Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.21 no.6 Lisboa dez. 2014
https://doi.org/10.1016/j.jpge.2014.09.001
GUIDELINES
A practical guide for antiviral therapy of chronic Hepatitis C
Guia Prático para a Terapêutica Antivírica da Hepatite C Crónica
José Velosaa,b,∗, Fátima Serejoa,b, Fernando Ramalhoa,b, Rui Marinhoa,b, Beatriz Rodriguesa, Cilénia Baldaiaa,b, Miguel Raimundoa, Paula Ferreiraa
a Gastroenterology and Hepatology Department, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte, Lisboa, Portugal
b Faculty of Medicine, University of Lisbon, Lisboa, Portugal
* Corresponding author.
ABSTRACT
The antiviral treatment of Hepatitis C virus (HCV) infection is changing rapidly. Peginterferon and ribavirin are no longer the standard of care therapy and are being replaced by new drugs with greater efficacy and fewer adverse effects. These new direct-acting antivirals (DAAs), incorporated in interferon-based or all-oral regimens, cure more than 90% of infections, even in patients before considered difficult to treat, such as cirrhotic and non-responders. These agents allow shortening the treatment duration, improving sustained response rates (SVR) and have reduced toxicity. The goal of this review is to discuss the current stage of HCV therapy. We describe the mechanisms of the new drugs, the efficacy of the therapeutic regimens, and the predictors of SVR. Individual approach should be based on the different combinations of drugs, and treatment strategy should take into account the profile of the patient, the efficacy and safety of drugs as well as the reimbursement policy.
Keywords: Chronic hepatitis C; Peginterferon; Triple therapy; Direct-acting antiviral interferon-free regimens
RESUMO
A terapêutica antivírica da infecção pelo vírus da Hepatite C (VHC) está em acelerada transformação. A combinação de peginterferão e ribavirina, até aqui a regra do tratamento, está em vias de ser substituída por fármacos mais eficazes e melhor tolerados. Estes novos antivíricos de acção directa (AADs), incorporados em esquemas terapêuticos com ou sem interferão, curam a infecção em mais de 90% dos casos, mesmo em doentes antes considerados como difíceis de tratar, como são os cirróticos e os não responsivos. Os AADs permitem encurtar a duração do tratamento, melhorar a resposta virológica mantida (RVM), e são praticamente isentos de toxicidade. Com esta revisão pretendemos discutir o estado actual da terapêutica da hepatite C, descrevendo o mecanismo de acção dos novos fármacos, a eficácia dos diversos regimes terapêuticos, e os factores predizentes de resposta. A escolha do regime terapêutico dependerá das combinações disponíveis, devendo a estratégia de tratamento ter em conta o perfil clínico do doente, a eficácia e a segurança dos fármacos, bem como da política de reembolso adoptada.
Palavras-Chave: Hepatite crónica C; Peginterferão; Terapêutica tripla; Antivirais de acção directa, regimes sem interferão
1. Introduction
Hepatitis C virus (HCV) affects 2.3-2.8% of the world population.1,2 It is a major cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC) and the main indication for liver transplantation.3 It carries a heavy burden with a significant increase in the incidence of cirrhosis and HCC expected over the next few years.4,5
Over the last thirty years outstanding advances were achieved in the management of chronic Hepatitis C, first with a peginterferon and ribavirin combination and more recently with the protease inhibitor-based triple therapy with boceprevir and telaprevir. Sustained virological response (SVR) rate, the clinical definition of disease cure, is above 70% in the patients with genotype 1 treated with firstgeneration protease-inhibitors.6,7 Viral eradication achieved with antiviral therapy results in relevant health gains, mainly in patients with cirrhosis, reducing hepatic decompensation, HCC and risk of death.8,9
Sofosbuvir,10,11 a pangenotypic HCV polymerase inhibitor, in combination with peginterferon and ribavirin or as an all-oral regimen associated either to ledipasvir12 or to daclatasvir13 - both NS5A replication complex inhibitors - increases the SVR rate to more than 90% in all genotypes. Difficult-to-treat patients, such as patients with cirrhosis and non-responders to double or even triple therapy with protease-inhibitors obtain similar SVR rates as non-treated patients.12 New direct-acting antiviral drugs, interferonfree, are coming on to the market, improving the revolution in Hepatitis C management. Therapeutic regimens with those drugs will be more comfortable, shorter and more efficient, generally free from adverse effects and drug interactions. In addition and just as important, this will allow for treatment of previously excluded patients such as those with decompensated cirrhosis, transplant recipients and all the remaining patients ineligible for interferon therapy.
Despite progress to better tolerated therapies, and multiple possible combinations, the choice of the most suitable regimen regarding genotype are yet to be clearly defined. In the present study we carried out a review of the efficacy of the different management alternatives, opening a window towards the near future. We recommend a management strategy for chronic Hepatitis C, focused on the patient and in the context of scarce access to direct-acting antiviral drugs (DAAs).
2. Double therapy with peginterferon and ribavirin
Peginterferon alfa and ribavirin combination, known as double therapy, has been the standard in chronic Hepatitis C management over the last twelve years. This regimen yields variable results, according to genotype, basal viraemia, stage of liver fibrosis and viral kinetics during treatment.14
2.1. Therapeutic regimen
Independently of viral genotype, peginterferon alfa-2a (PegIFN-2a) or peginterferon alfa-2b (PegIFN-2b) dosage should be 180mg/week and 1.5mg/kg body weight/week, respectively. Ribavirin (RBV) dosage depends on genotype: 15 mg/kg body weight (1200 or 1000 mg/day, depending on patients body weight above or below 75 kg, with PegIFN-2a, or 800-1400 mg/day, according to body weight, with PegIFN- 2b) in patients with genotypes 1, 4, 5 and 6. In patients with genotypes 2 and 3, the dosage is 800 mg (administered as 400 mg b.i.d.).
Treatment duration is 48 weeks in patients with genotypes 1, 4, 5 and 6, and 24 weeks with genotypes 2 and 3. Treatment may be extended to 72 weeks in patients with genotypes 1 and 4 when a delayed viral response occurs and up to 48 weeks in patients with genotypes 2 and 3 in the absence of rapid virological response (RVR), as long as an HCV RNA reduction > 2 log10 occurs by week 12 of treatment.14 In any event, therapy should be discontinued at week 24 when HCV RNA is still detectable.
Alternatively, patients should undergo a shorter duration of therapy under certain conditions: 24 weeks in patients with genotype 1 with basal viraemia < 400,000 IU/mL and RVR, i.e. with undetectable HCV RNA at week 4; 16 weeks in non-cirrhotic, low basal viraemia and RVR patients with genotype 2 or 3.14
In order to avoid futile treatment, therapy should be discontinued, independently of viral genotype, in the presence of an HCV RNA reduction < 2 log10 at week 12 or if HCV RNA is still detectable at week 24.
2.2. Efficacy
SVR in non-treated patients varies between 42% and 46% in patients with genotype 1 and between 76% and 84% in patients with genotype 2 and 3.15-17 In clinical practice, the efficacy of chronic Hepatitis C management with double therapy is around 50%.18 In Europe, the percentage of patients (approximately 50%) with genotype 1 with a positive response to therapy is higher than in the US, which means that half of the patients with the most prevalent genotype in the Western countries are not cured. This variability in SVR is related to the patients characteristics, compliance to treatment and medical expertise.
The response to retreatment is difficult to determine due to study heterogeneity, related to patients characteristics, the stage of the disease and mainly to the previous therapeutic regimen. In general, it is accepted that SVR rate with PegIFN/RBV retreatment is very low. HALT-C19 and EPIC20 studies, involving a high number of patients, the former including exclusively patients with advanced fibrosis, found a global SVR of 18% and 22%, respectively. Nevertheless, in relapsers after PegIFN/RBV therapy, a 23% SVR was obtained with genotype 1 and 57% with genotype 2/3; in addition, 4% SVR and 36% SVR were obtained in non-responders, respectively.20
Therefore, non-responders to a previous PegIFN and RBV therapy will unlikely respond to the same treatment and therefore the correct strategy involves using more eficiente drugs. When double therapy is the single option, non-responder genotype 1 patients should be maintained on treatment for at least 48 weeks if HCV RNA is undetectable at week 12. A global 56% SVR has been found in this context. In non-genotype 1 patients with an urgent need for retreatment, double therapy should be extended over 48 weeks in patients with genotype 2 and 3 and up to 72 weeks in patients with genotype 4.14
2.3. Factors related to treatment response
Double therapy SVR is influenced by demographic, genetic, metabolic and viral factors. Younger age,18,21 female gender,22 absence of visceral obesity, insulin resistance and diabetes,23-25 mild liver fibrosis,18,26 IL28B polymorphism CC,27 viraemia < 400,000 IU/mL28 and RVR29 are favourable response indicators. According to the existing number of factors, therapy success in patients with genotype 1 may vary between 27% and 100%.30 An estimated SVR rate above 80% with double therapy makes it competitive with first-generation protease inhibitors, when the patient with a genotype IL28B polymorphism CC or HCV RNA < 400,000 IU/mL achieves RVR.30 In non-cirrhotic naïve patients, with <600,000 IU/mL viraemia and RVR, the addition of a protease inhibitor does not seem to add any therapeutic benefit.31
A simple score combining some of the routine basal parameters for the assessment of patients who underwent treatment, such as age, body mass index, platelet count, ALT, AST and HCV RNA, allows for the identification of Caucasian patients with genotype 1 likely to achieve SVR with double therapy.21 In this score, patients with a coeficiente ≥5 achieved a 77% SVR, which increased to 87% if RVR was achieved.21 Nevertheless, the percentage of patients with favourable conditions for a response above 80% does not exceed 20%.21,30 The same applies to IL28B CC, present in only one quarter of the patients with genotype 1 and to RVR, which is achieved by only 16% of the same patients.29
3. Triple therapy with 1 st generation protease inhibitors
Since 2011, the association of pegylated interferon plus ribavirin with a NS3/4a protease inhibitor (PI), boceprevir (BOC) or telaprevir (TVR), became the standard treatment for patients with Hepatitis C infected with genotype 1.
3.1. Therapeutic regimen
Naïve patients:
I. Boceprevir: 800 mg (4 tablets) t.i.d., taken with meals, plus PegIFN/RBV, for 24 to 48 weeks. It includes a prior lead-in period (4 weeks of pegylated interferon plus ribavirin). This aims to reduce HCV RNA levels, in order to reduce the risk of virological escape or resistance, to assess sensitivity towards interferon and to estimate the chance of SVR.
A 28-week treatment should be considered for noncirrhotic patients in whom HCV RNA is undetectable at week 8 and 24.
Treatment should be discontinued with HCV RNA above 100 IU/mL at week 12 or detectable at week 24.
II. Telaprevir: 750 mg (3 tablets) b.i.d., with a high-fat meal, plus PegIFN/RBV, for 3 months, followed by double therapy over another 12-36 weeks.
A 24-week treatment should be considered in noncirrhotic telaprevir/PegIFN/RBV- treated patients, with undetectable HCV RNA at week 4 and 12.
III. Boceprevir or telaprevir-treated cirrhotic patients should undergo a 48-week treatment.
Non-responders:
I. The therapy regimen is similar to the naïve patients regimen. Boceprevir-retreated patients with HCV RNA > 100 IU/mL at week 12 should discontinue treatment, in order to avoid emerging viral resistance. Telaprevir-retreated patients with HCV RNA > 1000 IU/mL at week 4 or 12 should also discontinue therapy.
II. Non-responders treatment should include a lead-in stage. In the event of a HCV RNA reduction ≥1 log10 at week 4, boceprevir or telaprevir treatment should be continued according to the standard regimen.
Due to the predicted absence of response or to the risk of viral resistance, patients with no virological response or relapsers with one of the PI, should not be retreated with another inhibitor.
Triple therapy is associated with a higher number of adverse effects. In boceprevir clinical trials, anaemia and dysgeusia were the most common secondary effects, while in telaprevir clinical trials these included anaemia, rash, anorectal symptoms and pruritus.
Haemoglobin levels < 10 g/dL occurred in 49% and 36% of boceprevir and telaprevir-treated patients, respectively.
Monitoring of adverse effects should be particularly careful in patients with compensated liver cirrhosis, especially for haematological disorders, which are the most common. The CUPIC study32 showed that cirrhotic patients with platelet count ≤100,000mm-3 and albumin concentration <35 gr/L are more prone to development of serious complications, occurring in 44% of the patients and as such those patients should not undergo the triple therapy.
3.2. Efficacy
In registry studies, SVR varied according to patients prior therapy: naïve or previously treated patients. In naïve patients, the SVR increase regarding patients treated with PegIFN/RBV combination was 28% and 31% with BOC and TVR, respectively.6,7 In non-responders, the SVR rate increase with BOC reached 45% globally: 46% increase in relapsers and 45% in partial responders.33 In TVR-retreated patients, global SVR rate was 49%: 64% increase in relapsers, 39% in partial responders and 28% in null responders.34 In short, triple therapy is particularly useful in non-responders to a previous PegIFN/RBV treatment, mainly in relapsers and, to a lesser extent, in partial responders and null responders.33,34
The ILUMMINATE study35 showed that, in naïve patients, the 12-week triple therapy regimen with TVR, followed by 12 additional weeks of PegIFN/RBV treatment, was as effective as a 36-week regimen with PegIFN/RBV, as long as eRVR (extended rapid virological response) occurred (undetectable HCV RNA at week 4 and 12). Boceprevir treatment duration may also be guided by virological response and should be reduced to 28 weeks in naïve patients with undetectable HCV RNA at week 8 and 24. Patients with HCV RNA still detectable at week 8 and 24 should keep triple therapy until week 36 and PegIFN/RBV therapy until week 48.14 RVR, occurring in 58% of TVR-treated patients and in 47% of BOC-treated patients, predicts 89% and 96% SVR rate, respectively. Therefore, in non-cirrhotic patients with RVR therapy may be reduced to 24 weeks with TVR and 28 weeks with BOC. In cirrhotic naïve patients, therapy should not be shortened regardless of the viral kinetics.
In patients with advanced liver fibrosis (F3-F4), the SVR rates are generally higher in BOC or TVR therapy, when compared with the rates obtained in double therapy-treated patients, mainly in patients with RVR.6,7
Treatment should be discontinued, due to its inefficacy, in triple therapy with BOC, when HCV RNA is >100 IU/mL at week 12 or when detectable at week 24. Likewise, with TVR, when HCV RNA is >1000 IU/mL at week 4 or 12.
3.3. Factors related to treatment response
As for double therapy, we may also estimate which patients will likely achieve SVR with triple therapy (Table 1). SPRINT 26 and REALIZE34 studies showed that genotype IL28B CC is a strong SVR predictive factor, allowing for the shortening of treatment duration. Nevertheless, the T allele negative predictive value is not powerful enough to contra-indicate therapy, as SVR may be achieved, particularly in patients with RVR. HCV RNA undetectability at week 4 (RVR) showed to be as powerful or an even more powerful SVR predictive factor than the presence of genotype CC. In boceprevirtreated patients, a >1 log10 decrease in HCV RNA at week 4 (lead-in stage with PegIFN/RBV) showed to be a stronger SVR indicator than IL28B.36 SVR rate in these patients was nine times higher than SVR for patients with <1 log10 decrease.36 In addition, an undetectable HCV RNA at week 8 (fourth week of triple therapy) increases to 96% the SVR rate.36 Low basal viraemia, absence of cirrhosis and BMI (body mass index) ≤30 are also independent SVR factors.36 In patients with advanced liver fibrosis (F3-F4), triple therapy showed to be more efficient than double therapy, not only in naïve patients, but also in non-responders.37 Response to interferon in the lead-in stage, as well as undetectable HCV RNA at week 8, worked as SVR predictors in these patients.37 Nevertheless, we should remark that the major factor of response to triple therapy in non-responders is the type of response to double therapy, since relapsers, regardless of the fibrosis stage, achieve SVR rates consistently above 80%.38
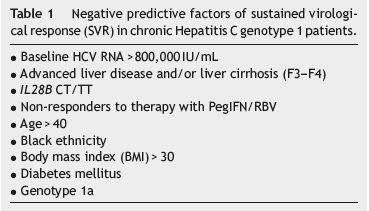
BOC and TVR-treated patients are commonly treated with other medication and, since these may inhibit liver enzymes, such as P450 cytochrome, they may potentially induce drug interactions. It is therefore important to be aware of drug interactions and to inform patients about this risk.39
4. Triple therapy with sofosbuvir or simeprevir
The sofosbuvir plus PegIFN/RBV combination is currently one of the most efficient associations for reaching cure of Hepatitis C infection. Sofosbuvir is a powerful nucleotide polymerase inhibitor with pangenotypic action.40,41 It has a predominant renal excretion and does not require dose adjustment when the creatinine clearance is above 30 mL/min.
Simeprevir, a 2nd generation NS3/NS4A protease inhibitor, one thousand times more powerful than the 1st generation protease inhibitors (PIs), is recommended for treatment of patients with genotype 1 and genotype 4, in a daily dose of 150 mg.42 It must be taken with meals (fatty or not), it undergoes liver metabolism through CYP3A4 and has a renal excretion below 1%. Although less frequently than the 1st generation PIs, it may also interact with other drugs that are enzyme inhibitors or inducers. Even though sérum concentrations increase in patients with liver failure, there is no need for dose adjustment in the presence of light to moderate hepatic impairment.43
4.1. Naïve patients
4.1.1. Sofosbuvir
Therapeutic regimen. In patients with genotype 1, 4, 5 and 6, sofosbuvir is recommended in a single daily dose of 400 mg associated to peginterferon alfa-2a and ribavirin in standard doses. Triple therapy must be followed for 12 weeks.14
In patients with genotype 3, when triple therapy is the option, sofosbuvir must be administered in a 400 mg single daily dose associated to peginterferon alfa and ribavirin in the standard doses for 12 weeks.
Efficacy. Global SVR was 91% in the NEUTRINO trial10: 90% in genotype 1, 96% in genotype 4 and 100% in genotype 5 and 6. In cirrhotic patients, SVR was 80%. SVR was slightly higher in patients with IL28B CC (98% vs. 87%), low viraemia (97%) and genotype 1a (92%) vs. 1b (82%). Sofosbuvir was well tolerated and allowed for a SVR improvement of approximately 30% vs. the PegIFN/RBV group (58%). The efficacy in genotype 4, 5 and 6 was very favourable although difficult to analyze given the reduced number of patients included.
4.1.2. Simeprevir
Therapeutic regimen. Simeprevir is recommended for patients with genotype 1 and 4, including those patients with liver cirrhosis. The daily dose is 150 mg in association with PegIFN/RBV for 12 weeks, followed by 12 or 36 weeks of PegIFN/RBV.44,45 In patients reaching HCV RNA < 25 IU/mL at week 4 and undetectable at week 24 (85% in QUEST-1 trial and 91% in QUEST-2 trial), treatment should be discontinued at week 24, while in the remaining patients it should be extended until week 48. Simeprevir should be discontinued if HCV RNA level is >1000 IU/mL at week 4, keeping PegIFN/RBV. This regimen is not recommended for patients with genotype 1a and a NS3 protease Q80K polymorphism.
Efficacy. The efficacy of simeprevir, peginterferon and ribavirin combination has been assessed in QUEST-1 and QUEST-2 trials.44,45 SVR12 (sustained virological response 12 weeks after completion of HCV treatment) was 80% and 81% in QUEST-1 and QUEST-2 trials (European patients), respectively. In the same studies, RVR was achieved in 80% and 79% vs. 12% and 13% in the PegIFN/RBV group. In QUEST-1 as in QUEST-2 trials, most patients, 85% and 86% respectively, underwent a 24-week treatment only, as they met the Response-Guided Therapy (RGT) criteria. From these, 91% and 86%, in QUEST-1 and QUEST-2 trials, respectively, achieved SVR-12. In each of these studies, the patients with genotype 1a with a Q80K polymorphism obtained lower SVR-12 rates than patients with genotype 1a without such polymorphism and with genotype 1b. SVR12 was significantly higher in every stage of liver fibrosis, including cirrhosis. Simeprevir is well tolerated, without any diferences in adverse effects incidence between simeprevir-treated patients and patients treated with PegIFN/RBV alone.44,45
4.2. Non-responders
4.2.1. Sofosbuvir
Therapeutic regimen. There are no randomized clinical trials in this group of patients and therefore optimal treatment duration has not been determined. Nevertheless, the drug SPC (Summary of Product Characteristics) considers the possibility of extending triple therapy with sofosbuvir in the standard doses up to 24 weeks, especially in more difficult-to-treat patients: liver cirrhosis, advanced age, black ethnicity, metabolic syndrome, IL28B non-CC or high basal viraemia. In these patients, extrapolation of known data from double therapy we anticipate lower SVR rates.
In patients with genotype 3 non-responders to previous therapy, sofosbuvir triple combination for 12 weeks, in a daily dose of 400 mg associated to PegIFN/RBV, is an alternative to interferon-free double therapy for 24 weeks with sofosbuvir and ribavirin.14
Efficacy. In the LONESTAR-2 II-b stage trial,12 sofosbuvir, peginterferon and ribavirin combination efficacy was assessed in patients with genotype 2 and 3 non-responders or relapsers to previous treatment with PegIFN/RBV, from which 55% were cirrhotic. SVR12 was 96% in patients with genotype 2 (with liver cirrhosis: 93%, non-cirrhotic: 100%) and 83% in patients with genotype 3 (with or without cirrhosis).
4.2.2. Simeprevir
Therapeutic regimen. No simeprevir phase III study has been published in non-responders. According to EASL (European Association for the Study of the Liver) guidelines,14 simeprevir should be used in a daily dose of 150 mg in association with peginterferon and ribavirin, for 12 weeks, in nonresponder genotype 1 patients, followed by 12 additional weeks of peginterferon and ribavirin for relapsers and 36 additional weeks (total duration of 48 weeks) for partial and null responders, including cirrhotic patients.
Efficacy. In the ASPIRE trial,46 SVR24 was higher in the groups treated with 150 mg/day of simeprevir for 12 weeks (patients with 24 and 48 weeks of treatment were grouped), in comparison with PegIFN/RBV group: relapsers 89% vs. 37%, partial responders 86% vs. 9%, null responders 59% vs. 19%. The IL28B polymorphism was not a SVR predictor. In difficult-to-treat patients (F3 and F4), the virological response was higher in all groups treated with simeprevir. SVR24 in cirrhotic patients was 77% in relapsers, 44% in partial responders and 28% in null responders. In the PROMISE phase III study47 that included genotype 1 patients who relapsed, simeprevir regimens efficacy was 79.2% vs. 36.1% in the PegIFN/RBV group.
Safety profile. Sofosbuvir has an excellent safety profile. No patient developed any resistance or presented viral reactivation during treatment. Sofosbuvir was well tolerated, without any additional adverse effects beyond those associated to interferon and ribavirin. The most frequent were insomnia, fatigue, nausea and headache. It is nevertheless recommended that sofosbuvir should not be used alone in the treatment of Hepatitis C and should be discontinued whenever the associated antiviral drug is discontinued. The dose of sofosbuvir should never be modified, even in elderly patients. As its liver metabolism does not require CYP3A4, the risk of drug interactions is smaller. In addition, there is no interaction with calcineurin inhibitors but when associated to cyclosporine a slight increase of serum level was documented.40
The most frequent adverse effects with simeprevir were fatigue, rash and neutropenia. The incidence of severe rash (grade 3 or 4), besides being rare, was not significantly different from those occurring in patients treated with peginterferon and ribavirin alone. Hyperbilirubinemia was more common in the first 2 weeks of treatment with simeprevir, but was not associated to other hepatic abnormalities nor did it interfere with SVR.
Factors related to treatment response. With sofosbuvir, baseline and on-treatment factors related to response to double therapy with peginterferon and ribavirin lost their impact. However, in patients with genotype 1, IL28B CC, a low basal viraemia, genotype 1b and the absence of liver cirrhosis remain favourable factors of response to triple therapy.10
Therapy may be shortened to 24 weeks using simeprevir and RGT if HCV RNA < 25 IU/mL at week 4 and undetectable HCV RNA at week 12 and 24.44,45 Therapy should be discontinued when HCV RNA is above 25 IU/mL at week 4, 12 or 24. Viral reactivation is assumed when there is >1 log10 HCV RNA increase in relation with the lowest obtained value or when HCV RNA is >100 IU/mL in patients previously with undetectable or < 25 IU/mL HCV RNA levels.42,43
5. Interferon-free therapy
An all-oral therapy will be the new paradigm in chronic Hepatitis C therapy in the near future (Table 2). The interferon-free therapy, with or without ribavirin, is more efficient, shorter and better tolerated by naïve48,49 and treatment-experienced patients.50,51 Oral therapy for all patients will be a matter of time, once economic constraints that are currently conditioning its use are overcome.

The EASL14 and AASLD/IDSA (American Association for the Study of Liver Diseases/Infectious Diseases Society of America)52 guidelines already include all-oral therapy as an option for almost all HCV genotype, including naïve and non-responders to previous therapy with peginterferon and ribavirin, alone or in combination with 1st generation protéase inhibitors and in cirrhotic patients.
Some doubts remain regarding the ideal therapy regimen in order to achieve a SVR rate above the desired 90%. In cirrhotic genotype 3 patients, naïve or treatment-experienced, it is not yet clear whether a 24-week treatment with sofosbuvir plus ribavirin53 or a 12-week of the same combination associated to interferon would be the best treatment. In addition, in cirrhotic genotype 1a null responders, the extension of treatment for another 24 weeks improves SVR rate.54 We should also remark the fact that there are some gaps in the treatment of non-1 genotype patients, due to insufficient research with some of the new direct-acting antiviral agents (DAAs).
5.1. Therapeutic regimen
Table 3 shows oral therapy regimens for which there is already agreement. Ribavirin may be associated in some cases to DAAs in patients with less favourable response indicators, mainly if cirrhotic.
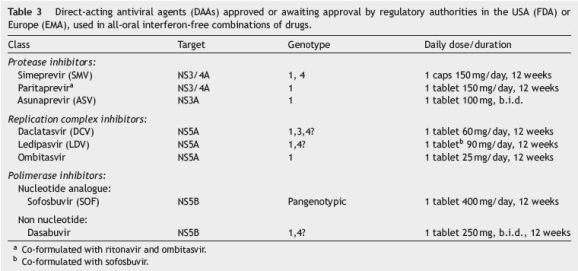
In genotype 1 non-responders to triple therapy with peginterferon, ribavirin and boceprevir or telaprevir, a 24-week sofosbuvir and daclatasvir combination has been shown to be very efficient.13
In naïve patients it seems possible to shorten to 8 weeks the treatment duration with sofosbuvir/ledipasvir combination, without compromising SVR rate.55
In patients with genotype 2, oral recommended therapy is the combination of sofosbuvir with ribavirin for 12 weeks.53 In non-responders, the association of interferon is recommended.
In genotype 3, two oral therapy alternatives are recommended: 24-week sofosbuvir plus ribavirin or sofosbuvir plus daclatasvir for 12 or 24 weeks in naïve and non-responders, respectively.13,53
In genotype 4, data are scarce, although it is assumed that 12-week sofosbuvir/simeprevir or sofosbuvir/daclatasvir combinations are efficient.14
5.2. Efficacy
Table 4 shows the different oral therapy regimens already approved, or awaiting approval by regulatory authorities, as well as their efficacy. The SVR rate is generally above 95%, regardless of prior treatment-experience and independently of response to previous therapy with PegIFN/RBV.51,56,57 In treatment-experienced patients with cirrhosis, the rates of SVR with sofosbuvir and ledipasvir combination were 88% and 100% after 12 or 24 weeks of therapy, respectively50; with paritaprevir/ritonavir plus ombitasvir plus dasabuvir were 98% and 97%, respectively.54 In the COSMOS study (sofosbuvir and simeprevir), the rates of SVR remained high in patients with cirrhosis with or without ribavirin, duration of treatment (12 or 24 weeks) or previous therapy.57
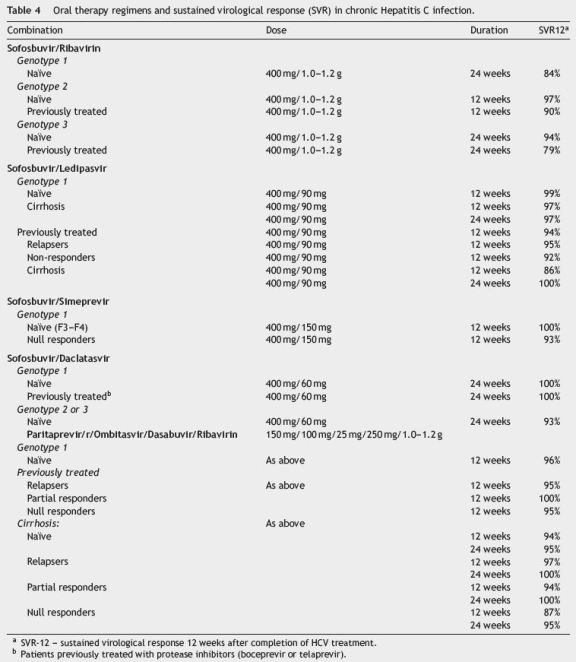
Current trend is towards excluding ribavirin from therapy regimens, despite the association of ribavirin with DAAs and the 24-week extension of therapy slightly increases the SVR rate in more difficult-to-treat patients: cirrhotic, null responder genotype 1a or genotype 3 patients.13,53,54,58
Shortening of treatment duration from 12 to 8 weeks does not seem to affect the efficacy of sofosbuvir/ledipasvir combination (94% vs. 95%, respectively), in non-cirrhotic genotype 1 patients.55 Genotype 3 patients had lower SVR rates with oral therapy than the remaining genotypes, particularly in cirrhotic patients.53 In this genotype, currently the most difficult to treat, peginterferon association may significantly improve the SVR rate and shorten treatment to 12 weeks.
Regarding patients with genotype 4, data are scarce, not allowing for an accurate determination of which is the best alternative oral therapy.14
The selection of simeprevir (Q80K) and daclatasvirresistant strains (NS5A-A30K) seems to only occur very rarely, when sofosbuvir is included in therapy regimens.
5.3. Factors related to response to treatment
The previously described factors influencing the response to the treatment with PegIFN/RBV become almost meaningless with the combination of DAAs. As described, only genotype 1a, genotype 3 and mainly liver cirrhosis may show slight reductions in SVR rate. These are susceptible to be overcome by more extensive therapy regimens.
6. Therapeutic strategy
In this time of transition towards all-oral therapies, the guidelines for treatment of Hepatitis C that we presente are close to the EASL proposal.14 The choice of therapeutic regimen should be based on clinical data and should aim to maximize cure, treating the highest possible number of patients, according to budget and established priorities (Table 5). Patients clinical profile, previous therapy and the stage of liver fibrosis will lead the decision.
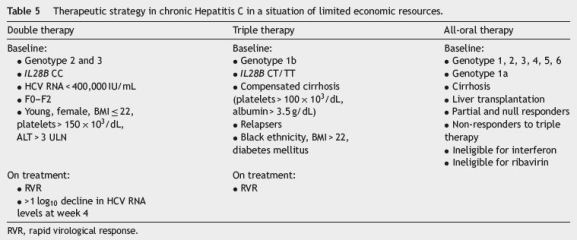
In patients with genotype 1, double therapy may be a therapeutic alternative in about 18% of the patients presenting a set of good SVR indicators: IL28B CC, F0-2, HCV RNA < 400.000 IU/mL and/or RVR. In countries with high budget restrictions, triple therapy with first generation protéase inhibitors, boceprevir or telaprevir, could be an affordable alternative. However, a response guided triple therapy with simeprevir in patients with genotype 1b and genotype 1a without Q80K polymorphism, naïve or relapsers, is a better alternative with an expected 80% SVR efficacy. Patients with interferon intolerance or contraindications to its use and in non-responder cirrhotic patients, the preferential option should be all-oral therapy with sofosbuvir associated to simeprevir or daclatasvir.
In patients with genotype 2, either double therapy or the association with sofosbuvir in cirrhotic or non-responders is recommended. In patients intolerant to interferon, a 12-week course of sofosbuvir with ribavirin obtains equivalente results in naïve patients.
In non-cirrhotic patients with genotype 3, double therapy is recommended or, in patients ineligible for interferon, a 12-week combination of sofosbuvir with ribavirin. In cirrhotic non-responders, with intolerance or contraindication to interferon, the best option would be the sofosbuvir/daclatasvir combination. In non-cirrhotic nonresponders, the most reasonable option would be to wait for more cost-effective therapies.
In patients with genotype 4, there are no welldocumented alternatives to double therapy. EASL recommends 12-week sofosbuvir, peginterferon and ribavirin regimen or, in the case of patients intolerant to interferon, a 12-week course of sofosbuvir associated to simeprevir or daclatasvir.
References
1. Levanchy D. The global burden of hepatitis C. Liver Int. 2009;29:74-81. [ Links ]
2. Hanafiah KM, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-42. [ Links ]
3. Razavi H, Elkhoury AC, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57:2164-70. [ Links ]
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513-21. [ Links ]
5. Deuffic-Burban S, Deltenre P, Buti M, Stroffolini T, Parkes J, Muhlberger N, et al. Predicted effects of treatment for HCV infection vary among European countries. Gastroenterology. 2012;143:974-85. [ Links ]
6. Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic genotype 1 infection. N Engl J Med. 2011;364:1195-206. [ Links ]
7. Jacobson IM, McHutchinson JG, Dusheiko GM, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-16. [ Links ]
8. Velosa J, Serejo F, Marinho R, Nunes J, Glória H. Eradication of hepatitis C virus reduces the risk of hepatocellular carcinoma in patients with compensated cirrhosis. Dig Dis Sci. 2011;56:1853-61. [ Links ]
9. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Ytter YF. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma. Ann Intern Med. 2013;158:329-37. [ Links ]
10. Lawitz AND, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-87. [ Links ]
11. Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-77. [ Links ]
12. Lawitz E, Poordad F, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naïve and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomized, phase 2 trial. Lancet. 2014;383:515-23. [ Links ]
13. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211-21. [ Links ]
14. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [ Links ]
15. Manns M, McHutchinson JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65. [ Links ]
16. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçalves FL, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82. [ Links ]
17. Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha 2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-55. [ Links ]
18. Marcellin P, Cheinquer H, Curescu M, Dusheiko GM, Ferenci P, Horban A, et al. Higher sustained virologic response in rapid virologic response patients in the large real-world PROPHESYS cohort confirms results from randomized clinical trials. Hepatology. 2012;56:2039-50. [ Links ]
19. Shiffman ML, Di Bisceglie AM, Lindsay, Morishima C, Wright EC,Everson GT, et al. Peginterferon alfa-2a and ribavirin in patients with chronic hepatitis C who have failed prior treatment. Gastroenterology. 2004;126:1015-23. [ Links ]
20. Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136:1618-28. [ Links ]
21. Ferenci P, Aires R, Ancuta I, Arohnson A, Cheinquer H, Delic D, et al. A tool for selecting patients with a high probability of sustained virological response to peginterferon alfa-2a (40KD)/ribavirin. Liver Int. 2014, http://dx.doi.org/10.1111/liv.12439. [ Links ]
22. Di Marco V, Covolo L, Calvaruso V, Levrero M, Puoti M, Suter F, et al. Who is more likely to respond to dual treatment with pegylated-interferon and ribavirin for chronic hepatitis C? A gender-oriented analysis. J Viral Hepat. 2013;20:790-800. [ Links ]
23. Petta S, Amato M, Cabibi D, Cammà C, Di Marco V, Giordano C, et al. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology. 2010;52:1543-52. [ Links ]
24. Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodriguez CM, et al. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636-41. [ Links ]
25. Giordanino C, Bugianesi E, Smedile A, Ciancio A, Abate ML, Olivero A, et al. Incidence of type 2 diabetes mellitus and glucose abnormalities in patients with chronic hepatitis C infection by response to treatment: results of a cohort study. Am J Gastroenterol. 2008;103:2481-7. [ Links ]
26. Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis S, et al. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51:388-97. [ Links ]
27. Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, et al. Genetic variation in IL28B predicts hepatitis C treatmentinduced viral clearance. Nature. 2009;461:399-401. [ Links ]
28. Zeuzem S, Rodrigues-Torres M, Reddy KR, Marcellin P, Diago M, Craxi A, et al. Optimized threshold for serum HCV RNA to predict treatment outcomes in hepatitis C patients receiving peginterferon alfa-2a/ribavirin. J Viral Hepat. 2012;19:766-74. [ Links ]
29. Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55:69-75. [ Links ]
30. Andriulli A, Di Marco V, Margaglione M, Ippolito AM, Fattovich G, Smedile A, et al. Identification of naive HCV-1 patients with chronic hepatitis who may benefit from dual therapy with peginterferon and ribavirin. J Hepatol. 2014;60:16-21. [ Links ]
31. Pearlman BL, Ehleben C. Hepatitis C genotype 1 virus with low viral load and rapid virologic response to peginterferon/ribavirin obviates a protease inhibitor. Hepatology. 2014;59:71-7. [ Links ]
32. Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, et al. Triple therapy in treatment experienced patients with HCV-cirrhosis in a multicentre cohort of the French early access programme. J Hepatol. 2013;59:434-41. [ Links ]
33. Bacon BR, Gordon SC, Lawitz AND, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-17. [ Links ]
34. Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-28. [ Links ]
35. Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, et al. Response guided Telaprevir combination treatment for Hepatitis C virus infection. N Engl J Med. 2011;365:1014-24. [ Links ]
36. Poordad F, Bronowicki JP, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608-18. [ Links ]
37. Bruno S, Vierling JM, Esteban R, Nyberg LM, Tanno H, Goodman Z, et al. Efficacy and safety of boceprevir plus peginterferon-ribavirin in patients with HCV G1 infection and advanced fibrosis/chirrosis. J Hepatol. 2013;58:479-87. [ Links ]
38. Bourlière M, Wendt A, Fontaine H, Hézode C, Pol S, Bronowicki JP. How to optimize HCV therapy in genotype 1 patients with cirrhosis. Liver Int. 2013;33 Suppl.:46-55. [ Links ]
39. www.hep-druginteractions.org [ Links ]
40. Marino Z, Van Bommel F, Forns, Berg T. New concepts of sofosbuvir-based treatment regimens in patients with hepatitis C. Gut. 2014;63(2):207-15. [ Links ]
41. Koff RS. The efficacy and safety of sofosbuvir, a novel oral nucleotide ns5b polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2014;39(5):478-87. [ Links ]
42. Asha V, Perry CM, Simeprevir: First global approval. Drug. 2013;73:2093-100. [ Links ]
43. Talwani R, Heil EL, Gillian BL, Temesgen Z. Simeprevir: a macrocyclic HCV protease inhibitor. Drugs Today. 2013;49(12):769-79. [ Links ]
44. Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naïve patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomized, double-blind, placebo-controlled trial. Lancet. 2014:3-4, http://dx.doi.org/10.1016/S0140-6736(14)60494-3. [ Links ]
45. Manns M, Marcellin P, Poordad F, Araujo SA, Buti M, Horsmans Y, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naïve patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomized, double-blind, placebo-controlled phase 3 trial. Lancet. 2014, http://dx.doi.org/10.1016/S0140-6736(14)60538-9. [ Links ]
46. Zeuzem S, Berg T, Gane AND, Ferenci P, Foster GR, Fried MW, et al. Simeprevir increase rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;46:430-41. [ Links ]
47. Forns X, Lawitz AND, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapses after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669-6679. [ Links ]
48. Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-98. [ Links ]
49. Feld JJ, Kowdley KV, Coakley AND, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594-603. [ Links ]
50. Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483-93. [ Links ]
51. Zeuzem S, Jacobson IM, Baykal T, Marinho R, Poordad F, Bourlière M, et al. Retreatment of HCV with ABT-450/rombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1604-14. [ Links ]
52. Recommendations for testing, managing, and treating hepatitis. ASSLD Guidelines. Available: http://www.hcvguidelines.org [ Links ]
53. Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993-2001. [ Links ]
54. Poordad F, Hézode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370:1973-82. [ Links ]
55. Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-88. [ Links ]
56. Sulkwoski MS, Jacobson IM, Ghalib R, et al. Once-daily simeprevir (TMC435) plus sofosbuvir (GS7977) with or without ribavirin in HCV genotype 1 prior null responders with METAVIR F0-2: COSMOS study subgroup analysis. J Hepatol. 2014;60:S4. [ Links ]
57. Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C vírus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomized study. Lancet. 2014, http://dx.doi.org/10.1016/S0140-6736(14)61036-9. [ Links ]
58. Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo YL, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-92. [ Links ]
*Corresponding author
E-mail address: josevelosa@fm.ul.pt (J. Velosa).
Ethical disclosures
Protecting people and pets. The authors declare that for this investigation, experiment is not conducted on humans and/or animals.
Confidentiality of data. The authors declare that they do not show patient data in this article.
Right to privacy and consent in writing. The authors declare that no patient data appear in this article.
Received 12 August 2014; accepted 29 September 2014














