Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
GE-Portuguese Journal of Gastroenterology
versão impressa ISSN 2341-4545
GE Port J Gastroenterol vol.21 no.5 Lisboa out. 2014
https://doi.org/10.1016/j.jpg.2014.05.001
ORIGINAL ARTICLE
Octreotide Long-Acting Release is effective in preventing gastrointestinal bleeding due to angiodysplasias
O octreótido Long-Acting Release é eficaz na prevenção de hemorragia por angiodisplasias gastrointestinais
Paulo Salgueiroa,∗, Ricardo Marcos-Pintoa,b,c, Rodrigo Liberal d, Paula Lagoa, Ricardo Magalhãesa, Maria Magalhãesa, José Ferreiraa, Isabel Pedrotoa,b
a Gastroenterology Department, Hospital Santo António, Porto, Portugal
b Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Portugal
c CINTESIS, Faculdade de Medicina da Universidade do Porto, Portugal
d Medicine Department, Hospital Santo António, Porto, Portugal
*Corresponding author
ABSTRACT
Background: Angiodysplasias are one of the most frequent causes of gastrointestinal bleeding. Pharmacological options, such as octreotide Long-Acting Release (LAR), do not yet have a defined role and are currently used for patients who are not candidates for or are refractory to endoscopic treatment.
Aims: (1) To evaluate the efficacy of octreotide LAR by considering transfusion requirements (units of packed erythrocytes (UPE)/month) and number of hospitalizations/month before and during therapy; (2) to verify whether the characteristics of patients and/or concurrent medication influenced response to therapy; and (3) to evaluate the safety of therapy by registering adverse effects.
Methods: A retrospective cohort of 16 patients with angiodysplasias treated with octreotide LAR was reviewed.
Results: (1) There was a significant decrease (follow up before vs. follow up during) in the median number of UPE/month (1.84 vs. 0.42, p = 0.008) and the number of admissions/month (0.21 vs. 0.00, p = 0.015). (2) Of the characteristics analyzed, only the presence of aortic stenosis (vs. other comorbidities) positively influenced the response to therapy in relation to the variation in transfusion requirements (−2.39 UPE/month vs. −0.61 UPE/month; p = 0.009). (3) Adverse effects: splenic infarction (1 patient) and gallstones (1 patient).
Conclusions: Octreotide LAR is effective as prophylaxis for gastrointestinal bleeding angiodysplasia by decreasing transfusion requirements and the need for hospitalizations. Patients with aortic stenosis were those who most benefited from the therapy. A dose of 20 mg/month did not prove more effective than a dose of 10 mg/month.
Keywords: Octreotide Long-Acting Release; Angiodysplasias; Gastrointestinal bleeding
RESUMO
Introdução: As angiodisplasias são uma das causas mais frequentes de hemorragia com ponto de partida no intestino delgado. As opções farmacológicas como o octreótido LAR não têm ainda um papel definido destinando-se a doentes não candidatos ou refratários à terapêutica endoscópica.
Objetivos: (1) Avaliar a eficácia terapêutica comparando: necessidades transfusionais (unidades de concentrado eritrocitário/mês) e número de internamentos/mês antes e durante a terapêutica; (2) Verificar se as características dos doentes e/ou da medicação tiveram influência na resposta à terapêutica; (3) Avaliar a segurança da terapêutica (registo de efeitos adversos).
Métodos: Efetuado estudo retrospetivo de coorte de 16 doentes com AD tratados com octreótido LAR.
Resultados: (1) Observou-se diminuição significativa (follow up before vs follow up during) do número mediano de UCE/mês (1.84 UCE/mês vs 0,42 UCE/mês; p=0.008) e do número de internamentos/mês (0,21 internamentos/mês vs 0,00 internamentos/mês; p=0.015). (2) Das características analisadas, apenas a presença de estenose aórtica (vs outras comorbilidades) influenciou positivamente a resposta à terapêutica no que concerne à variação das necessidades transfusionais (---2,39 UCE/mês vs ---0,61 UCE/mês; p=0,009). (3) Efeitos adversos: enfarte esplénico (1 doente); litíase vesicular (1 doente).
Conclusão: O octreótido LAR é eficaz como profilaxia de hemorragia por angiodisplasias gastrointestinais diminuindo as necessidades transfusionais e a necessidade de internamentos. Os doentes com estenose aórtica foram aqueles que mais beneficiaram com a terapêutica. A dose de 20 mg/mês não provou ser mais eficaz que a dose de 10 mg/mês.
Palavras-Chave: Octreótido Long-Acting Release; Angiodisplasias; Hemorragia Digestiva
Introduction
Angiodysplasias are the major vascular malformations of the digestive tract and are the most frequent cause of gastrointestinal bleeding originating in the small bowel in individuals over 50 years of age.1
The pathogenesis of angiodysplasias is unclear, although they are presumed to result from degenerative processes, neurohormonal dysfunction or complications of an underlying disease, such as chronic kidney failure (CKF), liver cirrhosis or aortic stenosis.1
About 40-60% of the patients have multiple angiodysplasias2,3 and, while most patients have vascular malformations in the same intestinal segment, synchronous lesions can be identified elsewhere in the gastrointestinal tract in approximately 20% of the patients.4,5
Diagnosis can be challenging, mainly because bleeding is intermittent or patients are anaemic or with hypotension which makes angiodysplasias less obvious on light endoscopy. In addition to this, angiodysplasias in the small bowel are relatively difficult to access by endoscopy, although this hasbeen aided by the introduction and widespread use of vídeo capsule enteroscopy (VCE) and balloon-assisted enteroscopy in clinical practice.
The aims of the treatment of gastrointestinal angiodysplasias are, firstly, the cessation of acute or chronic haemorrhage and, secondly, the prevention of recurrence of bleeding. Besides the correction of the underlying disease, when applicable, this treatment is aided by blood transfusion and correction of any coagulation disorders and/or the discontinuation of antiplatelet therapy or anticoagulants.1,6
The commitment of the clinician to avoid the recurrence of bleeding from angiodysplasias should be guided by knowledge of the natural history of this disease and the notion that about 50% of the patients relapse after a first episode of haemorrhage.6
The potentially most effective therapeutic interventions are: endoscopy (especially the destruction of angiodysplasias with argon plasma coagulation)7; therapeutic angiography (super-selective embolization) especially in the context of acute haemorrhage8 and surgery (in patients selected on the basis of clinical presentation, location of the lesions and the patients age and comorbidities).9 When these interventions are not possible, are contraindicated or have been ineffective, as often happens in patients with multiple lesions distributed across several segments of the digestive tract or when the patient has comorbidities that prevent performing invasive procedures, pharmacological treatment is the only alternative possibility.6,10
Various drugs have been reported, commonly in clinical case studies, to be beneficial in the treatment of gastrointestinal bleeding from angiodysplasias. The three most promising drugs for this indication are hormonal therapywith a combination of oestrogen-progesterone,11-16 octreotide17-21 and thalidomide.22,23
The objectives of our study were: (1) to determine the effectiveness of therapy with octreotide LAR in patients with recurrent episodes of gastrointestinal bleeding as a result of gastrointestinal angiodysplasia, assessing the need for transfusion and the number of hospitalizations for gastrointestinal bleeding by comparing periods before and during therapy, (2) to verify whether the characteristics of patients and/or the concurrent medication had an influence on the response to therapy, and (3) to evaluate the safety of this therapy (adverse effects).
Patients and methods
We retrospectively evaluated all patients followed in our hospital between 2003 and 2011 with recurrent episodes of gastrointestinal bleeding originating from gastrointestinal angiodysplasias characterized by iron-deficiency anaemia or overt gastrointestinal bleeding episodes in which angiodysplasias were observed by endoscopy and all other possible causes of gastrointestinal bleeding were excluded.
All patients included in the study underwent colonoscopy, endoscopy and VCE. Some patients also underwent doubleballoon enteroscopy (examination always of therapeutic purpose).
Whenever feasible, endoscopic therapy was performed with argon plasma coagulation. When, despite endoscopic treatment, patients had recurrent bleeding (defined as at least two episodes of gastrointestinal bleeding from angiodysplasias for a period of 6 months) or were not candidates for endoscopic treatment (due to multiple angiodysplasias dispersed through the digestive tract or due to contraindications to invasive procedures) they were initiated onto therapy with octreotide LAR (Sandostatin LAR, Novartis Pharma®).
Since the dose of this drug has varied between studies and because comparative studies are lacking, the dosage used (10 or 20 mg monthly octreotide LAR, intramuscular) was chosen according to the decision of the physician responsible for the patient (without taking into account any objective criteria such as age, weight, severity of the disease, number of blood transfusions or number of hospitalizations driven by gastrointestinal bleeding).
Blood transfusions were performed whenever the haemoglobin value fell below 7.5 g/dL (for any patient) or below 10 g/dL (in patients with ischaemic heart disease).
The follow-up period from the date of diagnosis of angiodysplasias haemorrhage to the start time of treatment with octreotide LAR was called follow up before and the time during which the patient received therapy was called follow up during.
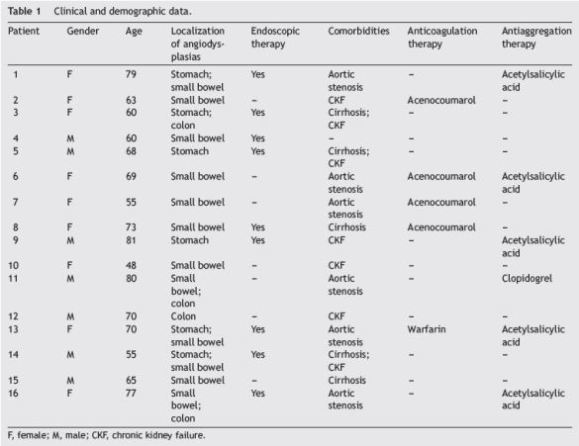
Patients were monitored clinically and analytically during the periods of follow-up by registering sex, age, location of angiodysplasias, whether or not an endoscopic treatment was performed, comorbidities associated with the presence of angiodysplasias (aortic stenosis, CKF and liver cirrhosis), concomitant medications (antiplatelets or hipocoagulants), number of hospitalizations (motivated by gastrointestinal bleeding), number of blood transfusions performed and adverse effects during therapy.
As the times follow up before and follow up during varied from patient to patient, the average monthly transfusion rate (units of packed erythrocytes (UPE)/month) and the average number of hospitalizations per month (number of visits divided by the number of months of follow up) were used. To verify whether the characteristics of patients and/or concurrent medications influenced response to therapy, we compared the variations in average transfusion rate (difference between the average UPE/month during follow up during and follow up before) and the number of admissions per month (the average difference between inpatient admissions/month during follow up during and follow up before) isolating each variable independently.
In the descriptive analysis of the variables we used the relative frequencies for dichotomous variables, measures of central tendency and dispersion for continuous variables.
To compare whether there was a difference between the values of continuous variables assessed at two points in time (before and during) we used the nonparametric Wilcoxon signed rank test.
When comparing quantitative variables from two independente groups we used the nonparametric Mann-Whitney U test.
To analyze the association between two qualitative variables we used the Fishers exact test.
Statistical tests were performed bilaterally considering a significance level of 5%.
The statistical analysis was performed on IBM SPSS Statistics 20.0 software.
Results
Sixteen patients were included in this study, nine were female (56.3%) and seven were male (43.8%) patients. Median age was 68.5 years (range: 48-81).
All patients had multiple vascular lesions and the most common location of these was the small bowel (n = 12; 75%). Six patients (37.5%) had angiodysplasias in multiple locations.
Endoscopic treatment with argon plasma coagulation was frequently performed (n = 9; 56.25%) and some patients were subjected to several sessions of endoscopic therapy.
Only 1 patient showed no evidence of comorbidity associated with angiodysplasias. Some patients had more than one comorbidity: CKF, n = 7 (43.75%); aortic stenosis, n=6 (37.5%); cirrhosis, n = 5 (31.25%).
Concomitant medication (anticoagulation/antiplatelet therapy) was very common, reflecting the multiple comorbidities present in this cohort of patients: five patients (31.25%) were on anti-coagulation therapy and six (37.5%) were on anti-aggregation therapy.
Table 1 summarizes the clinical and demographic characteristics of the included patients.
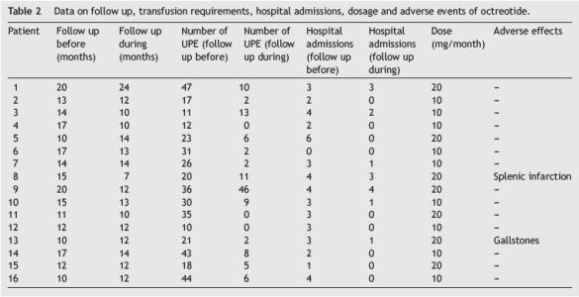
The median follow-up before the start of octreotide LAR was 14 months (range: 10-20) and patients remained on treatment with this drug (follow up during) for a median time of 12 months (range: 7-24).
The dosages used were 10 mg/month and 20 mg/month in 9 patients (56.25%) and 7 patients (43.75%) respectively.
In the period of follow up before 5.26 UPE were transfused on average (range: 10-47), which corresponds to an average transfusion rate of 1.93 UPE per month (median: 1.84 UPE/month) and patients were admitted for gastrointestinal bleeding on average 2.9 times (range: 0-6), corresponding to a median of 0.21 hospitalizations per month. In the period of follow up during 7.63 UPE were transfused on average (range: 0-46), corresponding to an average transfusion rate of 0.65 UPE per month (median: 0.42 UPE/month) and there were on average 0.94 admissions (range: 0-4) corresponding to a median of 0.00 hospitalizations per month.
Table 2 summarizes the data for the periods of follow-up, including transfusion requirements, hospitalization, doses and adverse effects.
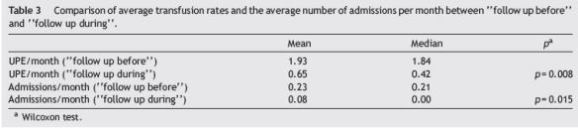
Considering rates of transfusion (Table 3), there was a significant decrease in the median UPE/month in the follow up before period compared to the follow up during period (1.84 UPE/month vs. 0.42 UPE/month; p = 0.008). When comparing the number of admissions per month between these two periods (Table 3), there was also a statistically significant decrease (0.21 hospitalizations/month vs. 0.00 admissions/month; p = 0.015).
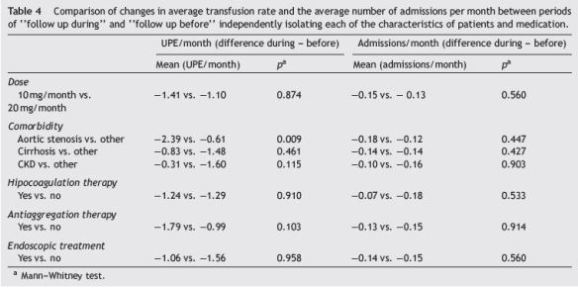
When considering the variation in the transfusion rate and average number of hospitalizations per month in the periods follow up before and follow up during, independently isolating each variable studied (univariate analysis), we observed that only the presence of aortic stenosis positively influenced the response to therapy in terms of reduced transfusion requirements compared with patients with other comorbidities (−2.39 UCE/month vs. −0.61 UCE/month, p = 0.009). No other characteristic analyzed affected the response to therapy including octreotide dose (Table 4). There were no clinical differences between the group of patients treated with 10 mg/month vs. those treated with 20 mg/month (Table 5).
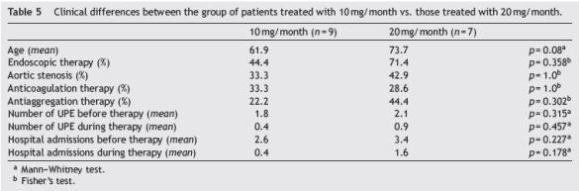
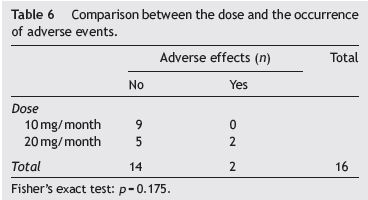
Adverse effects occurred in two of the patients studied (gallstones and splenic infarction). In the patient developing splenic infarction, medication was discontinued. There was no association between the dosage of octreotide
LAR and the occurrence of adverse events (p = 0.175) (Table 6).
Discussion
The drugs used as prophylaxis for haemorrhage due to gastrointestinal angiodysplasias with the greatest emphasis within the literature are oestrogen-progesterone, thalidomide and octreotide.
The efficacy of oestrogen-progesterone in preventing the recurrent haemorrhage of gastrointestinal angiodysplasias continues to be controversial.6,11 Although the mechanisms of action are not fully understood, the proposed effects of hormone therapy include restoration of endotelial integrity, procoagulant effects and stasis effect on mesenteric microcirculation.24-26 These effects are not immediate and they appear to be dependent on the dose of oestrogen (usually 10-60 micrograms/day of ethinylestradiol).27 Conflicting results were obtained in studies investigating the efficacy of this therapy for this indication, with significant differences in the methodology used in several published studies (different types of patients, doses and formulations of oestrogen-progesterone).12,28,29 In the largest prospective, randomized and controlled trial to date, which included 72 patients, Junquera et al.16 showed that hormone therapy was ineffective in preventing rebleeding or decreasing transfusion requirements. In this study, there was rebleeding or recurrent iron deficiency anaemia in 39% of the treated patients vs. 46% of the patients in the placebo group, after a follow-up period of 1-2 years (difference not significant). There are, however, important limitations of this study, such as the use of low doses of ethinylestradiol, errors in the analysis and stratification of patients (patients analyzed interchangeably with acute and chronic non-bleeding and haemorrhage by considering separately the angiodysplasias affecting the small bowel) and the exclusion of patients with angiodysplasias associated with liver cirrhosis and Rendu-Osler-Weber syndrome (subgroups that could possibly benefit more from this therapy6). In addition, the patients included in this study generally showed mild disease and had not received prior or concurrent endoscopic treatment.6,11,27 Thus, these discouraging results may not be applicable to all patients. Although hormone therapy is low cost and may possibly have major benefits in certain subgroups of patients,11 the efficacy and safety of other agents may place hormone therapy as a second-line therapy or adjunctive therapy in some cases.
The gastrointestinal angiodysplasias seem to be associated with increased serum levels of vascular endotelial growth factor (VEGF), this being touted as a possible etiologic factor of this important entity. High concentrations of VEGF are associated with aberrant angiogenesis and the formation of angiodysplasias lesions with a thinner muscle layer, which causes high vascular fragility and a tendency to haemorrhage.22 Thalidomide inhibits VEGF, which is the rationale for its use for this indication. Recently, Zhi-Zheng Ge et al.23 showed in a prospective randomized study involving 28 patients treated with thalidomide (100 mg daily PO for 4 months) and control patients treated only with iron, a significant reduction of bleeding episodes (response rates in thalidomide-treated group and the control group of 71.4% and 3.7% respectively) during the first year of followup. In this study there was also a significant decrease in serum levels of VEGF in the group of patients on therapy with thalidomide. There were no serious adverse effects although minor adverse effects were frequently observed within the therapeutic group. Thus, probably by decreasing the concentration of VEGF, thalidomide may be an effective therapeutic option that is relatively safe in patients with gastrointestinal bleeding angiodysplasias.
Octreotide is a synthetic octapeptide analogue of somatostatin with similar pharmacologic effects, but with a considerably longer half-life. The proposed mechanisms by which somatostatin analogues can act to decrease gastrointestinal haemorrhage resulting from angiodysplasias are: inhibition of angiogenesis, splanchnic vasoconstriction, increased vascular resistance and increased platelet aggregation.17 In the largest series published to date,18 32 non-cirrhotic patients with diagnosis of intestinal bleeding secondary to angiodysplasias were treated with octreotide at a dose of 50 micrograms subcutaneously (twice a day) for a period of 1-2 years. In the present cohort, patients treated with octreotide had a lower frequency of recurrent bleeding compared to those who received placebo (23% vs. 48%); moreover, treatment with octreotide reduced the need for further treatment with iron.
The requirement for long-term daily subcutaneous administrations is a disadvantage of treatment with octreotide. To overcome this, a sustained-release formulation (Octreotide LAR), requiring only monthly intramuscular administration, was developed maintaining the same safety profile and efficacy of subcutaneous octreotide in the treatment of diseases such as acromegaly and neuro-endocrine tumours.30,31
Octreotide LAR was tested in the treatment of chronic bleeding secondary to gastrointestinal angiodysplasias in a small series of 3 patients,19 wherein the authors reported a significant decrease in the number of hospitalizations and blood transfusions and an increase in average haemoglobin after initiation of octreotide LAR treatment (20 mg/month) during a follow-up period of 15-17 months. In another retrospective study, including 13 patients treated for one year with 10 mg octreotide LAR per month,20 nine patients did not require blood transfusions or iron supplementation during the observation period (12-60 months). More recently, Bon et al.21 published a study including 15 patients with angiodysplasias treated with octreotide LAR for a median time of 12 months (range: 6-36). The authors observed a decrease in the transfusion requirements (median: 10 (range: 6-24) vs. 2 (range: 0-14), p < 0.001) and the proportion of patients experiencing new episodes of gastrointestinal bleeding (20% vs. 73%, p = 0.01), on comparing the period before therapy with the period during therapy. In this study the mean haemoglobin level increased during the period in which patients received treatment with octreotide LAR (median: 7 (range: 5-8.5) vs. 10 g/dl (range: 9-13), p < 0.001).
Similarly, in our study, this therapy was effective in reducing the need for transfusion and hospitalization when comparing the periods follow up before and follow up during. We note that, in our study, the transfusion needs of three patients were reduced to zero (patients 4, 11 and 12) and eight patients (patients 2, 4, 5, 11, 12, 14, 15 and 16) no longer needed to be hospitalized for gastrointestinal bleeding after the initiation of treatment. Only two patients failed to respond to therapy, and these patients actually increased their requirement for transfusion (patients 3 and 9). In one of these patients (patient 3), poor compliance to treatment potentially accounts for this lack of response, although the reason behind the lack of response in the other case (patient 9) is unknown.
A comparison of patient characteristics revealed that the presence of valvular heart disease (aortic stenosis) was the only factor positively influencing the response to therapy. In patients with aortic stenosis, turbulent passage of blood through the stenosed valve results in mechanical disruption of vonWillebrand factor multimers, which causes the development of an acquired form of vonWillebrands disease (Heydes syndrome).32 Octreotide, by improving platelet aggregation,17 directly rectifies the mechanism by which aortic stenosis leads to increased bleeding episodes by digestive angiodysplasias, and this may be the explanation for the best response to therapy of these patients in our series.
Additionally, for the first time, we compared two doses of octreotide LAR and we found that higher doses (20 mg/month) did not translate to therapeutic benefit. The response to this drug depends upon the binding of octreotide to several receptor subtypes. The somatostatin receptor subtype 2 binds octreotide with the greatest affinity, and this receptor is expressed at the highest density in the gastrointestinal tract.33,34 Thus, it is likely that the gastrointestinal effects mediated by this receptor are achieved at low dosages of octreotide, explaining the similar efficacy of the two dosages used in our study.
Adverse effects occurred in 2 patients (12.5%) who were treated with the highest dose of the drug. However, we found no statistical association between dosage and adverse effects, although this may be due to the small number of patients with this outcome.
It can be concluded, based on our study, that octreotide LAR is an effective therapy for prophylaxis of gastrointestinal bleeding resulting from angiodysplasia, by decreasing transfusion requirements and the need for hospitalization for gastrointestinal bleeding. Patients with aortic stenosis benefited most from the therapy.
There was no increase in efficiency when higher doses of the drug were used, therefore we recommend lower dosages (10 mg/month) to avoid excess expense and lower the incidence of potential adverse effects.
Our series, despite being the largest reported to date, have some limitations; it is a retrospective cohort study with a small number of patients (n = 16) and lacks a control group. Prospective, double blind and randomized studies are warranted to validate the efficacy and safety of this therapy for this indication.
References
1. Regula J, Wronska E, Pachlewski J. Vascular lesions of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2008;22:313-28. [ Links ]
2. Clouse RE, Costigan DJ, Mills BA, Zuckerman GR. Angiodysplasia as a cause of upper gastrointestinal bleeding. Arch Intern Med. 1985;145:458-61. [ Links ]
3. Moreto M, Figa M, Ojembarrena E, Zaballa M. Vascular malformations of the stomach and duodenum: an endoscopic classification. Endoscopy. 1986;18:227-9. [ Links ]
4. Cappell MS. Spatial clustering of simultaneous nonhereditary gastrointestinal angiodysplasia. Small but significant correlation between nonhereditary colonic and upper gastrointestinal angiodysplasia. Dig Dis Sci. 1992;37:1072-7. [ Links ]
5. Steger AC, Galland RB, Hemingway A, Wood CB, Spencer J. Gastrointestinal haemorrhage from a second source in patients with colonic angiodysplasia. Br J Surg. 1987;74:726-7. [ Links ]
6. Raju GS, Gerson L, Das A, Lewis B, American Gastroenterological Association. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-717. [ Links ]
7. Kwan V, Bourke MJ, Williams SJ, Gillespie PE, Murray MA, Kaffes AJ, et al. Argon plasma coagulation in the management of symptomatic gastrointestinal vascular lesions: experience in 100 consecutive patients with long-term follow-up. Am J Gastroenterol. 2006;101:58-63. [ Links ]
8. Uflacker R. Transcatheter embolization for treatment of acute lower gastrointestinal bleeding. Acta Radiol. 1987;28:425-30. [ Links ]
9. Meyer CT, Troncale FJ, Galloway S, Sheahan DG. Arteriovenous malformations of the bowel: an analysis of 22 cases and a review of the literature. Medicine (Baltimore). 1981;60:36-48. [ Links ]
10. Molina Infante J, Perez Gallardo B, Fernandez Bermejo M. Update on medical therapy for obscure gastrointestinal hemorrhage. Rev Esp Enferm Dig. 2007;99:457-62. [ Links ]
11. Madanick RD, Barkin JS. Hormonal therapy in angiodysplasia: should we completely abandon its use? Gastroenterology. 2002;123:2156 (author reply 2156-7). [ Links ]
12. van Cutsem E, Rutgeerts P, Vantrappen G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen-progesterone. Lancet. 1990;335:953-5. [ Links ]
13. Junquera F, Santos J, Saperas E, Armengol JR, Malagelada JR. Estrogen and progestagen treatment in digestive hemorrhage caused by vascular malformations. Gastroenterol Hepatol. 1995;18:61-5. [ Links ]
14. Barkin JS, Ross BS. Medical therapy for chronic gastrointestinal bleeding of obscure origin. Am J Gastroenterol. 1998;93:1250-4. [ Links ]
15. Lewis BS, Salomon P, Rivera-MacMurray S, Kornbluth AA, Wenger J, Waye JD. Does hormonal therapy have any benefit for bleeding angiodysplasia? J Clin Gastroenterol. 1992;15: 99-103. [ Links ]
16. Junquera F, Feu F, Papo M, Videla S, Armengol JR, Bordas JM, et al. A multicenter, randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology. 2001;121:1073-9. [ Links ]
17. Brown C, Subramanian V, Wilcox CM, Peter S. Somatostatin analogues in the treatment of recurrent bleeding from gastrointestinal vascular malformations: an overview and systematic review of prospective observational studies. Dig Dis Sci. 2010;55:2129-34. [ Links ]
18. Junquera F, Saperas E, Videla S, Feu F, Vilaseca J, Armengol JR, et al. Long-term efficacy of octreotide in the prevention of recurrent bleeding from gastrointestinal angiodysplasia. Am J Gastroenterol. 2007;102:254-60. [ Links ]
19. Orsi P, Guatti-Zuliani C, Okolicsanyi L. Long-acting octreotide is effective in controlling rebleeding angiodysplasia of the gastrointestinal tract. Dig Liver Dis. 2001;33:330-4. [ Links ]
20. Scaglione G, Pietrini L, Russo F, Franco MR, Sorrentini I. Long-acting octreotide as rescue therapy in chronic bleeding from gastrointestinal angiodysplasia. Aliment Pharmacol Ther. 2007;26:935-42. [ Links ]
21. Bon C, Aparicio T, Vincent M, Mavros M, Bejou B, Raynaud JJ, et al. Long-acting somatostatin analogues decrease blood transfusion requirements in patients with refractory gastrointestinal bleeding associated with angiodysplasia. Aliment Pharmacol Ther. 2012;36:587-93. [ Links ]
22. Bauditz J, Schachschal G, Wedel S, Lochs H. Thalidomide for treatment of severe intestinal bleeding. Gut. 2004;53:609-12. [ Links ]
23. Ge ZZ, Chen HM, Gao YJ, Liu WZ, Xu CH, Tan HH, et al. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology. 2011;141:1629-2370. [ Links ]
24. Menefee MG, Flessa HC, Glueck HI, Hogg SP. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease). An electron microscopic study of the vascular lesions before and after therapy with hormones. Arch Otolaryngol. 1975;101:246-51. [ Links ]
25. Liu YK, Kosfeld RE, Marcum SG. Treatment of uraemic bleeding with conjugated oestrogen. Lancet. 1984;2:887-90. [ Links ]
26. Foutch PG. Angiodysplasia of the gastrointestinal tract. Am J Gastroenterol. 1993;88:807-18. [ Links ]
27. Szilagyi A, Ghali MP. Pharmacological therapy of vascular malformations of the gastrointestinal tract. Can J Gastroenterol. 2006;20:171-8. [ Links ]
28. Tran A, Villeneuve JP, Bilodeau M, Willems B, Marleau D, Fenyves D, et al. Treatment of chronic bleeding from gastric antral vascular ectasia (GAVE) with estrogen-progesterone in cirrhotic patients: an open pilot study. Am J Gastroenterol. 1999;94:2909-11. [ Links ]
29. Manzanera MJ, Gutierrez E, Dominguez-Gil B, García JA, González E, Praga M. Digestive haemorrhage due to angiodysplasia in dialysis patients. Treatment with conjugated estrogens. Nefrologia. 2005;25:412-5. [ Links ]
30. McKeage K, Cheer S,Wagstaff AJ. Octreotide long-acting release (LAR): a review of its use in the management of acromegaly. Drugs. 2003;63:2473-99. [ Links ]
31. Anthony LB. Long-acting formulations of somatostatin analogues. Ital J Gastroenterol Hepatol. 1999;31 Suppl. 2:S216-8. [ Links ]
32. OBrien JR, Etherington MD, Brant J, Watkins J. Decreased platelet function in aortic valve stenosis: high shear platelet activation then inactivation. Br Heart J. 1995;74:641-4. [ Links ]
33. Hogenauer C, Aichbichler B, Santa Ana C, Porter J, Fordtran J. Effect of octreotide on fluid absorption and secretion by the normal human jejunum and ileum in vivo. Aliment Pharmacol Ther. 2002;16:769-77. [ Links ]
34. Gugger M, Waser B, Kappeler A, Schonbrunn A, Reubi JC. Cellular detection of sst2A receptors in human gastrointestinal tissue. Gut. 2004;53:1431-6. [ Links ]
*Corresponding author
E-mail address: paulosalgueiro@gmail.com (P. Salgueiro).
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Conflicts of interest
The authors have no conflicts of interest to declare.
Received 4 October 2013; accepted 5 May 2014














