Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Gestão Costeira Integrada
versão On-line ISSN 1646-8872
RGCI vol.15 no.3 Lisboa set. 2015
https://doi.org/10.5894/rgci594
ARTICLE / ARTIGO
Biomarkers responses in different body regions of the polychaeta Hediste diversicolor (Nereidae, Polychaete) exposed to copper*
Resposta de Biomarcadores em Hediste diversicolor (Nereidae, Polychaete): Efeitos da exposição ao Cobre
Z. Bouraoui@, 1; J. Ghedira1; H. Boussetta1
@Corresponding author, to whom correspondence should be addressed: <bouraoui_zied@yahoo.fr>
1University of Sousse,1Laboratory of Biochemistry and Environmental Toxicology, I.S.A. Chott-Mariem, 4042, Tunisia. Phone: 00 216 73 327 544. Fax: 00 216 73 327 591
ABSTRACT
This study aims to evaluate the effects of exposure to 1 µM of copper during a period of test of 48 h, on enzymatic and lipid peroxidation biomarkers in anterior (A), middle (M) and posterior (P) body regions of the polychaeta (Nereididae) Hediste diversicolor. The biomarkers selected in this work were the NADPH cytochrome c reductase (NADPH red) as phase I bio-transformation enzyme, glutathione-S-transferase (GST) as phase II enzyme, and the oxidative stress markers using catalase activity (CAT) and malondialdehyde accumulation (MDA). The NADPH red activity was not significantly affected by copper exposure in the different body regions. Glutathione-S-transferase (GST) was significantly augmented (p< 0.05) only in the A region of Cu group compared to control group. The higher and significant CAT activity (p< 0.05) was noted in the P region of treated group paralleled by a lack of MDA production in the same region. A higher MDA content was observed in A region compared with the same body region of treated worm supporting the idea of a highest oxidant condition in this region.
Keywords: Copper, NADPH cytochrome c reductase, glutathione-S-transferase, catalase, Hediste diversicolor.
RESUMO
Com este trabalho pretende-se estudar a exposição ao cobre (1 µM) em testes de 48 h, com biomarcadores enzimáticos e de peroxidação lipídica nas regiões corporais anterior (A), mediana (M) e posterior (P) no poliqueta (Nereididae) Hediste diversi-color. Os biomarcadores selecionados para este estudo foram NADPH cytochrome c reductase (NADPH red) como enzimas biotransformadoras nas reações de fase I, glutathione-S-transferase (GST) como enzima da fase II, e os marcadores do stress oxidativo usando a atividade da enzima antioxidante catalase (CAT) e a acumulação de malondialdehyde (MDA). A atividade do NADPH não foi significativamente afetada pela exposição ao cobre nas diferentes regiões do corpo do poliqueta. Glutathione-S-transferase (GST) aumentou significativamente (p< 0.05) na região A comparativamente com o grupo controle. A maior e significativa atividade de CAT (p< 0.05) foi registada na região P paralelamente à ausência de produção de MDA nessa mesma região. Maiores concentrações de MDA foram registadas na região A evidenciando uma maior condição de stress oxidativo nessa região.
Keywords: Cobre, NADPH cytochrome c reductase, glutathione-S-transferase, catalase, Hediste diversicolor
1. Introduction
Many species of annelids are commonly used in toxicological studies. Polychaetes are the dominant macrofauna within fine sediments and the presence or absence of specific polychaetes is considered as an excellent indication of the condition of the benthic environment (Marcano et al. 1996; Lucan-Bouché et al. 1999; Carvalho et al., 2013). In the group of the polychaeta, many species seem to exhibit an extraordinary tolerance to various environmental contaminants, being also the most common invertebrates found in polluted areas (Eriksen et al., 1988; Bouraoui et al., 2014). Hediste diversicolor is a marine annelid which lives in estuary sediments rich in microorganisms and toxic agents resulting from pollution. It has been the subject of numerous studies, focusing on diverse aspects of its biology and ecology, including a range of pollution related subjects. This polychaete is characterized by high physiological tolerance to extreme variation of many environmental parameters such as temperature and salinity (Ait Alla et al., 2006;) and seems to exhibit tolerance to various environmental contaminants (Eriksen et al., 1988).
Copper pollution in the aquatic environment results from natural and anthropogenic sources such as mine washing or agricultural leaching (Rainbow and Smith, 2013). Although copper is a trace element essential to life it is also one of the most toxic metals (Ferreira-Cravo et al. 2009). This metal is required for maintaining cellular function and is an integral part of a number of copper-containing enzymes. One of the main toxic mechanisms of this metal is due to the alterations in intracellular protein machinery either directly via denaturation of enzymes or indirectly via generation of reactive oxygen species (ROS) through Fenton and Haber–Weiss reaction (Furuno et al., 1996; Bouraoui et al., 2009).
The use of biomarkers has been reported to be very informative about the organism’s stress response to pollutants (Jebali et al., 2013). NADPH cytochrome c reductase is a phase I biotransformation enzyme (CYP-dependent monooxygenase) playing a main role in the detoxification of organics xenobiotics (Arun and Subramanian, 2003). Glutathione-S-transferase (GST) is a phase II enzyme involved in the metabolism of lipophilic organic contaminants. This enzyme also plays a role in cellular protection against oxidative stress (Guidi et al., 2010). Catalase (CAT) is a well-known anti-oxidant enzyme, its activity increasing in organisms submitted to oxidative stress (Durou et al., 2007). One of the well-known lipid peroxidation products is malondialdehyde (MDA), this markers was usually used to evaluate the state of lipid peroxidation of the membrane (Alexandrova and Bochev, 2005, Ben Kheder et al., 2014) Polycheates are invariably exposed to pollutants. Studies examining bioaccumulation and subsequent toxicity of contaminants have often focused on whole organism. However, the interactions pollutants-different body regions are poorly known and need to be considered. Taking into consideration the existence of a differential response along the body of H. diversicolor and also the previous reports showing a response gradient in different body regions of annelids (Rosa et al 2005; Ferreira-Cravo et al 2009), the aim of this study was to evaluate the effects of exposure to copper on enzymatic biomarkers and lipid peroxidation level in anterior, middle and posterior body regions of H. diversicolor.
2. Material and methods
2.1. Animal treatment
Specimens of the polychaete H. diversicolor, with mass between 0.4 and 0.6g were collected from Teboulba (Tunisia), which was reported to be a clean site (Banni et al., 2005, 2007; Jebali et al., 2007; Bouraoui et al., 2009, 2014). The animals together with the surrounding sediments were put in polyethylene bottles. Once in the laboratory the worms were separated from sediment, cleaned from debris, and then placed in glass dishes at 14°C with aerated clean sea water to ambient photoperiod regimes for 3 days; this acclimation period was used for excluding specimens with exoskeleton or skin infections.
After this period, worms were exposed for 48 h to1 µM of Copper (CuSO4). A control group was run in parallel, employing only saline water (10‰) with the same characteristics cited above. The number of worms were used per experimental group varied between 40 (control) and 50 (copper). After 48 h of exposure, the organisms were sacrificed and based on the anatomic structure, were subdivided in three regions: anterior region (A, first 20 setiger segments), middle region (M, next 20 setiger segments) and posterior region (P, the rest of the body) (Rosa et al., 2005). A, M or P sections were pooled into five replicate samples of 5 to 7 worms each, washed briefly in ice-cold, and conserved in liquid nitrogen until analysis.
2.2. Biochemical analyses
A pool of each region (n=5-7) were homogenized (1:5, w/v) in phosphate buffer 100 mM, pH 7.5, NaCl (2.5%). Homogenates were then centrifuged at 9000g for 30 min (4°C). The supernatant (S9 fraction) of each sample was stored at -20°C, and employed later to determine total protein content, CAT and GST activities and MDA accumulation. Subsequently, the supernatant was centrifuged at 100.000g for 50 min at 4° C. The pellet was resuspended in 10 mM HEPES, pH 7.4, containing 250 mM sucrose in 20% glycerol, to obtain a microsomal fraction. This microsomal suspension was used for NADPH cytochrome c activity measurements. Total protein content in the homogenate was measured following the Bradford method (Bradford, 1976), at 595 nm, using bovine serum albumin as standard.
2.3. NADPH cytochrome c reductase determination
NADPH cytochrome c activity was determined according to Hayes (1982). Reaction mixture contained the stock microsomal enzyme, 20 mM NADPH and 10 mM of cytochrome c. The specific activity was determined by spectrophotometric method at 550 nm. The results were expressed as nmoles cytochrome c reduced/min/mg proteins.
2.4. Glutathion-S-transferase determination
GST activity was assayed by the method described by Habig et al. (1974) using the 1-chloro-2,4-dinitrobenzene (CDNB) as substrate, and GSH (1 and 4 mM final concentration, respectively), in 100 mM sodium phosphate buffer, pH 7.5. All GST activity assays were realized in conditions of linearity with respect to incubation time. The results were expressed as nmole produced/min/mg proteins.
2.5. Catalase determination
Catalase activity was determined by the method of Claiborne (1985) measuring the rate of enzymatic decomposition of H2O2 determined as absorbance decrements at 240 nm. The assay mixture consisted of 750 µL of sodium phosphate buffer (0.1 M, pH 7.5 and 25°C), 200 µL solution of 0.5 mM H2O2 and 50 µL of cytosolic fraction. Results were expressed as µmol H2O2 consumed/min/mg proteins.
2.6. Malondialdehyde accumulation
Lipid peroxidation was estimated in terms of thiobarbituric acid reactive species (TBARS) with use of 1,1,3,3-treaethyloxypropane as a standard. The reaction was determined at 532 nm, using TBA reagent as described by Buege and Aust (1978). MDA content was expressed as nmoles equivalent MDA/mg proteins.
2.7. Statistical analysis
The results were expressed as means ± SD. SPSS software (version 20.0) was used for statistical analysis. The data were first tested for normality and homogeneity of variance to meet statistical demands. Data from different groups were compared by a one-way analysis of variance (ANOVA) and Gabriel’s test were used to analyze raw biomarker data for comparison of responses between groups. All differences were considered significant at p < 0.05. Different letters a, b and c indicated significant differences between groups.
3. Results
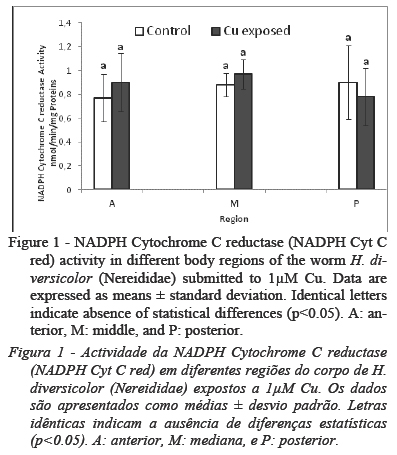
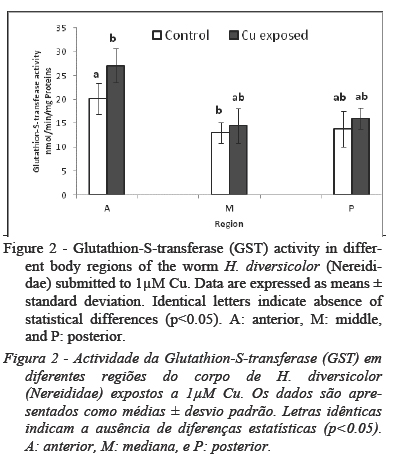
No significant worm mortality (<10%) was observed during the exposure period. The effect of 1µM Cu on NADPH Cyt C red was reported in Fig.1. This enzymatic activity showed no differences between control and Cu groups and between the body regions (p<0.05). However, GST activity (Fig. 2) was higher (p<0.05) in the A region, both in control and Cu group in respect of the M regions and P regions. Also GST activity in the A region of Cu group was higher (p<0.05) than GST activity in the same body region of control group.
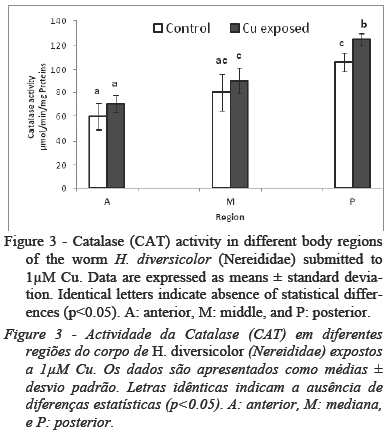
In terms of oxidative stress marker, CAT activity was significantly different along the body regions of controls and treated group. It was lower (p < 0.05) in the A region, intermediate in the M region, and higher in the P region, both in control and Cu group (Fig.3).
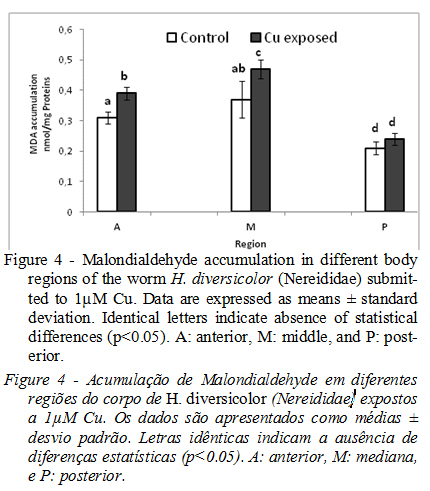
Concerning oxidative damage, the posterior region presented the lower MDA content and no statistical difference (p <0.05) was observed in both control and Cu groups. The MDA accumulation was significantly higher (p <0.05) in A and M regions in respect of the control group (Fig.4).
4. Discussion
The effects of metals on the polychaeta H. diversicolor were largely documented, however, and to our knowledge, no studies investigated the effects of subacute concentrations of copper in different body regions of the polychaeta H. diversicolor. The present work reported the acute effects of 1µM of Cu on anterior, middle and posterior body regions of H. diversicolor using a multimarker approach comprising a set of enzymatic and lipid peroxidation markers.
In this study, worms were exposed to 1 µM of Cu, this concentration was reported to be sublethal for terrestrial (Gastaldi et al., 2007; Hankard et al., 2004) and marine worms (Ferreira-Cravo et al., 2009). Indeed, Moreira et al. (2005) were reported acute toxicity of copper in H. diversicolor. They suggested that 48-h LC50 value was 241 ug/L and 48-h IC50 was 52 ug/L
Yang et al. (2012) were reported that the LC50 of Cu in polychaeta Perinereis aibuhitensis is approximately 475 µg/L after 96 h of exposure.
Metals as copper have been of great concern in marine and coastal ecosystems, since they cause several biological alterations from molecular to tissue level depending to their concentrations and time exposure (Banni et al., 2009; Ben Khedher et al., 2014). Therefore, they may be also, accumulated in various tissues of living organisms and lead to several orders of magnitude higher than those of the surrounding water (Ghedira et al., 2011; Jebali et al., 2014).
Toxic effects of pollutants often depend on their capacity to increase the cellular levels of reactive oxygen species (ROS). Cu was reported to create an oxidative stress status by the Harber-Weiss and Fenton reaction of Cu cations (Bouraoui et al., 2009; Caldwell et al., 2011), resulting in cellular damages due to hydroxyl radicals (HO•). Some enzymatic and non-enzymatic tests have been proved to be suitable for monitoring the effects of pollutants. In the present work, the level of NADPH Cyt C red along the body was not affected by exposure to copper. The same absence of response was observed in previous studies (Bouraoui et al., 2009) when this worm was exposed for 12, 24, 36 and 48h to the same concentration. Moreover, several authors were reported that the level of NADPH cyt c red could increase after exposure mainly to hydrocarbons compounds (Christensen et al., 2002; Bouraoui et al., 2009, 2010) suggesting a high increase of the organic compound metabolic processes.
Worm phase II conjugation, measured as GST activity, was significantly increased in the A region of Cu group compared to the same region in control group. It is known that GSTs constitute an anti-oxidant enzyme involved in GSH conjugation to xenobiotics, fatty acids hydroperoxides and aldehydic products of lipid peroxidation (Hermes-Lima, 2004; Jebali et al., 2014). The higher GST in the A region indicates a higher antioxidant capacity against peroxyl and hydroxyl radicals. In other hand, the lack of difference between control and treated group in M and P region can be explained by other antioxidant mechanisms as glutathione peroxydase (GPX) and/or superoxide dismutase (SOD). In this view, Rosa et al., 2005 reported high SOD and GPX activity in M and P region of Laeoneris acuta (anelida polychaeta) after exposure to 50 µM of hydrogen peroxide (H2O2) accompanied by low activity of GST in these regions.Concerning another anti-oxidant enzyme, CAT, our result demonstrate that, in H. diversicolor, exposure to sublethal concentrations of Cu, induced significant changes in CAT activity only in P region. In other hand, CAT was significantly different along the body regions of controls and treated group indicating that H. diversicolor deals with oxidative stress employing different strategies among these body regions. Indeed and as suggested by our data, Rosa et al., (2005) and Ferreira-Cravo et al. (2009) described an increase of CAT activity in posterior region of Laeoneris acuta exposed respectively to 10 µM of H2O2 and 62.5 ug/L of copper.
The content of malondialdehyde (MDA) is a way to evaluate the lipid peroxidation level, which occurs in the absence of sufficient antioxidant defense (Guidia et al., 2010; Ghedira et al., 2011; Buffet et al., 2014). A Lower MDA values for posterior regions is due to a sufficient antioxidant defense such as CAT in this region. In fact, when, CAT activity was induced in P region of worms exposed to 1µM of Cu, the MDA accumulation followed a decreasing trend. A clear evidence of oxidative perturbations reported in previous studies in whole body of H. diversicolor treated with sublethal Cd, Cu and B&[a]P proved a reduced capability to prevent lipid peroxidation generated by pollutants as metals, hydrocarbons and nanomaterials (Catalano et al., 2012; Bouraoui et al., 2014; Moschino et al., 2014).
5. Conclusion
H. diversicolor exposed to 1µM of Cu, presents differential biomarkers responses in the anterior, middle, and posterior region of its body: In the A region, higher activity of GST, whereas in the P region higher activity of CAT with a low MDA level. Our study can be used not only to understand the response of polychaete exposed in situ to copper, but also, to ensure a better sustainable management of coastal areas. However, studies with other toxicological responses (enzymatic or non enzymatic) are needed for a better understanding of the results obtained in the present work.
Acknowledgements
This work was supported by the Research Unit of Biochemistry and Environmental Toxicology, UR 04AGR05, Higher Institute of Agronomic Sciences of Chott-Mariem (IRESA, Tunisia).
References
Ait Alla, A.; Mouneyrac, C.; Durou, C.; Moukrim, A.; Pellerin, J. (2006) - Tolerance and biomarkers as useful tools for assessing environmental quality in the Oued Souss estuary (Bay of Agadir, Morocco). Comparative Biochemistry and Physiology C: Toxicology & Pharmacology, 143(1):23-29. DOI: 10.1016/j.cbpc.2005.11.015 [ Links ]
Alexandrova, M.L.; Bochev, P.G. (2005) - Oxidative stress during the chronic phase after stroke. Free Radical Biology & Medicine, 39(3):297–316. DOI: 10.1179/135100003225001548
Arun, S.; Subramanian, P. (2003) - Cytochrome P450 and other biotransformation activity in aquatic organisms: potential bio-markers to environmental pollution. In: Tripathi, G., Kumar, A. (eds.), Potentials of Living Resources, pp.459–488, Discovery Publishers, New Delhi, India. ISBN: 978-8171417186.
Banni, M.; Jebal,, J.; Daubeze, M.; Clerendeau, C.; Guerbej, H.; Narbonne, J.F.; Boussetta, H. (2005) - Monitoring Pollution in Tunisian coasts: Application of a classification scale based on biochemical markers. Biomarkers, 10(2-3):105-116. DOI: 10.1080/13547500500107497 [ Links ]
Banni, M.; Dondero, F.; Jebali, J.; Guerbej, H.; Boussetta, H.; Viarengo, A. (2007) - Assessment of heavy metal contamination using real time PCR analysis of mussel metallothionein mt10 and mt20 expression: avalidation along the Tunisian coasts. Biomarkers, 12(4):369–383. DOI: 10.1080/13547500701217061
Banni, M.; Bouraoui, Z.; Clerandeau, C.; Narbonne, J.F.; Boussetta, H. (2009) - Mixture toxicity assessment of cadmium and benzo[a]pyrene in the sea worm Hediste diversicolor. Chemosphere, 77(7):902–906. DOI: 10.1016/j.chemosphere.2009.08.041
Ben-Khedher, S.; Jebali, J.; Houas, Z.; Nawéli, H.; Jrad, A.; Banni, M.; Boussetta, H. (2014) - Metals bioaccumulation and histopathological biomarkers in Carcinus maenas crab from Bizerta la-goon, Tunisia. Environmental Science and Pollution Research, 21(6):4343-4357. DOI: 10.1007/s11356-013-2399-x [ Links ]
Bouraoui, Z.; Banni, M.; Ghedira, J.; Clerandeau, C.; Narbonne, JF.; Boussetta, H. (2009) - Evaluation of enzymatic biomarkers and lipoperoxidation level in Hediste diversicolor exposed to copper and benzo[a]pyrene. Ecotoxicology and Environmental Safety, 72(7):1893-1898. DOI: 10.1016/j.ecoenv.2009.05.011 [ Links ]
Bouraoui, Z.; Banni, M.; Chouba, L.; Ghedira, J.; Clerandeau, C.; Jebali, J.; Narbonne, J.F.; Boussetta, H. (2010) - Monitoring pollution in Tunisian coasts using a scale of classification based on biochemical markers in worm Nereis (Hediste) diversicolor. Environmental Monitoring and Assessment, 164(1-4):691-700. DOI: 10.1007/s10661-009-0921-x [ Links ]
Bouraoui, Z.; Ghedira, J.; Capri, F.; Chouba, L.; Boussetta, H. (2014) - Cytochemical responses of Hediste diversicolor (Nereidae, Polychaete) sampled from polluted sites along the Tunisian coast. Revista de Gestão Costeira / Journal of Integrated Coastal Zone Management, 14(1):119-127. DOI: 10.5894/rgci480 [ Links ]
Bradford, M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 72(1-2):248-254. DOI: 10.1016/0003-2697(76)90527-3
Buege, J.A.; Aust, S.D. (1978) - Microsomal lipid peroxidation. In: Packer, L. (Ed.), Methods in Enzymology, 52:302–310. DOI: 10.1016/S0076-6879(78)52032-6
Buffet, P.E.; Poirier, L.; Zalouk-Vergnoux, A.; Lopes, C.; Amiard, J.C.; Gaudin, P.; Rissode Faverney, C.; Guibbolini, M.; Gilliland D.; Perrein-Ettajani, H.; Valsami-Jones, E.; Mouneyrac, C. (2014) - Biochemical and behavioural responses of the marine polychaete Hediste diversicolor to cadmium sulfide quantum dots (CdS QDs): Waterborne and dietary exposure. Chemosphere, 100:63–70. DOI: 10.1016/j.chemosphere.2013.12.069
Caldwell, G.S.; Lewis, C.; Pickavance, G.; Taylor, R.L.; Bentley, M.G. (2011) - Exposure to copper and cytotoxic polyunsaturated aldehyde induces reproductive failure in the marine polychaete Nereis virens (Sars). Aquatic Toxicology, 104(1-2):126–134. DOI: 10.1016/j.aquatox.2011.03.018
Catalano, B.; Moltedo, G.; Martuccio, G.; Gastaldi, L.; Virno-Lambert, C.; Lauria, L.; Ausili A. (2012) - Can Hediste diversicolor (Nereidae, Polychaete) be considered a good candidate in evaluating PAH contamination? A multimarker approach. Chemosphere, 86(9):875-882. DOI: 10.1016/j.chemosphere.2011.10.040 [ Links ]
Carvalho, A.N.; Lino Vaz, A.S.; Boto Sérgio, T.I.; Talhadas dos Santos, P.J. (2013) - Sustainability of bait fishing harvesting in estuarine ecosystems - Case study in the Local Natural Reserve of Douro Estuary, Portugal. Revista de Gestão Costeira Integrada / Journal of Integrated Coastal Zone Management, 13(2):157-168. DOI: 10.5894/rgci393 [ Links ]
Christensen, M.; Andersen, O.; Banta, G. (2002) - Metabolism of pyrene by the polychaetes Nereis diversicolor and Arenicola marina. Aquatic Toxicology. 58(1-2):15-25. DOI: 10.1016/S0166-445X(01)00217-X [ Links ]
Claiborne, A. (1985) - Catalase activity. In: Robert A. Greenwald (ed.), CRC Handbook of Methods in Oxygen Radical Research, pp.283–294, CRC Press, Florida, U.S.A. ISBN: 978-0849329364
Durou, C.; Poirier, L.; Amiard, J.C.; Budzinski, H.; Gnassia-Barelli, M.; Lemenach, K.; Peluhet, L.; Mouneyrac, C.; Romeo, M.; Amiard-Triquet, C. (2007) - Biomonitoring in a clean and a multi-contaminated estuary based on biomarkers and chemical analyses in the endobenthic worm Nereis diversicolor. Environmental Pollution, 148(2):445-458. DOI: 10.1016/j.envpol.2006.12.022 [ Links ]
Eriksen, K.D.H.; Daae H.L.; Andersen, R.A. (1988) - Evidence of presence of heavy metal-binding proteins in polychaete species. Comparative Biochemistry and Physiology, Part C: Comparative Pharmacology, 91(2):377–384. DOI: 10.1016/0742-8413(88)90045-X
Ferreira-Cravo, M.; Ventura-Lima, J.; Sandrini, J.Z.; Amado, L.L.; Geracitano, L.A.; Rebelo, M.; Bianchini, A.; Monserrat, J.M. (2009) - Antioxidant responses in different body regions of the polychaeta Laeonereis acuta (Nereididae) exposed to copper. Ecotoxicology and Environmental Safety, 72(2):388–393. DOI: 10.1016/j.ecoenv.2008.07.003
Guidia, P.; Frenzilli, G.; Benedettib, M.; Bernardeschia, M.; Falleni, A.; Fattorini, D.; Regoli, F.; Scarcelli, V.; Nigro, M. (2010) - Antioxidant, genotoxic and lysosomal biomarkers in the fresh-water bivalve (Unio pictorum) transplanted in a metal polluted river basin. Aquatic Toxicology, 100(1):75-83. DOI: 10.1016/j.aquatox.2010.07.009 [ Links ]
Gastaldi, L.; Ranzato, E.; Capri, F.; Hankard, P.;, Peres, G.; Canesi, L.; Viarengo, A.; Pons, G. (2007) - Application of a biomarker battery for the evaluation of the sublethal effects of pollutants in the earthworm Eisenia andrei. Comparative Biochemistry and Physiology, Part C: Toxicology & Pharmacology, 146(3):398-405. DOI: 10.1016/j.cbpc.2007.04.014 [ Links ]
Ghedira, J.; Jebali, J.; Banni M.; Chouba, L, Boussetta, H.; López-Barea, J.; Alhama, J. (2011) - Use of oxidative stress biomarker sin Carcinus maenas to assess littoral zone contamination in Tunisia. Aquatic Biology, 14(1):87–98. DOI: 10.3354/ab00377
Hankard, P.K.; Svendsen, C.; Wright, J.; Wienberg, C.; Fishwick, S.K.; Spurgeon, D.J. (2004) - Biological assessment of contaminated land using earthworm biomarkers in support of chemical analysis. Science of The Total Environment, 330(1-3):9-20. DOI: 10.1016/j.scitotenv.2003.08.023 [ Links ]
Habig, W.H.; Pabst, M.J.; Jakoby, W.B. (1974) - Glutathione S-transferases, the first step in mercapturic acid formation. Journal of Biological Chemistry, 249(22):7130-7139, American Society of Biological Chemists (ISSN: 0021-9258), Rockville, MD, U.S.A. Available on-line at http://www.ncbi.nlm.nih.gov/pubmed/4436300 [ Links ]
Hayes, AW. (1982) - Principles and Methods of Toxicology. 750p., Raven Press, New York, U.S.A. ISBN: 978-0890044704 [ Links ]
Hermes-Lima, M. (2004) - Oxygen in biology and biochemistry: role of free radicals. In: Kenneth B. Storey (ed.), Functional Metabolism: Regulation and Adaptation, pp.319–368, Wiley, New York, NY, U.S.A. ISBN: 978-0471410904 DOI: 10.1002/047167558X.ch12
Jebali, J.; Banni, M.; Alves de Almeida, E.; Boussetta, H. (2007) - Oxidative DNA damage levels in the clams Ruditapes decussatus as pollution biomarkers of Tunisian marine environment. Environmental Monitoring and Assessment, 124(1-3):195-200. DOI: 10.1007/s10661-006-9217-6. [ Links ]
Jebali, J.; Ben Khedher, S.; Sabbagh, M.; Kamel, N.; Banni, M.; Boussetta, H. (2013) - Cholinesterase activity as biomarker of neurotoxicity: utility in the assessment of aquatic environment contamination. Revista de Gestão Costeira Integrada / Journal of Integrated Coastal Zone Management, 13(4):525-537. DOI: 10.5894/rgci430. [ Links ]
Jebali, J.; Chicano-Gálvez, E.; Fernández-Cisnal, R.; Banni, M.; Chouba, L.; Boussetta H.; Barea, H.L.; Alhama, J. (2014) - Proteomic analysis in caged Mediterranean crab (Carcinus maenas) and chemical contaminant exposure in Téboulba Harbour. Tunisia. Ecotoxicology and Environmental Safety, 100:15–26. DOI: 10.1016/j.ecoenv.2013.11.025
Lucan-Bouché, M.L.; Biagianti-Risbourg, S.; Arsac, F.; Vernet, G.; (1999) - An original decontamination process developed by aquatic oligochaete Tubifex tubifex exposed to copper and lead. Aquatic Toxicology, 45(1):9-17. DOI: 10.1016/S0166-445X(98)00091-5 [ Links ]
Marcano, L.; Nusetti, O.; Rodrıguez-Grau, J.; Vilas, J. (1996) - Uptake and depuration of copper and zinc in relation to metal-binding protein in the polychaete Eurythoe complanata. Comparative Biochemistry and Physiology, Part C: Pharmacology, Toxicology and Endocrinology, 114(3):179-184. DOI: 10.1016/0742-8413(96)00016-3 [ Links ]
Moreira, S.M.; Moreira-Santos, M.; Guilhermino, L.; Ribeiro, R. (2005)- A short-term sublethal in situ toxicity assay with Hediste diversicolor (Polychaeta) for estuarine sediments based on postexposure feeding. Environmental Toxicology and Chemistry, 24(8): 2010-2018. DOI: 10.1897/04-473R1.1 [ Links ]
Moschino, V.; Nestoa, N.; Barisonb, S.; Agresti, F.; Colla, L.; Fedele, L.; Da Ros, L. (2014) - A preliminary investigation on nanohorn toxicity in marine mussels and polychaetes. Science of the Total Environment, 468-469:111-119. DOI: 10.1016/j.scitotenv.2013.08.020. [ Links ]
Rainbow, P.S.; Smith, B.D. (2013) - Accumulation and detoxification of copper and zinc by the decapods crustacean Palaemonetes varians from diets of field-contaminated polychaetes Nereis diversicolor. Journal of Experimental Marine Biology and Ecology, 449:312-320. DOI: 10.1016/j.jembe.2013.09.022 [ Links ]
Rosa, C.E.; Iurman, M.G.; Abreu, P.C.; Geracitano, L.A.; Monserrat, J.M..(2005) - Antioxidant mechanisms of the nereidid Laeonereis acuta (Annelida: Polychaeta) to cope with environmental hydrogen peroxide. Physiological and Biochemical Zoology, 78(4):641–649. DOI: 10.1086/430229
*Submission: 13 MAR 2015; Peer review: 16 APR 2015; Revised: 29 APR 2015; Accepted: 5 MAY 2015; Available on-line: 6 MAY 2015














