lesion resection. In all cases no submucosal or local nodes invasion was suspected. One endoscopist (P. B.) with previous training in ESD performed all the procedures. This cohort presented a mean age of 70±9 years with no gender prevalence (17 men, 17 women). Resection duration ranged from 25 to 260 min (mean 100±65 min). Twenty-nine resections (85%) were considered en bloc and R0 was also achieved in 26/34 (76%) cases. Mean follow-up was 14±10 months (1-36) with one local residual lesion reported treated with snare EMR.
Due to different clinical implications, results will be divided according to lesion origin.
3.1. Gastric resections
Gastric ESD represented 53% (n = 18) of the resected lesions (Fig. 1). Ten of them were located in the antrum, five were in the body, two were in the angular incisure and one was in the cardia. The average size was 21±5mm, with a variable endoscopic morphology (Table 2). Resection duration ranged from 30 to 140 min (mean 67±34 min).
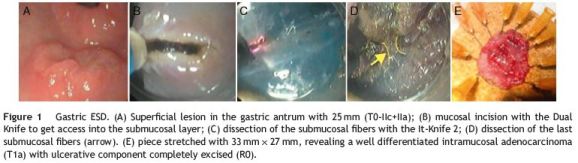
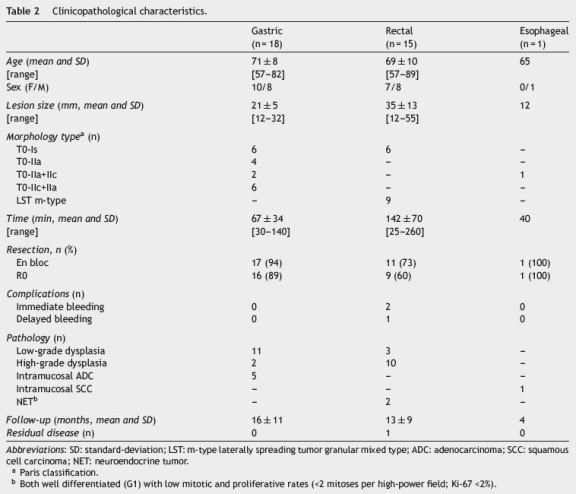
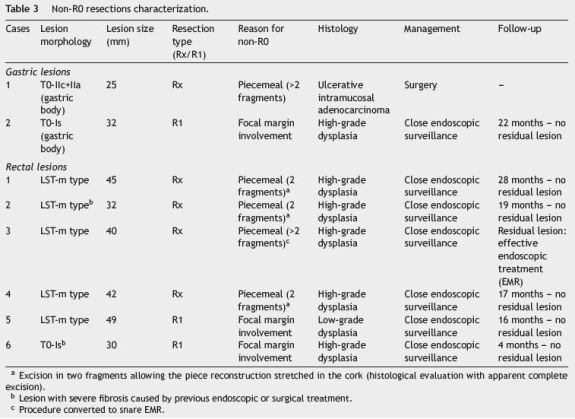
En bloc resection was feasible in 17/18 (94%) and R0 was achieved in 16/18 (89%) lesions, without any complication. In just one patient, with an ulcerative lesion, en bloc resection was not achieved due to submucosal fibrosis. In this case, histological examination of the resected pieces showed an ulcerative intramucosal adenocarcinoma and surgery was proposed (Table 3). Mean follow-up was 16±11 months (1-36) with no local recurrence reported (Table 2).
Post-procedure pathologic examination revealed high grade dysplasia in 11 lesions (61%), low grade dysplasia in two lesions and differentiated intramucosal adenocarcinoma in the remaining five.
3.2. Rectal resections
Fifteen rectal lesions (44%) were removed by ESD (Fig. 2). Six of them were located in the distal rectum, six in the middle and three in the proximal part of the rectum. The average size was 35±13mm and most of them were granular mixedtype lateral spreading tumors. Resection duration ranged from 25 to 260 min (mean 142±70 min). Eleven resections (73%) were considered en bloc and R0 was achieved in 9/15 (60%). In three of the four piecemeal resections the lesion was obtained in two fragments allowing the piece reconstruction stretched in cork. Histological evaluation showed apparent complete excision, however by definition it was considered Rx resection. Two cases of en bloc resection were considered R1 (Table 3).
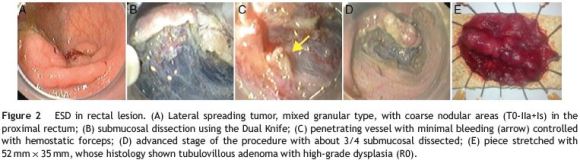
There were three cases of minor bleeding: two during the procedure and controlled endoscopically and one delayed bleeding in an anti-coagulated patient (managed conservatively without any local treatment required). Mean follow-up was 13±11 months (2-28) with one local residual lesion reported, endoscopically treated with snare EMR (Table 2).
Post-procedure pathologic examination revealed high grade dysplasia in 10 lesions (67%), low grade dysplasia in three and neuroendocrine tumor (carcinoid) in two.
3.3. Esophageal resection
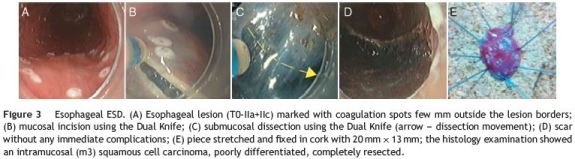
One esophageal lesion was treated at our institution (Fig. 3). It was located in the middle esophagus and its size was 12mm. Resection duration was 40 min. Resection was considered en bloc and R0 without complications. Pathologic examination revealed poor differentiated intramucosal (m3) squamous cell carcinoma. Therefore, surgery was proposed but the patient refused it. After a follow-up time of 4 months, no local recurrence was reported.
4. Discussion
The major advantage of ESD procedures is its high rate of en bloc resection, regardless of lesions size, which contributes to a decrease in recurrence rate and leads to a better pathological diagnosis and curative resections.2,3 The lack of experience in western countries may explain the differences in success rates when compared to Japanese series (mainly in the past series).14
The current study represents a retrospective evaluation of superficial gastrointestinal tumors treated by ESD in a single Portuguese center. To the best of our knowledge it is the first Portuguese report including rectal and esophageal lesions.
Due to its technical difficulties, a period of training in animal was first performed (more than 30 procedures all under expert supervision). The next step involved the participation in more than 15 human ESD procedures, with performance of some phases under expert supervision. As early proficiency was gained, we started. At first, most of the lesions treated were located in the gastric antrum. As more extensive experience was carried out, more challenging resections were performed (larger size, proximal gastric lesions and then in different gastrointestinal locations). This kind of approach led us, in gastric lesions, to a success rate (94% en bloc and 89% R0) and an average resection time (67 min) equivalent to other international and Portuguese series reported.5-7 Traditionally, ESD emerged in order to allow en bloc resection of lesions with more than 2 cm, normally not possible by conventional EMR techniques. However, further analysis showed that even smaller gastric lesions may benefit from ESD excision compared with EMR. For gastric lesions, Watanebe et al. and Simura et al. reported significantly higher complete resection rates with ESD than with EMR for lesions larger than 10mm.15,16 In other study, Hoteya et al. showed that complete resection rates were overwhelmingly better for ESD than for EMR even for lesions between 5 and 10mm in diameter, regardless of gastric location.17 These excellent results in the stomach, namely over EMR, made ESD the therapy of choice for well differentiated non-ulcerative early gastric cancers, irrespective of its size and location, and for small ulcerative early cancers (less than 3 cm), as recently proposed by Spanish guidelines.18
Also in the esophagus, after proper training, ESD has been showing high technical success rates associated with a low incidence of complications.19-21 Although most published data is from eastern countries, a recent large European series confirms these results.22 Our experience in esophageal lesion resection is still scarce. Only one patient was treated. Technical success was achieved although resection was considered not curative due to histopathological features (poor differentiated intramucosal squamous cell carcinoma).20 In contrast to gastric lesions, esophageal ESD and EMR have shown similar complete resection rates for lesions with less than 15mm with better outcomes for ESD over EMR just for larger lesions.13 In our case the lesion had only 12mm, however we chose an ESD resection to ensure proper free-tumor margins due to the suspicion of poorly differentiated neoplasm in diagnostic biopsies (dimension of the resected piece: 21mm×18 mm).
Compared with others locations, in our series, rectal lesion resections were more time consuming (ranging from 25 to 260 min) with a lower en bloc (n = 11, 73%) and R0 (n = 9, 60%) resections. These results can in part be explained by features of resected lesions such as large dimension (excluding the two neuroendocrine cases - smaller lesions - mean lesion dimension: 38mm (20-55 mm)) and heavier submucosal fibrosis in some cases due to previous endoscopic/surgical treatment attempts (two cases). Nevertheless, we just had one case of residual tissue in our follow-up period, successfully treated by snare EMR. Although R0 resection was achieved just in 9/15 lesions, there were three additional cases where lesions were obtained in two fragments allowing the piece reconstruction. In these cases the histological evaluation showed apparent complete excision, considered R0 in some series.7,23 In two cases, resection was considered R1. However, in the follow-up period, no residual lesion was documented in both cases, which make complete excision likely. We hypothesize that cautery artifacts or small fragmentation of the tumor-free margins during the procedure might be responsible for a non-R0 resection in both cases. We had no cases of major complications, reporting just three minor bleeding cases without clinical impact. Our results are similar to other European series reported.24 Despite the growing acceptance in Japan, the benefit of ESD over EMR in the colon continues to be contested by many western endoscopists. Indeed, in spite of a higher en bloc rate, better histological examination and lower relapse rate, colorectal ESD is usually more time consuming, technically difficult and has a higher risk of complications when comparing to EMR.25 Therefore, it is recognized the need for structured training system to enhance trainee experience and to reduce the risks of complications. However, there are differences from West to Japan, such as the availability of qualified mentors, the pathology seen and trainees background. An algorithm for western physicians which integrates both hands-on training courses, animal model work as well as visits to expert centers has been proposed. After having experience with gastric ESD, endoscopists can gradually expand to cases of increasing difficulty such as those with colon location. Then, a training continuum with books, journals, conferences, live demonstrations, and visits to expert centers is essential to maintain proficiency.26 Recognizing its technical challenges, at this time we believe that colorectal ESD should be performed in selected patients, particularly in rectal lesions (lower risk of free perforation and surgical option potentially more crippling) when curative resection is expected just after a careful histological assessment (risk of minimal submucosal invasion). Moreover, ESD in rectum has shown to be as efficient as surgical transanal resections, with a lower morbidity and shorter hospital stay.25,27,28
When compared to surgery, ESD is also a minimal invasive approach that seems to be preferred by patients. Indeed, in our series one patient accepted ESD but refused surgical approach when it was proposed due to a non-curative endoscopic resection. This choice can be a reflection of ESD safety profile. We report only minor complications (bleeding) in three patients (8%), all of them from rectal lesion resections, one of them previously treated by surgery. Our results are similar to those reported by other Western and Japanese series.21,29,30
Although not formally recommended, biopsy specimens were routinely taken from post-ESD scar during this initial experience period in great part due to differences in scarring between patients. However, we report only one residual rectal lesion (after piecemeal resection) which was endoscopically seen. Routine follow-up biopsies at the endoscopic resection scar may not be necessary, unless it is an Rx or R1 resection or if there is an endoscopic suspicious of residual/recurrent disease during a follow-up endoscopy.31
There are some limitations to our data analysis. Firstly, our population is small compared to other Eastern series. Secondly, our follow-up time is short. So we still cannot correctly analyze our long-term outcome. In conclusion, ESD represents a significant advance in therapeutic endoscopy by increasing the capacity of endoscopic curative resections. Given our present results, we believe that the ESD technique is feasible and safe in our environment.
5. Author contributions
All authors have seen and approved the manuscript being submitted. All authors listed contributed significantly to the work.
References
1. Hirao M, Masuda K, Ananuma T, Masuda K. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc. 1988;34:264-9. [ Links ]
2. Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962-7. [ Links ]
3. Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, et al. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-8. [ Links ]
4. Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77:23-8. [ Links ]
5. Uedo N, Takeuchi Y, Ishihara R. Endoscopic management of early gastric cancer: endoscopic mucosal resection or endoscopic submucosal dissection: data from a Japanese high-volume center and literature review. Ann Gastroenterol. 2012;25:281-90. [ Links ]
6. Pimentel-Nunes P, Mourão F, Veloso N, Afonso L, Jácome M, Moreira-Dias L, et al. Long-term follow-up after endoscopic resection of gastric superficial neoplastic lesions in Portugal. Endoscopy. 2014;46:933-40. [ Links ]
7. Coda S, Trentino P, Antonellis F, Porowska B, Gossetti F, Ruberto F, et al. A western single-center experience with endoscopic submucosal dissection for early gastrointestinal cancers. Gastric Cancer. 2010;13:258-63. [ Links ]
8. Chaves DM, Filho FM, Moura EGH, Santos MEL, Arrais LRG, Kawaguti F, et al. Endoscopic submucosal dissection for the treatment of early esophageal and gastric cancer - initial experience of a western center. Clinics. 2010;65:377-82. [ Links ]
9. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1 2002. Gastrointest Endosc. 2003;58:S3-43. [ Links ]
10. Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [ Links ]
11. Kuwano H, Nishimura Y, Ohtsu A, Kato H, Kitagawa Y, Tamai S, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2007 edition: part I edited by the Japan Esophageal Society. Esophagus. 2008;5:61-73. [ Links ]
12. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010. Gastric Cancer. 2011;14:113-23. [ Links ]
13. Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in western countries? J Gastroenterol Hepatol. 2013;28:406-14. [ Links ]
14. Bergman JJ. How to justify endoscopic submucosal dissection in the Western world. Endoscopy. 2009;41:988-90. [ Links ]
15. Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776-82. [ Links ]
16. Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, Ogasawara N, et al. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22:821-6. [ Links ]
17. Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Benefits of endoscopic submucosal dissection according to size and location of gastric neoplasm, compared with conventional mucosal resection. J Gastroenterol Hepatol. 2009;24:1102-6. [ Links ]
18. Fernández-Esparrach G, Calderón A, de-la-Peña J, Díaz-Tasende José, Esteban J, Gimeno-García A, et al. Endoscopic submucosal dissection. Endoscopy. 2014;46:361-70. [ Links ]
19. Isomoto H. Updates on endoscopic therapy of esophageal carcinoma. Thoracic Cancer. 2014;3:125-30. [ Links ]
20. Higuchi K, Koizumi W, Satoshi T, Sasaki T, Katada C, Azuma M, et al. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res. 2009;3:153-61. [ Links ]
21. Joo DC, Kim G, Park Y, Jhi J, Song G. Long-term outcome after endoscopic submucosal dissection in patients with superficial esophageal squamous cell carcinoma: a single-center study. Gut Liver. 2014;8:612-8. [ Links ]
22. Probst A, Aust D, Märkl B, Anthuber M, Messmann H. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy. 2015;47:113-21. [ Links ]
23. Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350-5. [ Links ]
24. Rahmi G, Hotalyt B, Lepillez V, Giovannini M, Coumaros D, Charachon A, et al. Endoscopic submucosal dissection for superficial rectal tumors: prospective evaluation in France. Endoscopy. 2014;46:670-6. [ Links ]
25. Hon SS, Ng SS, Chiu PW, Chan FK, Ng EK, Li JC, et al. Endoscopic submucosal dissection versus local excision for early rectal neoplasms: a comparative study. Surg Endosc. 2011;25:3923-7. [ Links ]
26. Coman R, Gotoda T, Draganov P. Training in endoscopic submucosal dissection. World J Gastrointest Endosc. 2013;5:369-78. [ Links ]
27. Kiriyama S, Saito Y, Matsuda T, Nakajima T, Mashimo Y, Joeng HK, et al. Comparing endoscopic submucosal dissection with transanal resection for non-invasive rectal tumor: a retrospective study. J Gastroenterol Hepatol. 2011;26:1028-33. [ Links ]
28. Kawaguiti F, Nahas C, Marques C, Martins B, Retes F, Medeiros R, et al. Endoscopic submucosal dissection versus transanal endoscopic microsurgery for treatment of early rectal cancer: a comparative study. Surg Endosc. 2011;25:3923-7. [ Links ]
29. Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, et al. Endoscopic and oncologic outcomes of early gastric cancer after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-93. [ Links ]
30. Sattianayagam PT, Desmond PV, Jayasekera C, Chen RY. Endoscopic submucosal dissection: experience in an Australian tertiary center. Ann Gastroenterol. 2014;27:212-8. [ Links ]
31. Lee JY, Choi IJ, Cho SJ, Kim CG, Kook MC, Lee JH, et al. Routine follow-up biopsies after complete endoscopic resection for early gastric cancer may be unnecessary. J Gastric Cancer. 2012;12:88-98. [ Links ]
* Corresponding author.
E-mail address: pedrobarreiro@msn.com (P. Barreiro).
Conflict of interest
The authors report no potential conflict of interest.
Ethical disclosures
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Received 19 February 2015; accepted 5 May 2015














