Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Gestão Costeira Integrada
versão On-line ISSN 1646-8872
RGCI vol.14 no.1 Lisboa mar. 2014
https://doi.org/10.5894/rgci480
ARTICLE / ARTIGO
Cytochemical responses of Hediste diversicolor (Nereidae, Polychaete) sampled from polluted sites along the Tunisian coast*
Respostas Citoquímicas em Hediste diversicolor (Nereidae, Polychaeta) de locais poluídos na Zona Costeira Tunisina**
Zied Bouraoui@, I, Jihene GhediraI, Flavia CapriII, Lassaad ChoubaIII, Hamadi BoussettaI
@Corresponding author
ILaboratory of Biochemistry and Environmental Toxicology, Higher Institute of Agronomic Sciences. Chott-Mariem, 4042, Sousse, Tunisia
IIDepartment of Environmental and Life Sciences, University of Piemonte Orientale Amedeo Avogadro, 15100, Alessandria, Italy
IIILaboratory of Marine Environment, National Institute of Sciences and Technologies of the Sea, La Goulette, 2060, Tunis, Tunisia
ABSTRACT
The polychaete worm Hediste diversicolor was collected in several sites from the Tunisian coast. The aim of our study was to study several cytochemical biomarkers in this species in response to a pollution gradient caused by various discharges a long the Tunisian coast. Worms were collected from six sites: Bizerta Lagoon, Gargour, Nakta, Mahres, Skhira and from Teboulba, witch is considered a reference site.
H. diversicolor. They are consistent with the chemical analysis and that worms from Bizerta and Mahres have been submitted to high levels of pollution.
Keywords: Biomarkers, lysosomal membrane stability, neutral lipids, lipofuscin, Ca2+-ATPase activity, Hediste diversicolor.
RESUMO
Com o objectivo de estudar vários marcadores citoquímicos em Hediste diversicolor ao longo de um gradiente de poluição foram colhidos indivíduos dessa espécie na costa Tunisina (Bizerta Lagoon, Gargour, Nakta, Mahres, Skhira e Teboulba como local de referência).
H. diversicolor. Estes resultados mostraram-se consistentes com as análises químicas e ainda com a maior exposição a poluentes nos locais Bizerta e Mahres.
Palavras-Chave: Biomarcadores, estabilidade membranar dos lisossomas, lípidos neutros, lipofuscina, atividade Ca2+-ATPase, Hediste diversicolor.
1. INTRODUCTION
Estuaries and coastal waters are particularly at risk from anthropogenic pollution including industrial, agricultural and/or urban development, receiving toxic effluents. Compared to current practice in aquatic monitoring based on recording the concentrations of a small range of pollutants in biological matrices in a given environment, a probably more realistic way to assess the ‘‘health status’’ of the environment is to measure a suite of biomarkers. Chemical analyses reveal only the bioaccumulated concentrations of particular pollutants in the organisms. While an integrated response approach involving a suite of the in situ responses of populations at each particular site, demonstrates their interactive and combined effects with regard to the numerous environmental factors characterizing the location. It is always very difficult from only contamination body burden data to obtain information about their significance upon animal health. Therefore, techniques for measuring biological effects are critical for any pollution monitoring program. A large number of biomarkers have been tested and validated for their applicability to detect biological effects as indicators of chemical stress at different levels of biological organization (Amiard et al., 2006; Magni et al., 2006; Nigro et al., 2006; Bouraoui et al., 2009). However the use of enzyme activities, performs a risk of being inhibited by high contaminant loads (Narbonne et al., 2005), and it is therefore important to also monitor the overall health status by making use of biomarkers of effect and stress. Several of the most sensitive cellular stress markers are lysosomal parameters, e.g. lysosomal membrane stability (LMS), lipofuscin (LF) and neutral lipid (NL) contents, lysosomal volume by assessing the lysosome/cytoplasm ratio (Lowe et al., 1981; Moore, 1988; Viarengo et al., 1991; Dondero et al., 2006) and Ca2+-ATPase activity. Sediments (both suspended and deposited) constitute the main reservoir for most of the chemicals introduced into aquatic environments by human activities. Then, it is necessary to develop the use of biomonitors more representative of the sedimentary compartment of aquatic habitats.
The polychaetes are the dominant species of macrofauna within fine sediments. In this group, many species seem to exhibit an extraordinary tolerance to various environmental contaminants, being also the most common invertebrates found in polluted areas (Eriksen et al., 1988). H. diversicolor is a marine annelid which lives in estuary sediments rich in microorganisms and toxic agents resulting from pollution. It has been the subject of numerous studies, focusing on different aspects of its biology and ecology, including a range of pollution related subjects. This polychaete is characterized by a high physiological tolerance to extreme variation of many environmental parameters such as temperature and salinity (Bartels-Hardege & Zeeck, 1990; Ait Alla et al., 2006). Therefore, the use of polychaete worms as bioindicators for estuarine ecosystems has proved efficiency to be a useful tool in the assessment of environmental quality, being not insensitive to stressful environmental conditions (Dean, 2008).
The aim of this work was to employ cellular stress markers in H. diversicolor to assess the marine environment quality along the Tunisian coasts.
2. MATERIAL AND METHODS
2.1. Sampling sites
Sampling sites along the Tunisian coast were chosen for the presence of a natural population of the polychaete H. diversicolor and their geographical locations near urban, industrial and agricultural areas (Fig. 1); they are constantly threatened by contamination due to their proximity to human settlements and high economic and industrial activities. Menzel Abdelrahmen is located in the north of Tunisia in the Bizerta Lagoon (S1), a Mediterranean lagoon covering roughly 15 km2 that represents an economically important body of water due to a variety of fishing and aquaculture activities. The other four sites, Nakta (S2), Gargour (S3) and Mahres (S4) and Skhira (S5), are located in the gulf of Gabés, in the southeastern coast of Tunisia, which is considered as a great Tunisian aquatic resource, contributing to more than half of the national production. Important industrial activities, mainly crude phosphate treatments, chemical industries and tannery, are being developed along this region, possibly affecting this marine ecosystem (Hamza-Chaffai et al., 1995; Boujelben, 1998; Banni et al., 2005). The control worms were collected from Teboulba, located on the mid of Tunisia; this site is characterized with no apparent contamination sources and thus considered in many field works as reference site (Banni et al., 2007; Jebali et al., 2007; Bouraoui et al., 2010).
2.2. Collection
Samples were collected in the intertidal zone at low tide from six sites in Tunisian coastal areas during September 2009; worms were sampled from Bizerta lagoon, Gargour, Nakta, Mahres, Skhira and from Teboulba (natural population). The surface oxygenated layer (a few mm deep and about 300 cm2 area) of sediments destined for metal analysis was collected and placed in aluminum box. Once in the laboratory, the worms were examined (specimens with exoskeleton or skin infections were excluded) and flash-frozen in N-hexane, chilled in liquid nitrogen and then stored at -80 °C.
2.3. Metal analysis in sediments
Sediment were digested with 2:5 (V:V) 33% HCl and 65% HNO3 (UNEP/COI/AIEA/FAO 1994). Cu, Cd and Zn were analyzed by atomic absorption spectrophotometry AAS (Zeeman effect) in these acid solutions after dilution with deionised water. The calibration was carried out using the following standards for all of the three metals: 125, 250, 500 ng.ml-1. For each sediment sample, three replicates were analysed concomitantly. All metal concentration was reported in micrograms per gram dry weight of sediment.
2.4. Cytochemical analysis
2.4.1. Lysosomal membrane stability (LMS) assay
Pieces of excised worms were placed on two different aluminum cryostat chucks for a total of six individuals, and sections (10µM) were cut with a cryostat (Leica CM3050) and flash-dried by transferring them to room temperature. The cryostat sections were incubated at 37 °C in 0.1 M citrate buffer containing 2.5% NaCl for various pre-treatment times (0, 2, 5, 10, 15, 20, 25, 30, 35 min) in order to labilise the lysosomal membrane. Then, sections were incubated at 37 °C in 50 mL of 0.1 M citrate buffer with 2.5% NaCl, containing 20 mg naphthol AS-BI-N-acetyl-β-glucosaminide previously dissolved in 2.5 mL 2-methoxyethanol and 3.5 g of polypeptide, rinsed at 37 °C in 3% NaCl, treated at room temperature with 1 mg/mL Fast Violet B in 0.1 M phosphate buffer and fixed for 15 min in Baker’s fixative at 4 °C (Moore, 1976). Staining intensity of lysosomes was determined by studying the slide at 400×magnification with an inverted Axiovert microscope (Zeiss), connected to an Axiocam digital camera (Zeiss). Digital image analysis was carried out using the Scion Image software package (Scion Corp).
2.4.2. Neutral lipids content
This analysis was made from sections prepared the same as for the lysosomal membrane stability assay by fixing the sections in calcium-formaldehyde (2% Ca-acetate (w/v), 10% formaldehyde (v/v)) for 15 min at 4◦C, followed by a rinsing step with de-ionised water, and incubation with 60% triethylphosphate (TEP) for 3 min. The sections were then stained with Oil Red- O (1% in 60% TEP) for 30 s, rinsed with de-ionised water, and mounted in 20% (v/v) glycerol (Moore, 1988). Neutral lipid content was quantified by digital image analysis, as described for the LMS assay.
2.4.3. Lipofuscin content
Cryostat sections were fixed in calcium-formaldehyde and rinsed with de-ionised water, as described for the neutral lipid assay, followed by a 5 min incubation step with 1% Fe2Cl3, 1% potassium ferrocyanide in a 3:1 ratio (Moore, 1988). The sections were rinsed with 1% acetic acid and mounted in 20% (v/v) glycerol. Lipofuscin content was quantified by digital image analysis of stained sections, as described for the LMS assay.
2.4.4. Ca2+-ATPase activity assay
This enzyme activity was quantified from cryostat sections obtained as for the LMS assay, using the histochemical method described by Pons et al. (2002). Cryostat sections were washed in 0.05M cacodylate buffer (pH 7.4), fixed in 1% paraformaldehyde (pFA) in 0.05 M cacodylate buffer (pH 7.4) for 30 min at 4 °C and washed again in 0.05M cacodylate buffer. Samples were then dehydrated in increasing acetone concentrations at 4°C and embedded in Technovit 7100 resin. Serial cross sections (2μm) were cut using a microtome, transferred onto glass slides and incubated for 6 h at room temperature in a medium containing 2.4 mM ATP, 18 mM CaCl2, 8 mM levamisole, 0.2 mM ouabain,1 mM Pb(NO3)2, and 20 mM sodium barbiturate. After incubation, the medium was removed and slides washed in water and rinsed in an ammonium sulfide-saturated water solution (3 min) to reveal the brown lead sulfide. Pb3(PO4)2 precipitates stained with ammonium sulfide was quantified on sections by digital imaging as described above.
2.4.5. Statistics
Cytochemical data (n = 6) were analyzed for difference between two groups, the reference site (Teboulba site) versus each other site, by the Student’s t-test, using the Instat 4.0 software (Graph Pad, USA).
3. RESULTS
Considering the total determinations of metals in sediments (Table 1), Bizerta Lagoon and Mahres are the most heavily contaminated sampling site. In fact, sediments from the latter two sites showed the highest content of Cd, Cu and Zn, respectively 0.77, 116.6 and 1208.9 µg/g dry weight (dw) from Bizerta Lagoon and 0.63, 105.32 and 1016.11 µg/g dw in samples from Mahres. Relatively high concentrations of these same metals were also observed in samples from Skhira. Teboulba showed the lowest heavy metal contents with only a level of 0.08 µg Cd/g dw, 12.49 µg Cu/g dw and 176.9 µg Zn/g dw.
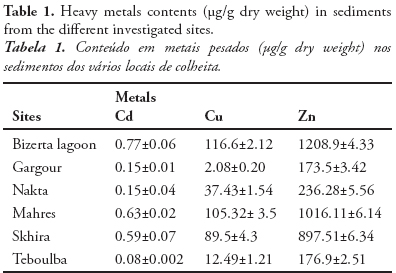
Evaluation of a stress syndrome in worms collected from Tunisian coasts was made by using a total of four biomarkers. Three of these are dedicated to the cellular level: lysosomal membrane stability (LMS), lysosomal contents of neutral lipids (NL) and lipofuscin (LF) and one to the tissue level: Ca2+-ATPase activity. A significant decrease in LMS with respect to controls (Teboulba site) was observed in intestinal cells of worms collected from each site, except for Nakta site (Fig. 2). LMS in polychaetes sampled in Mahres site is approximately 74% less then reference individuals. Worms from Bizerta, Gargour and Skhira have also showed a significant reduce of LMS compared to controls by respectively 43%, 50% and 64%.
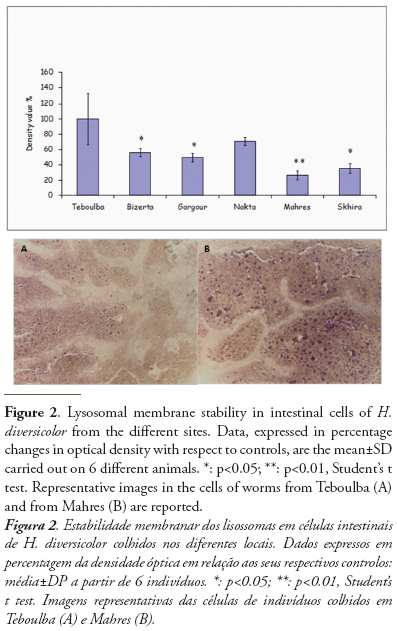
The results of neutral lipid (NL) and lipofuscin content in H. diversicolor are reported in figures 3 and 4.
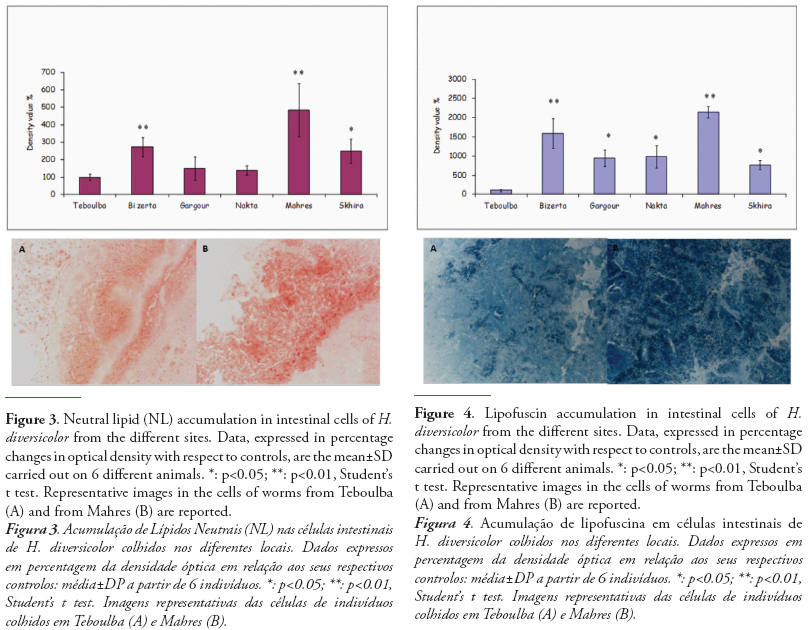
Lysosomal contents of neutral lipids and lipofuscin showed similar trend. In comparison to polychaetes from the reference site, contents of both parameters increased in the individuals sampled at each site then peaked at Mahres and were still high at Bizerta site. The content of lysosomal lipofuscin proved a more sensitive parameter than neutral lipids, with significantly higher levels in individuals from all the study area. For neutral content, significantly higher value with respect to reference worms were measured in individuals from Mahres (483%), from Bizerta (271%) and from Skhira (248%). A not significant increase was recorded in specimens collected at Gargour and Nakta.
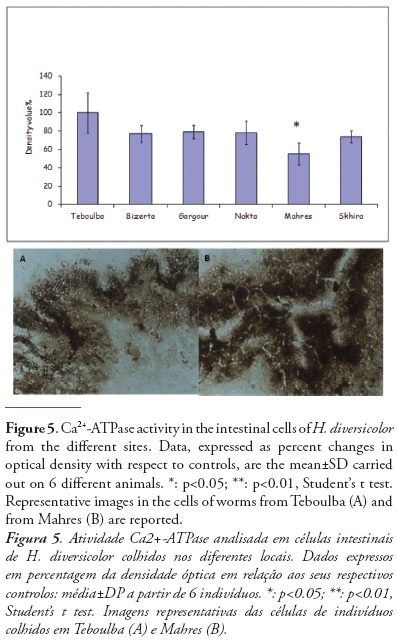
The results of Ca2+-ATPase activity is reported in figure 5. It has significantly decreased only in worms from Mahres (44%) with respect to reference individual.
4. DISCUSSION
The study of the biological responses of organisms to different environmental conditions and the quantitative evaluation of their physiological status are being considered as a successful approach for the assessment of environmental quality (Banni et al., 2009, 2010; Dondero et al., 2010). Invertebrates, such as polychaetes are suitable organisms for studying the biological effects of pollutants (Viarengo et al., 2007; Carvalho et al., 2013; Díaz-Jaramillo et al., 2013). In fact, the use of H. diversicolor as an experimental model is widely advised. This polychaete is an endobenthic worm species widespread in brackish water environments throughout Tunisian coasts. It is thus among the first species exposed to terrestrial pollutants, and consequently, environmental contamination occurs earlier and could be considered more accentuated than in bivalve species (Scaps, 2002).
In this study, we employed a battery of four biomarkers to polychaetes H. diversicolor that had been collected from polluted site along the Tunisian coast (Fig. 1). We selected six sites characterized by different levels of heavy metal contamination. Our result clearly showed different degrees of heavy metal loads in the sediment sampled along the Tunisian sites (Table 1). Zinc content was higher at Bizerta lagoon, Marhes and Skhira. Cadmium content was very low in Teboulba and still low in Gargour and Nakta, the latter three showing also the lowest copper amounts. However Bizerta lagoon, Skhira and Mahres were characterized by relative high heavy metal loads (Cd and Cu), as previously reported by several study on the Tunisian coast (Hamza-Chaffai & Pellerin, 2003; Banni et al., 2007; Bouraoui et al., 2010; Jebali et al., 2013). Based on the present chemical analysis and our previous results, we maintain Teboulba as a reference site and we confirm that Bizerta lagoon, Skhira and Mahres contains metal-tolerant populations of H. diversicolor. Numerous metals, among them Zn, Cu, and Cd, can be sequestered by invertebrates as electron-dense concretions. In invertebrates, these granules are present in all major phyla, including annelids (Bryan, 1974; Mouneyrac et al., 2003).
The lysosomal system of many marine organisms such as molluscs, annelids, crustaceans and fishes, is known to be particularly sensitive to environmental perturbations and, for this reason, its alterations are widely used as indicators of physiological stress. Lysosomes, particularly in the digestive cells, are involved in various cellular processes including digestion, defence, and reproduction (Moore, 1988; Viarengo et al., 2007). Moreover, they are actively implied in the detoxification metabolisms, being involved in the sequestration and accumulation of a wide range of chemicals, such as metal ions, polycyclic aromatic hydrocarbons PAH, pharmaceuticals as well as nanoparticles (Moore, 2006; Koehler et al., 2008).
Several reports have already demonstrated activation of lysosomes in response to environmental stress (Gastaldi et al., 2007; Banni et al., 2009; Catalano et al., 2012). All the lysosomal parameters evaluated in poychaetes digestive cells, were clearly affected by a pollution gradient. A few reports have already demonstrated activation of lysosomes in response to environmental stress in polychaetes H. diversicolor (Catalano et al., 2012; Moschino et al., 2014). The effects on lysosomal membrane stability in H. diversicolor were evaluated in digestive cells following the reaction for N-acetyl-β-hexosaminidase using histochemical procedures applied on frozen tissue sections. This method is currently chosen by many researchers; for example it was selected in the BEEP EU project in the Baltic Sea as well as in the MARS project (Broeg et al., 2002; Koehler et al., 2002). However, while membrane destabilization is considered as a parameter of general stress, an increase in the contents of neutral lipids and lipofuscin indicates a situation of oxidative stress leading to lipid peroxidation of biological membranes (Moore, 1988; Gorbi et al., 2012; Raftopoulou & Dimitriadis, 2012). In fact, Reactive Oxygen Species (ROS) produced in both physiological and stress conditions can stimulate the peroxidation of membrane lipids. The end-products accumulated within the lysosomes as insoluble pigments known as lipofuscins (LF) (Brunk & Collins, 1981). Measurement of lysosomal lipofuscin accumulation represents an index of peroxidation processes in a tissue (Moore et al., 2006). Another general response to contaminant-induced stress is the alteration of fatty acid metabolism and the lysosomal accumulation of high levels of unsaturated neutral lipids (NL) that are internalised into lysosomes by autophagic uptake (Moore, 1985).
The global trend of lysosomal responses in the present study (LMS, NL and LF), clearly indicates accelerated oxidative stress in intestinal cells as a result of increasing metals loads as demonstrated by the chemical analysis. A previous study performed by our group on H. diversicolor sampled at field sites along the Tunisian coast and based on a biomarker battery included NADPH cytochrome C reductase, glutathione-S-transferase, catalase and malondialdehyde has reported the same trend in these studied areas (Bouraoui et al., 2010). In fact, using the scale of classification based on biochemical markers established by Narbonne et al. (1999), our group showed that the most polluted site reflected by a higher multi-marker pollution index (MPI) is Mahres. The second MPI was recorded in Bizerta and Skhira and the lowest in Gargour and Nakta. Our Lysosomal responses data confirms those of other authors to emphasize the pollution statue of Sfax city coasted area due essentially to the presence of continuous discharge of heavy metals and also of organic compounds from local industrial activities (Smaoui- Damak et al., 2004; Jebali et al., 2007; Banni et al., 2009). As for the region of Bizerta, our results are also consistent with those of Dellali et al. (2001) which reported a remarkable pollution status of this site by pesticides (organophosphorous and carbamates) and heavy metals.
Finally, another parameter of general stress, Ca2+-ATPase was used. This enzyme is membrane SH-containing proteins that play a pivotal role in Ca2+ homeostasis. In the present work, Ca2+-ATPase activity is the less significant parameter to discriminate between sites (Fig. 5). The Ca2+-ATPase activity decreased levels of the intestinal cells of H. diversicolor from Mahres is, once again, an indication of oxidative stress induced by in field contaminant exposure. In fact, oxidative stress conditions and heavy metals such as Cu, Hg and Cd may lead to a partial inhibition of Ca2+-ATPases in both aquatic invertebrates, such as mussels and seaworms (Viarengo et al., 1998; Ermak & Davies, 2001; Burlando et al., 2004, Catalano et al., 2012) and terrestrial invertebrates such as earthworm Eisenia andrei (Gastaldi et al., 2007).
5. CONCLUSION
Biomarkers have been assessed and evaluated in many field surveys. However, studies on lysosomal responses remain scarce. Our results provide an important mean for characterizing the potential effects of contaminant impact on organisms living at polluted areas. This study has demonstrated that physiological effect, as represented by lysosomal biomarkers, is able to assess a stress syndrome and discriminate sites with various degrees of pollution. Our study may thus be used to better protect the health of Tunisian coasts to ensure sustainable management of coastal areas.
ACKNOWLEDGEMENTS
This study was supported financially by the Ministry of Higher Education and Scientific Research, Tunisia (Research Unit of Biochemistry and Environmental Toxicology, UR 04AGR05) and Institution of Agricultural Research and Higher Education (IRESA, Tunisia).
REFERENCES
Ait Alla, A.; Mouneyrac, C.; Durou, C.; Moukrim, A.; Pellerin, J. (2006) - Tolerance and biomarkers as useful tools for assessing environmental quality in the Oued Souss estuary (Bay of Agadir, Morocco). Comparative Biochemistry and Physiology Part C, 143(1):23-29. Doi: 10.1016/j.cbpc.2005.11.015. [ Links ]
Amiard, J.C.; Amiard-Triquet, C.; Barka, S.; Pellerin, J.; Rainbow, P.S. (2006) -Metallothioneins in aquatic invertebrates: Their role in metals detoxification and their use as biomarkers. Aquatic Toxicology, 76:160-202. Doi: 10.1016/j.aquatox.2005.08.015 [ Links ]
Banni, M.; Negri, A.; Dagnino, A.; Jebali, J.; Ameur, S.; Boussetta, H. (2010) - Acute effects of benzo[a]pyrene on digestive gland enzymatic biomarkers and DNA damage on mussel Mytilus galloprovincialis. Ecotoxicology and Environmental Safety, 73(5):842-848. Doi: 10.1016/j.ecoenv.2009.12.032 [ Links ]
Banni, M.; Bouraoui, Z.; Ghedira, J.; Clearandeau, C.; Jebali, J.; Boussetta, H. (2009) - Seasonal variation of oxidative stress biomarkers in clams Ruditapes decussatus sampled from Tunisian coastal areas. Environmental Monitoring and Assessment, 155(1-4):119-128. Doi: 10.1007/s10661-008-0422-3. [ Links ]
Banni, M.; Dondero, F.; Jebali, J.; Guerbej, H.; Boussetta, H.; Viarengo, A. (2007) - Assessment of heavy metal contamination using real-time PCR analysis of mussel metallothionein mt10 and mt20 expression: A validation along the Tunisian coast. Biomarkers, 12(4):369-383. Doi: 10.1080/13547500701217061. [ Links ]
Banni, M.; Jebali, J.; Daubeze, M.; Clerendeau, C.; Guerbej, H.; Narbonne, J.F.; Boussetta, H. (2005) - Monitoring Pollution in Tunisian coasts: Application of a classification scale based on biochemical markers. Biomarkers, 10(2-3):105-116. Doi:10.1080/13547500500107497. [ Links ]
Bartels-Hardege, H.D.; Zeeck, E. (1990) - Reproductive behaviour of Nereis diversicolor (Annelida: Polychaeta). Marine Biology, 106:409-412. Doi:10.1007/BF01344320. [ Links ]
Boujelben, B., (1998) - Dosage de métaux lourds (Plomb, Cadmium, Cuivre, Zinc) dans les poissons péchés dans la région de Sfax. 79p., Doctorat en Médecine Vétérinaire, Faculté de Médecine Vétérinaire de Sidi-Thabet, Tunisia. [ Links ]
Bouraoui, Z.; Banni, M.; Ghedira, J.; Clerandeau, C.; Narbonne, JF.; Boussetta, H. (2009) - Evaluation of enzymatic biomarkers and lipoperoxidation level in Hediste diversicolor exposed to copper and benzo[a]pyrene. Ecotoxicology and Environmental Safety, 72(7):1893-1898. Doi: 10.1016/j.ecoenv.2009.05.011 [ Links ]
Bouraoui, Z.; Banni, M.; Chouba, L.; Ghedira, J.; Clerandeau, C.; Jebali, J.; Narbonne, J.F.; Boussetta, H. (2010) - Monitoring pollution in Tunisian coasts using a scale of classification based on biochemical markers in worm Nereis (Hediste) diversicolor. Environmental Monitoring and Assessment, 164(1-4):691-700. Doi: 10.1007/s10661-009-0921-x. [ Links ]
Broeg, K.; Koehler, A.; Westernhagen, H.V. (2002) - Disorder and recovery of environmental health monitored by means of lysosomal stability in liver of European flounder (Platichthys flesus L.). Marine Environmental Research, 54(3-5):569-573. Doi:10.1016/S0141-1136(02)00174-5. [ Links ]
Brunk, U.T.; Collins, V.P. (1981) - Lysosomes and age pigments in cultured cells. In: R. S. Sohal (Ed.), Age pigments, pp. 243-264, Elsevier, Amsterdam, the Netherlands. ISBN: 978-0444802774 [ Links ]
Burlando, B.; Bonomo, M.; Capri, F.; Mancinelli, G.; Pons, G.; Viarengo, A. (2004) - Different effects of Hg2+ and Cu2+ on mussel (Mytilus galloprovincialis) plasma membrane Ca2+-ATPase: Hg2+ induction of protein expression. Comparative Biochemistry and Physiology. Part C: Toxicology & Pharmacology, 139: 201-207. Doi:10.1016/j.cca.2004.11.001. [ Links ]
Bryan, GW. (1974) - Adaptation of an estuarine polychaete to sediments containing high concentrations of heavy metals. In: F. J. Vernberg & W. B. Vernberg (eds), Pollution and physiology of marine organisms, pp. 123–135, Academic Press, New York, NY, U.S.A.
Carvalho, A.N.; Lino Vaz, A.S.; Boto Sérgio, T.I.; Talhadas dos Santos, P.J. (2013) - Sustainability of bait fishing harvesting in estuarine ecosystems - Case study in the Local Natural Reserve of Douro Estuary, Portugal. Journal of Integrated Coastal Zone Management, 13(2):157-168. Doi:10.5894/rgci393. [ Links ]
Catalano, B.; Moltedo, G.; Martuccio, G.; Gastaldi, L.; Virno-Lamberti, C.; Lauria, L.; Ausili, A. (2012) - Can Hediste diversicolor (Nereidae, Polychaete) be considered a good candidate in evaluating PAH contamination? A multimarker approach. Chemosphere, 86:875-882. Doi: 10.1016/j.chemosphere.2011.10.040 [ Links ]
Dean, HK. (2008) - The use of polychaetes (Annelida) as indicator species of marine pollution: a review. International. Journal of Tropical Biology and Conservation, 56(4):11-38. Available at http://www.redalyc.org/pdf/449/44919934004.pdf. [ Links ]
Dellali, M.; Gnassia-Barelli, M.; Roméo, M.; Aissa, P. (2001) - The use of acetylcholinesterase activity in Ruditapes decussatus, Mytilus galloprovincialis in the biomonitoring of Bizerta lagoon. Comparative Biochemistry and Physiology Part C, 130(2): 227-235. Doi: 10.1016/S1532-0456(01)00245-9. [ Links ]
Díaz-Jaramillo, M.; Da Rocha, A.M.; Chiang, G.; Buchwalter, D.; Monserrat, J.M.; Barra, R. (2013) - Biochemical and behavioral responses in the estuarine polychaete Perinereis gualpensis (Nereididae) after in situ exposure to polluted sediments. Ecotoxicology and Environmental Safety, 89:182-188. Doi: 10.1016/j.ecoenv.2012.11.026. [ Links ]
Dondero, F.; Negri, A.; Boatti, L.; Marsano, F.; Mignone, F.; Viarengo, A. (2010) - Transcriptomic and proteomic effects of a neonicotinoid insecticide mixture in the marine mussel (Mytilus galloprovincialis, Lam). Science of the Total Environment, 408(18):3775-3786. Doi: 10.1016/j.scitotenv.2010.03.040. [ Links ]
Dondero, F.; Dagnino, A.; Jonsson, H.; Capri, F.; Gastaldi, L.; Viarengo, A. (2006) - Assessing the occurrence of a stress syndrome in mussels (Mytilus edulis) using a combined biomarker/gene expression approach. Aquatic Toxicology, 78(1):13-24. Doi: 10.1016/j.aquatox.2006.02.025 [ Links ]
Ermak, G.; Davies, K.J. (2001) - Calcium and oxidative stress: from cell signaling to cell death. Molecular Immunology, 38(10):713-721. Doi: 10.1016/S0161-5890(01)00108-0. [ Links ]
Eriksen, K.D.H.; Daae, H.L.; Andersen, R.A. (1988) - Evidence of presence of heavy metal-binding proteins in polychaete species. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 91(2):377-384. Doi: 10.1016/0742-8413(88)90045-X [ Links ]
Gastaldi, L.; Ranzato, E.; Capri, F.; Hankard, P.; Peres, G.; Canesi, L.; Viarengo, A.; Pons, G. (2007) - Application of a biomarker battery for the evaluation of the sublethal effects of pollutants in the earthworm Eisenia andrei. Comparative Biochemistry and Physiology Part C, 146(3): 398-40. Doi:10.1016/j.cbpc.2007.04.014. [ Links ]
Gorbi, S.; Bocchetti, R.; Binelli, A.; Bacchiocchi, S.; Orletti, R.; Nanetti, L.; Raffaelli, F.; Vignini, A.; Accoroni, S.; Totti, C.; Regoli, F. (2012) - Biological effects of palytoxin-like compounds from Ostreopsis cf. ovata: A multibiomarkers approach with mussels Mytilus galloprovincialis. Chemosphere, 89(5):623-632. Doi:10.1016/j.chemosphere.2012.05.064 [ Links ]
Hamza-Chaffai, A.; Cosson, R. P.; Amiard-Triquet, C.; El Abed, A. (1995) - Physico-chemical forms of storage of metals (Cd, Cu and Zn) and metallothionein like protein in fish from the Tunisian coast, ecotoxicological consequences. Comparative Biochemistry and Physiology Part C, 111(2):329-341. Doi: 10.1016/0742-8413(95)00058-V. [ Links ]
Hamza-Chaffai, A.; Pellerin, J. C. (2003) - Health assessment of a marine bivalves Ruditapes decussatus from the Gulf of Gabés (Tunisia). Environment International, 28(7):609-617. Doi:10.1016/S0160-4120(02)00102-2. [ Links ]
Jebali, J.; Banni, M.; Alves de Almeida, E.; Boussetta, H. (2007) - Oxidative DNA damage levels in the clams Ruditapes decussatus as pollution biomarkers of Tunisian marine environment. Environmental Monitoring and Assessment, 124(1-3):195-200. Doi:10.1007/s10661-006- 9217-6. [ Links ]
Jebali, J.; Ben Khedher, S.; Sabbagh, M.; Kamel, N.; Banni, M.; Boussetta, H. (2013) - Cholinesterase activity as biomarker of neurotoxicity: utility in the assessment of aquatic environment contamination. Journal of Integrated Coastal Zone Management, 13(4):525-537. Doi: 10.5894/rgci430. [ Links ]
Koehler, A; Marx, U; Broeg, K; Bahns, S; Bressling, J. (2008) - Effects of nanoparticles in Mytilus edulis gills and hepatopancreas- a new threat to marine life? Marine Environmental Research, 66(1):12-4. Doi:10.1016/j.marenvres.2008.02.009. [ Links ]
Koehler, A.; Soeffker, K.; Wahl, E. (2002) - Functional and morphological changes of lysosomes as prognostic biomarker of toxic injury in a marine flatfish Platichthys flesus (L.). Environmental Toxicology and Chemistry, 21(11):2434-2444. Doi:10.1002/etc.5620211124. [ Links ]
Lowe, D.M.; Moore, M.N.; Clarke, K.R. (1981) - Effects of oil on digestive cells in mussels: quantitative alterations in cellular and lysosomal structure. Aquatic Toxicology, 1(3-4):213-226. Doi: 10.1016/0166-445X(81)90016-3 [ Links ]
Magni, P.; De Falco, G.; Falugi, C.; Franzoni, M.; Monteverde, M.; Perrone, E.; Sgro, M.; Bolognesi, C. (2006) - Genotoxicity biomarkers and acetylcholinesterase activity in natural populations of Mytilus galloprovincialis along a pollution gradient in the Gulf of Oristano (Sardinia, western Mediterranean). Environmental Pollution, 142(1):65-72. Doi:10.1016/j.envpol.2005.09.018. [ Links ]
Moore, M.N. (1976) - Cytochemical demonstration of latency of lysosomal hydrolases in digestive gland cells of the common mussel Mytilus edulis, and changes induced by thermal stress. Cell and Tissue Research, 175(3):279-287. Doi:10.1007/BF00218706. [ Links ]
Moore, M.N. (1985) - Cellular responses to pollutants. Marine Pollution Bulletin, 16(4):134-139. Doi: 10.1016/0025-326X(85)90003-7. [ Links ]
Moore, M.N. (1988) - Cytochemical responses of the lysosomal system and NADPH-ferrihemoprotein reductase in molluscan digestive cells to environmental and experimental exposure xenobiotics. Marine Ecology Progress Series, 46(1-3):81-89. Available at http://www.int-res.com/articles/meps/46/m046p081.pdf. [ Links ]
Moore, MN. (2006) - Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environment International, 32(8):967-76. Doi:10.1016/j.envint.2006.06.014. [ Links ]
Moschino, V.; Nestoa, N.; Barisonb, S.; Agresti, F.; Colla, L.; Fedele, L.; Da Ros, L. (2014) - A preliminary investigation on nanohorn toxicity in marine mussels and polychaetes. Science of the Total Environment, 468-469:111-119. Doi:10.1016/j.scitotenv.2013.08.020. [ Links ]
Mouneyrac, C.; Mastain, O.; Amiard, J.C.; Amiard-Triquet, C.; Beaunier, P.; Jeantet, A.Y.; Smith, B.D.; Rainbow, P.S. (2003) - Trace-metal detoxification and tolerance of the estuarine worm Hediste diversicolor chronically exposed in their environment. Marine Biology, 143:731-744. Doi: 10.1007/s00227-003-1124-6 [ Links ]
Narbonne, J.F.; Daubèze, M.; Clérandeau, C.; Garrigues, P. (1999) - Scale of classification based on biochemical markers in mussels: application to pollution monitoring in European coasts. Biomarkers, 4(6):415-424. Doi: 10.1080/135475099230589. [ Links ]
Narbonne, J.F.; Aarab, N.; Clérandeau, C.; Daubèze, M.; Narbonne, J.; Champeau, O.; Garrigues, P. (2005) - Scale of classification based on biochemical markers in mussels: application to pollution monitoring in Mediterranean coasts and temporal trends. Biomarkers, 10(1):58-71. Doi:10.1080/13547500500214392. [ Links ]
Nigro, M.; Falleni, A.; Del Barga, I.; Scarcelli, V.; Lucchesi, P.; Regoli, F.; Fren zilli, G. (2006) - Cellular biomarkers for monitoring estuarine environments: transplanted versus native mussels. Aquatic Toxicology, 77(4):339-347. Doi:10.1016/j.aquatox.2005.12.013. [ Links ]
Pons, G.; Evangelisti, V.; Capri, F.; Mozzone, S.; Viarengo, A. (2002) - Cytochemical localization and quantification of plasma membrane Ca2+- ATPase activity in mollusc digestive gland cells. European Journal of Histochemistry, 46(1):31-40. Available at http://www.ncbi.nlm.nih.gov/pubmed/12044046. [ Links ]
Raftopoulou, E.K; Dimitriadis, V.K. (2012) - Aspects of the digestive gland cells of the mussel Mytilus galloprovincialis, in relation to lysosomal enzymes, lipofuscin presence and shell size: Contribution in the assessment of marine pollution biomarkers. Marine Pollution Bulletin, 64(2):182-188. Doi:10.1016/j.marpolbul.2011.12.017. [ Links ]
Scaps, P. (2002) - A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor (O.F. Muller) (Annelida: Polychaeta). Hydrobiologia, 470(1-3):203-218. Doi:10.1023/A:1015681605656. [ Links ]
Smaoui-Damak, W.; Hamza-Chaffaia, A.; Bebianno, M.-J.; Amiard, J.-C. (2004) - Variation of metallothioneins in gills of the clam Ruditapes decussatus from the Gulf of Gabes (Tunisia). Comparative Biochemistry and Physiology, Part C: Toxicology & Pharmacology, 139(4)181-188. Doi: 10.1016/j.cca.2004.09.015. [ Links ]
UNEP/COI/AIEA/FAO. (1994) - Programme de surveillance continue des contaminants utilisant des organismes marins. Assurance de la qualité et bonnes pratiques de laboratoire. Méthodes de référence pour les études de la pollution marine no. 57, PNUE, 25 pp. Available at http://195.97.36.231/acrobatfiles/ NonMAP/RefMethods/57fre.pdf. [ Links ]
Viarengo, A.; Canesi, L.; Pertica, M.; Livingstone, D.R. (1991) - Seasonal variations in the antioxidant defence systems and lipid peroxidation of the digestive gland of mussels. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 100(1-2):187-190. Doi:10.1016/0742-8413(91)90151-I. [ Links ]
Viarengo, A.; Blasco, J.; Burlando, B.; Ponzano, E.; Marchi, B.; Trielli, F. (1998) - Metallothionein and oxidative stress in marine organisms. Marine Environmental Research, 46(1):606-607. Doi:10.1016/j. cbpc.2007.04.011. [ Links ]
Viarengo, A.; Lowe, D.; Bolognesi, C.; Fabri, E.; Koehler, A. (2007) - The use of biomarkers in biomonitoring: A 2-tier approach assessing the level of pollutant induced stress syndrome in sentinel organisms. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 146(3):281-300. Doi.org/10.1016/j.cbpc.2007.04.011.
*Submission: 2 January 2014; Evaluation: 9 February 2014; Reception of revised manuscript: 17 February 2014; Accepted: 14 March 2014; Available on-line: 21 March 2014
**Tradução para Português do Título, Resumo e Legendas das Figuras e Tabelas da responsabilidade do Editor Associado Ulisses Miranda Azeiteiro.














