Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Gestão Costeira Integrada
versão On-line ISSN 1646-8872
RGCI vol.14 no.1 Lisboa mar. 2014
https://doi.org/10.5894/rgci435
ARTICLE / ARTIGO
Population biology of Callichirus major (Say, 1818) (Crustacea: Callianassidae) at Piedade Beach, Brazil*
Biologia populacional de Callichirus major (Say, 1818) (Crustacea: Callianassidae) na Praia de Piedade, Brasil
Flavio de Almeida Alves-JúniorI, Marina de Sá Leitão Câmara de AraújoII, Petrônio Alves Coelho†
@Corresponding author
ILaboratório de Carcinologia, Museu de Oceanografia da Universidade Federal de Pernambuco (UFPE). Av. Arquitetura, s/n, Cidade Universitária, Recife – PE. E-mail: bioflavio@hotmail.com (FAAJ)
IIDepartamento de Ciências Exatas e Naturais, Faculdade de Ciências, Educação e Tecnologia de Garanhuns, Universidade de Pernambuco (UPE). Rua Capitão Pedro Rodrigues, 105, São José, Garanhuns - PE. E-mail:mslc.araujo@gmail.com (MSLCA)
RESUMO
O objetivo deste trabalho foi estudar a ecologia populacional de Callichirus major (Say, 1818) na praia de Piedade, Estado de Pernambuco, Brasil, através da análise de parâmetros como proporção sexual, período reprodutivo e o recrutamento de juvenis nesta população. As coletas foram realizadas mensalmente entre dezembro de 2010 e novembro de 2011. A temperatura do ar e das tocas, além da salinidade da água do mar foram medidas in situ. O teste t de Student foi aplicado para comparar os valores dos fatores abióticos entre as estações seca e chuvosa. A proporção sexual foi analisada para cada mês e durante todo o período estudado, e o teste do qui-quadrado foi aplicado para verificar se desvia significativamente da proporção esperada. O período reprodutivo foi determinado com base na frequência de fêmeas ovígeras. O recrutamento foi obtido com base na frequência de indivíduos imaturos. A influência de fatores abióticos na população foi avaliada através do coeficiente de Pearson. A temperatura do ar e das tocas variou significativamente entre os períodos seco e chuvoso, com valores mais elevados no período seco. Um total de 389 indivíduos C. major foram amostrados (174 ♂ e 215 ♀). A razão sexual foi de 1:1,24 (♂:♀), as fêmeas foram mais abundantes que os machos. No presente estudo, foi observado que o pico da fase reprodutiva e frequência de fêmeas ovígeras de C. major ocorreu na estação seca (verão equatorial). O recrutamento de jovens na população ocorreu durante todo o ano, especialmente entre o verão e o outono. De acordo com a matriz de correlação, o fator abiótico mais influente na abundância de C. major, especialmente em fêmeas ovígeras, é a temperatura das tocas. Este estudo cria uma linha de base para futuras pesquisas com C. major no Nordeste do Brasil.
Palavras-chave: Axiidea, camarão fantasma, ecologia de praias arenosas, período reprodutivo.
ABSTRACT
The aim of this paper was to study the population ecology of Callichirus major (Say, 1818) at Piedade Beach, State of Pernambuco, Brazil, through the analysis of parameters such as sex ratio, reproductive period and recruitment of juveniles into this population. Sampling was taken monthly from December 2010 to November 2011. The burrow and air temperatures, as well as the sea water salinity, were measured in situ. A Student t test was applied to compare the value of abiotic factors between the dry and rainy seasons. The sex ratio was analyzed for each month and for the total studied period, and a Chi-square test was applied to verify if it deviated significantly from the expected proportion. The reproductive period was determined based on the frequency of ovigerous females. The recruitment was obtained based on the frequency of non-mature individuals. The influence of the abiotic factors was evaluated through a Pearson’s coefficient. The air and burrow temperatures varied significantly between the dry and rainy periods, with the highest values in the dry period. A total of 389 individuals of C. major (174 ♂ and 215 ♀) were sampled. The sex ratio was 1:1.24 (♂:♀), with females being more abundant than males. In this study, the most active reproductive period of C. major and frequency of ovigerous females was observed in the dry period (equatorial summer). Recruitment of juveniles into the population occurred throughout the year, between the summer and the autumn. According to the correlation matrix, the main abiotic factor influencing the abundance of C. major, especially of ovigerous females, was the burrow temperature. This study creates a baseline for further research with C. major in Northeastern Brazil.
Keywords: Axiidea, ecology of sand beaches, ghost shrimp, reproductive period.
1. INTRODUCTION
Macrocrustaceans are important components of coastal ecosystem communities, with an important role in artisanal, commercial and recreational fisheries. Besides their importance for human consumption, they represent an important food resource for many carnivores, both in the larval or adult phases (Teixeira & Sá, 1998).Studies on these marine and estuarine animals are fundamental to understand their ecology and to raise data for maintenance of natural populations, through conservation mechanisms (Araújo et al., 2011), and focuses the following aspects: abundance, sex ratio, reproductive period and recruitment of juveniles (Noro & Buckup, 2008; Araújo et al., 2012). As examples, Araújo & Calado (2008) studied the population biology of Ucides cordatus (Linnaeus, 1763), and Bezerra et al. (2010), who described the spatial distribution of Uca maracoani (Latreille, 1802-1803).
Reproduction is one of the most important phenomena in the life of a given species (Cobo & Fransozo, 2000). Besides the hormonal control, in many crustaceans, reproductive processes are related to environmental conditions (Laufer & Landau, 1991; Quackenbush, 1994; Fingerman, 1995). After spawning, the larvae usually undergo a series of molts in the open sea and then return to the coast to settle. The recruitment of marine benthos is defined as the number of new individuals that settle and survive in the substrate (Keough & Downes, 1982; Caley et al., 1996). In the case of species with planktonic larvae, it implies the transformation of its habit to a benthic form and survival until the time of settlement (Done, 1982).
Burrowing crustaceans of the Infraorder Axiidea have as their main ecological feature the construction of deep galleries in the sandy substrate of shallow waters (Griffis & Chavez, 1988). Their presence is detected by small apertures frequently surrounded by fecal pellets (Weimer & Hoyt, 1964; Frankenberg et al., 1967; Rodrigues & Shimizu, 1997). Among species of the family Callianassidae, Callichirus major (Say, 1818),popularly known as ghost shrimps and ‘corrupto’ in Portuguese, stands out. Their distribution includes the littoral of the Western Atlantic, from North Carolina State, United States of America, to Santa Catarina State, Brazil (Coelho & Ramos-Porto, 1986; Manning & Felder, 1986; Coelho, 1997; Melo, 1999).
Studies with species of Callianassids are extremely important due to their utilization as bait in coastal areas. Thus, the removal of these burrowing organisms may interfere with the local environment, causing impacts in the target species or in the organisms of the local meiofauna, because the removal revolves the structure of the sediment, causing the death of these individuals (Wynberg & Branch, 1991). The overexploitation of the target species may cause alterations in the density of the animal or even its disappearance (Borzone & Souza, 1996; Rodrigues & Shimizu, 1997).
In Brazil, the reproductive patterns of the callianassids are poorly documented, despite of the importance of this group in coastal benthic communities. Studies on C. major have been mainly from the Southern and Southeastern Brazilian coasts (Borzone & Souza, 1996; Shimizu, 1997; Rodrigues & Shimizu, 1997; Souza et al., 1998; Souza & Borzone, 2003), areas with distinct climatic conditions when compared to the North and Northeast Brazilian coasts, which results in different inter and intraspecific relations (Rodrigues, 1985). In the Northeast of Brazil, studies on this species were accomplished by Araújo et al. (2000), at Sobral Beach, Alagoas State, and by Botter-Carvalho (2001) and Botter-Carvalho et al. (2007), at Piedade Beach, Pernambuco State. Since these studies, there have been no others from the coastal Pernambuco segment. Thus, studies on reproduction, recruitment and sex ratio of these animals are still scarce.
The aim of this paper was to analyze some aspects of the life cycle of C. major, including the abundance, sex ratio, reproductive period and recruitment at Piedade Beach, Pernambuco, and to compare the results with the available literature.
2. MATERIALS AND METHODS
2.1.Study area
The municipality of Jaboatão dos Guararapes is located at the South of Pernambuco, Northeast of Brazil (Fig. 1). It has a megathermal climate with rainfall concentrated from March to August and a well-defined dry period (September to February), characterizing the As’ climate (Hot Humid Tropical) (Köeppen, 1948; Cavalcanti & Kempf, 1967/69). Piedade beach is located at 8° 09’ 40.80” S and 34° 54’ 08.98” W, and is considered to have intermediate dynamics, with the presence of beachrocks in the intertidal zone.
2.2.Sampling
Samplings were under taken monthly from December 2010 to November 2011, in the intertidal zone of Piedade beach, Pernambuco, Brazil, except in September, since no ghost shrimp was found at the beach. Three equidistant points were selected in the initial, median and final portions of the beach: P01 (8° 08’ 47.22” S and 34° 54’ 19.03” W), P02 (8° 54’ 39.11” S and 34° 54’ 19.03” W) and P03 (8° 10’ 30.47” S and 34° 54’ 56.99” W). At each point, four transects of 1m² were sampled, being distant 1m from each other, until the waterline of spring low tide (new moon).The animals were monthly sampled with one suction pump (Hailstone & Stephenson, 1961; Rodrigues, 1976), i.e. the number of pumps was always the same. They were preserved in 70% and transported to the laboratory. Some individuals were integrated into the carcinological collection of the Museu de Oceanografia Petrônio Alves Coelho (voucher number MOUFPE 14.863). The burrow temperature was measured with a digital thermometer, and the sea water salinity, with a refractometer. The air temperature and the rainfall were obtained at the ITEP/LAMEPE (Instituto de Tecnologia de Pernambuco - Laboratório de Meteorologia de Pernambuco).
2.3.Laboratory procedures
At the laboratory, the individuals were identified and sexed according to Melo (1999). They were classified as juvenile males, juvenile females, adult males, non-ovigerous females and ovigerous females. The total length (TL), from the rostrum to the telson, was measured with a vernier caliper (0.01 mm).
Statistical analysis
The minimum, mean ± standard deviation and maximum values of the abiotic parameters (burrow temperature, air temperature, sea water salinity and rainfall) were estimated. The Student t test was applied to compare the abiotic factors between the dry and rainy periods.
The sex ratio was analyzed by months and by the total study period, and a Chi-square test was applied to determine if the sex ratio deviated significantly from the expected proportion (1:1) (χ2= 3.84).
The distribution by size class was obtained(using 5 mm size classes), to determine the mode of each sex. Individuals with TL smaller than 40 mm were considered juveniles (based on Alves-Júnior et al., 2013), based on the visual observations of maturity.
The determination of the reproductive period was based on the monthly percentages of ovigerous females (Vazzoler, 1996). The reproduction was characterized, according to Pinheiro & Fransozo (2002), as: seasonal (ovigerous females in only some months or seasons), continuous (ovigerous females in all months with similar intensity) or seasonal-continuous (ovigerous females in all months, with distinguishable peaks of high reproductive activity in some of them).
The monthly proportion of juveniles and adults was estimated and a Chi-square test was applied to determine if they differed significantly from the expected proportion (1:1) (χ2= 3.84). The determination of the recruitment period was based on the months where the juveniles were significantly more abundant than adults.
A correlation matrix, with Pearson’s coefficient of linear correlation (r) was applied to verify the influence of the abiotic factors in the total abundance of C. major, as well as in the abundance of ovigerous females.
All statistical analyses were performed at α = 0.05.
3. RESULTS
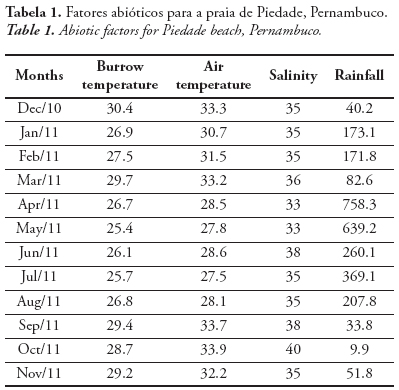
The burrow temperature varied from 25.4 to 30.4 °C (27.7 ± 1.6 °C). The air temperature varied from 27.5 to 33.9 °C (30.7 ± 2.5 °C). The salinity varied from 33.0 to 40.0 (35.6 ± 2.0) and the rainfall, from 9.9 to 758.3 mm (75.8 ± 89.6 mm) (Tab. 1). The following abiotic factors varied significantly between dry and rainy periods: burrow temperature (t = 2.36; p = 0.03), air temperature (t = 4.15;p = 0.01) and rainfall (t = 2.00; p = 0.01). The burrow and air temperatures were higher in the dry period, and the rainfall was higher in the rainy period. The salinity, however, did not vary significantly between dry and rainy periods (t = 1.13;p = 0.28).
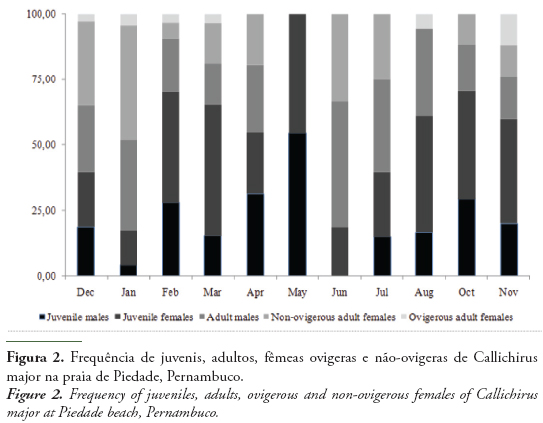
A total of 389 individuals of C. major were sampled during the study period,174 males (80 juveniles and 94 adults) and 215 females (114 juveniles and 101 adults, of which11 were ovigerous females). From these ghost shrimps, 202 were sampled in the dry period, and 183 in the rainy one. The sex ratio was 1:1.24 (♂:♀), with females being significantly more abundant than males (χ2 = 4.37; p < 0.05), especially in March (χ2 = 8.34; p < 0.05) (Fig. 2), when the females represented 68.97% of the ghost shrimps.
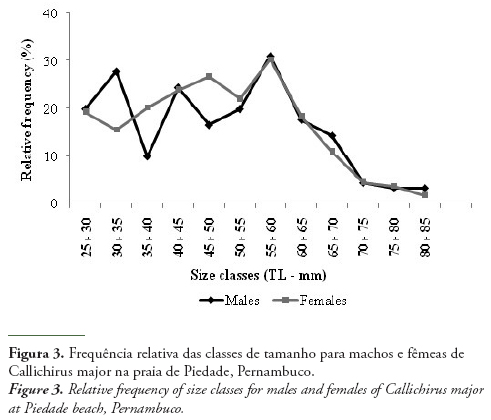
The mean TL was 50.56 ± 13.77 mm for males (Min. 27.10 mm and Max. 90.10 mm) and 47.55 ± 13.40 mm for females (Min. 25.40 mm and Max. 87.30 mm). Considering the distribution by size class, it could be observed that males were more frequent at 30 ˫ 35 and 55 ˫ 60 mm, while females were frequent at 45 ˫ 50 and 55 ˫ 60 mm (Fig. 3).
The frequency of ovigerous females (Fig. 2) was higher in the months of December 2010 to March and November 2011, mostly months of the dry period. A small peak was observed in August, transition from the rainy to the dry period.
The recruitment of juveniles into the population (Fig. 2) occurred in all months of the year, especially in February, March and May 2011, between the summer and the autumn, when the juveniles were significantly more frequent than adults (χ2 = 9.62, 4.41 and 11.00, respectively; p < 0.05). In April and from July to November 2011, no difference in the frequency of juveniles and adults was detected. The adults were significantly dominant in December 2010, January and June 2011 (χ2 = 9.14, 9.78 and 14.29, respectively; p < 0.05).
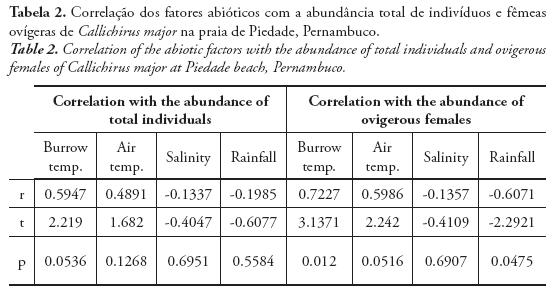
According to the correlation matrix, the abiotic parameter that most influenced the total abundance and abundance of ovigerous females of C. major was the burrow temperature (Tab. 2). Although not significant, it was considered regularly correlated. However, considering only the ovigerous females, the correlation with the burrow temperature was considered strong and significant. The air temperature was also positively correlated, despite not significant, to the abundance of total individuals and ovigerous females. The rainfall was negatively correlated to the abundance of ovigerous females, being significant. Positive correlations mean that the parameters are related to an increase in the animal abundance, while negative correlations, cause the decrease in the animal abundance.
4. DISCUSSION
The air and burrow temperatures varied significantly between dry and rainy periods, with the highest values in the dry period (September and February). Thus, the temperature followed the seasonal pattern of the littoral of Pernambuco and the Northeast of Brazil, corroborating Aragão (1998), Botter-Carvalho (2001) and Cavalcanti et al. (2006). According to Cavalcanti & Kempf (1969), salinity was lowest in the rainy period, and highest, in the dry period, such as observed in this study. However, no significant difference was observed between the periods. In this study, the rainfall differed significantly between dry and rainy periods, and according to Cavalcanti & Kempf (1967/69), there is an inverse relation between salinity and rainfall. According to the classification of Köeppen (1948), the climate of the metropolitan region of Recife is of the type As’, with rains from March to August, showing that during this period there is a greater input of freshwater in the sea, influencing the salinity. In the dry period, from September to February, the salinity of the coastal surface water remains relatively uniform.
Studies with callianassids show deviations in the sex ratio, with females being more abundant in the populations (Tunberg, 1986; Dworschak, 1988; Hanekom & Baird, 1992; Dumbauld et al., 1996; Rodrigues & Shimizu, 1997; Shimizu, 1997; Araújo et al., 2000). The sex ratio observed in our study corroborates these studies. At Piedade beach, however, Botter-Carvalho et al. (2007) observed a sex ratio of 0.98 ♂:1.0 ♀, with no significant differences between sexes. However, they observed monthly oscillations in the sex ratio; the females were more abundant from September to February and the males, from March to September, and in this study, the females were more abundant in March, with ovigerous females in March and November, and males more abundant in August, October, February and May. Similar results were observed by Hernáez & Wehrtmann (2007) for Callichirus seilacheri (Bott, 1955), with no significant differences in the annual sex ratio, but with females significantly more abundant than males in January and September. According to Wenner (1974), the deviations in the sex ratio can be a result of the differences in the life cycle, migration, mortality and growth. These deviations may also be due to biogeographic factors, as well as intra and interspecific factors (Rodrigues, 1985).
The capture of individuals with a suction pump can result in an undesirable sampling selectivity. The ovigerous females move to the most superior part of the galleries to release the eggs or to improve their ventilation (Nates & Felder, 1999; Botter-Carvalho et al., 2007),thus, they could be captured more easily. This is supported by Rowden & Jones (1994), for Callianassa subterranea (Montagu, 1808), where adult females were captured more frequently, since they occupied the higher portion of the burrows. Agonistic behavior occurs in adult males of many callianassids (Tunberg, 1986; Felder & Lovett, 1989; Tamaki et al., 1997; Shimoda et al., 2005), including C. major (Rodrigues & Shimizu, 1997). Thus, the males can build deeper burrows than females, and may also escape with greater agility. Both phenomena also explain the higher proportion of females in the population. Nevertheless, the burrows of the juveniles are shallower and they do not have the escape capacity as the adults. Thus, they are easily captured, which may explain their high abundance at Piedade beach, as also observed by Witbaard & Duineveld (1989) for Callianassa subterranea in the North Sea. Of the Callianassidea species studied in Brazil, females were larger than males in Neocallichirus mirim (Rodrigues, 1971) (Pezzuto,1993) and in C. major. That is not the pattern for all these animals, and males were significantly larger than females in many other studies, such as Hailstone & Stephenson (1961), Forbes (1973), Tamaki et al. (1996) and Shimizu (1997).
The distribution of ghost shrimps by size class was not normal, i.e., did not follow a Gaussian distribution. Besides, a decline in the frequency of individuals from 60 mm was observed. During the field sampling, a large number of fishermen was observed at the area, and they selected the larger ghost shrimps to use as live bait. This superexploitation of the species may not allow the population to reach their maximum sizes. Hernáez & Wehrtmann (2007) showed that the fishing of C. seilacheri to use as live bait considerably affected its reproduction and distribution in intertidal and sublittoral areas.
In this study, the highest frequency of ovigerous females was observed in the dry period (equatorial summer - September and February) (Fig. 2), and in the transition between the rainy period (equatorial winter- March and August) and the summer, in Northeast Brazil (Cavalcanti & Kempf, 1967/69). Araújo et al. (2000) and Botter-Carvalho et al. (2007) observed a similar period for C. major as well (Table 3). According to Botter-Carvalho (2001), the reproductive period of C. major is associated with the summer months in the Brazilian Northeast, where the highest temperatures were observed from December of 2010 to February of 2011, decreasing in March of 2011. Reproductive periods usually occur in seasons with high temperatures, since the temperature favors the sexual activity and embryonic development, as described by Hill (1977) for Upogebia africana (Ortmann, 1894) and Tamaki et al. (1996) for Nihonotrypaea japonica (Ortmann, 1891). Low temperatures are related to the diminution of the burrowing activity of callianassids. According to Posey (1986), low temperatures reduce the metabolism of ghost shrimps. Felder & Griffis (1994) also found higher densities of Lepidophthalmus louisianensis (Schmitt, 1935) at Mississippi, and Bilodeau et al. (2005), for Callichirus islagrande (Schmitt, 1935) at Louisiana, United States of America, in the dry period, as observed in the present study, showing that high temperatures favor the burrowing activity, reproduction and recruitment of the species.
In this study, the total abundance of C. major was not related to salinity, but the abundance of ovigerous females was negatively correlated. According to Hill (1977) and Posey (1986), salinity can exert a strong effect in the population of callianassids, due to its seasonal variations.
Seasonal changes in the density of C. major in sandy beaches are attributed to changes in the sediment deposition and coastal erosion that alters the beach profile (Botter-Carvalho et al., 2002) and events of recruitment (Tamaki & Ingole, 1993; Dumbauld et al., 1996). Souza et al. (1998) observed intense recruitment at the beginning of the summer in beaches in the State of Paraná, while Rodrigues & Shimizu (1997) found intense recruitment in October and in June (spring and autumn, respectively) at Barequeçaba beach, State of São Paulo. The seasonal variations of juveniles in a population are natural for callianassids, due to predation, environmental and biogeographic factors acting on the larval phases (Rodrigues, 1976). In this study, recruitment was intensified between the summer and autumn, as a result of reproductive activity in the summer. The embryonic development of C. major occurs in 10 stages during 30 days (Rodrigues, 1976). Larval development has from 3 to 5 zoeal stages withduration of 10 to 15 days to reach the decapodid stage (Strasser & Felder, 1999; Abrunhosa et al., 2008). Such short larval development explains the peak of recruitment right after the peak of ovigerous females. Annual variations in the quantity of recruits are also related to the degree of overexploitation of the population (Souza & Borzone, 2003). The sampling of adults favors the settlement of juveniles, since it reduces the competition for space in the substratum (Rodrigues & Shimizu, 1997).
We can conclude that the females are more abundant in the population, which is a pattern for callianassids of the Brazilian coast. A well-defined reproductive period in the summer was observed, when high temperatures favor the reproductive processes and contribute to the larval development of the animal. The recruitment of the species occurs in almost the entire study period, but it is intensified right after the reproductive peak, due to the short larval development of this species. This paper creates a baseline for further researches on C. major in Northeastern Brazil.
REFERENCES
Abrunhosa, F.A.; Arruda, D.C.B.; Simith, D.J.B.; Palmeira, C.A.M. (2008) - The importance of feeding in the larval development of the ghost shrimp Callichirus major (Decapoda: Callianassidae). Anais da AcademiaBrasileira deCiências (ISSN 0001-3765), 80(3):445- 453, Rio de Janeiro, RJ, Brasil. Available at http://www.scielo.br/pdf/aabc/v80n3/a06v80n3.pdf [ Links ]
Alves-Júnior, F.A.; Araújo, M.S.L.C.; Feitosa, F.A.N. (2013) - Crescimento Alométrico de Callichirus major (SAY 1818) (Crustacea: Callianassidae) em uma praia arenosa do Nordeste brasileiro.Tropical Oceanography (ISSN: 1679-3013), 41. [no prelo]. Recife, PE, Brasil. [ Links ]
Aragão, J.O.R. (1998) - O impacto do Enso e do Dipolo do Atlântico no Nordeste do Brasil. Bulletin de l’Institut Français d’Études Andines (ISSN: 0303-7495), 27(3): 839-844, Lima, Perú. Available at http://www.ifeanet.org/publicaciones/boletines/27(3)/839.pdf
Araújo, J.S.; Calado, T.C.S.; Sá, M.F.P. (2000) - Aspectos reprodutivos de Callichirus major (SAY 1818) (Crustacea: Callianassidae) da praia do Sobral, Maceió- Alagoas. Boletim de Estudos de Ciências do Mar (ISSN: 0102-8596), 11:101-112, Maceió, AL, Brasil. [ Links ]
Araújo, M.S.L.C.;Calado, T.C.S.(2008) - Bioecologia do Caranguejo-Uçá Ucides cordatus (Linnaeus) no Complexo Estuarino Lagunar Mundáu/Manguaba (CELMM), Alagoas, Brasil. Revista da Gestão Costeira Integrada, 8:169-181. doi:10.5894/rgci141 [ Links ]
Araújo, M.S.L.C.; Negromonte, A.O.; Barreto, A.V. (2011) - Reproductive period of the swimming crab Callinectes danae at the Santa Cruz Channel, a highly productive tropical estuary in Brazil. Nauplius (ISSN: 0104-6497), 19(2):155-162, Cruz das Almas, BA, Brasil. Available at http://www.crustacea.org.br/artigos/830_27_Article_7_Araujo_et_al_Reproduction_of_Callinectes_danae.pdf [ Links ]
Araújo, M.S.L.C.; Barreto, A.V.; Negromonte, A.O.; Schwamborn, R. (2012) - Population ecology of the blue crab Callinectes danae (Crustacea: Portunidae) in a Brazilian tropical estuary. Anais da AcademiaBrasileira deCiências, 84(1):129-138. DOI: 10.1590/S0001-37652012000100013 [ Links ]
Bezerra, L.E.A.;Dias, C.B.; Moraes, J. O.; Matthews-Cascon, H. (2010) - Distribuição espacial do caranguejo Uca maracoani (Latreile, 1802-1803) (Brachyura: Ocypodidae) em três manguezais do Nordeste do Brasil. Revista da Gestão Costeira Integrada, Número especial 2, Manguezais do Brasil. No prelo. Available at http://www.aprh.pt/rgci/pdf/rgcimang63_Bezerra.pdf [ Links ]
Bilodeau, A.L.; Felder, D.L.; Neigel, J.E. (2005) - Multiple paternity in the thalassinidean ghost shrimp, Callichirus islagrande (Crustacea: Decapoda: Callianassidae). Marine Biology, 146:381-385. DOI: 10.1007/s00227-004-1444-1 [ Links ]
Borzone, C.A.; Souza, J.R.B. (1996) - A extração de corrupto Callichirus major (Decapoda: Callianassidae) para uso como iscas em praias do litoral do Paraná: características da pesca. Nerítica (ISSN: 1806-969X), 10:67-79, Pontal do Sul, PR, Brasil. [ Links ]
Botter-Carvalho, M.L. (2001) - Ecologia de Callichirus major (Say 1818) (Crustacea, Callianassidae) na praia de Piedade, Jaboatão dos Guararapes-PE.Recife (PE). 114p., Dissertação de Mestrado em Biologia Animal, Universidade Federal de Pernambuco,Brasil. Não publicado. [ Links ]
Botter-Carvalho, M.L.; Santos, P.J.P.; Carvalho, P.V.V.C. (2002) - Spatial Distribuition of Callichirus major (Say 1818) (Decapoda: Callianassidae) on a sandy beach, Piedade, Pernambuco, Brazil. Nauplius (ISSN: 0104-6497), 10(2): 97-109, Cruz das Almas, BA, Brasil. Available at http://decapoda.nhm.org/pdfs/13801/13801.pdf [ Links ]
Botter-Carvalho, M.L.; Santos, P.J.P.; Carvalho, P.V.V.C. (2007) - Population dynamics of Callichirus major (Say, 1818) (Crustacea, Thalassinidea) on a beach in northeastern Brazil. Estuarine, Coastal and Shelf Science (ISSN: 0272-7714), 71:508-516. doi:10.1016/j.ecss.2006.09.001 Available at http://decapoda.nhm.org/pdfs/27735/27735.pdf [ Links ]
Caley, M.J.; Carr, M.H.; Hixon, M.A.; Hughes, T.P.; Jones, G.P.; Menge, B.A. (1996) - Recruitment and the local dynamics of open marine populations. Annual Review of Ecology and Systematics, 27:477-500. doi: 10.1146/annurev.ecolsys.27.1.477 [ Links ]
Cavalcanti, L.B.; Kempf, M. (1967/69) - Estudo da Plataforma Continental na Área do Recife (Brasil). II. Meteorologia e Hidrologia. Trabalhos Oceanográficos da Universidade Federal de Pernambuco (ISSN: 0374-0412), 9(11):149-158, Recife, PE, Brasil. Available at http://www.ufpe.br/tropicaloceanography/resumos/9_10_11_cavalcanti.html [ Links ]
Cavalcanti, E.P.; Silva, V.P.R.; Sousa, F.A.S. (2006) - Programa computacional para a estimativa da temperatura do ar para a Região Nordeste do Brasil. Revista Brasileira de Engenharia Agrícola e Ambiental (ISSN: 1415-4366), 10:140-147, Campina Grande, PB, Brasil. Available at http://www.scielo.br/pdf/rbeaa/v10n1/v10n1a21.pdf [ Links ]
Cobo, V.J.; Fransozo, A. (2000) - Fecundity and reproduction period of the red mangrove crab Goniopsis cruentata (Brachyura, Grapsidae) São Paulo State, Brazil. In: Klein, J.C.V. and Schram ,F.R. (org.), Crustacean Issues, The Biodiversity Crisis and Crustacea, Vol.12, pp. 527-533, A.A. Balkema, Rotterdam, the Netherlands. ISBN: 9054104783. [ Links ]
Coelho, P.A. (1997) - Revisão das espécies de Thalassinidea encontradas em Pernambuco, Brasil (Crustacea, Decapoda). Trabalhos Oceanográficos da Universidade Federal de Pernambuco (ISSN: 0374-0412), 25:137-161, Recife, PE, Brasil. Available at http://www.revista.ufpe.br/tropicaloceanography/artigos_completos_resumos_t_d/25_1997_coelho_5.pdf [ Links ]
Coelho, P.A.; Ramos-Porto, M. (1986) - Sinopse dos crustáceos decápodos brasileiros (Familias Callianassidae, Callianadeidae, Upogebiidae, Diogenidae). Trabalhos Oceanográficos da Universidade Federal de Pernambuco (ISSN: 0374-0412), 19:27-53 , Recife, PE, Brasil. Available at http://www.revista.ufpe.br/tropicaloceanography/artigos_completos_resumos_t_d/19_1985_1986_coelho.pdf [ Links ]
Done, T.J. (1982) - Patterns in the distribution of coral communities across the central great barrier reef. Coral Reefs, 1:95-107. doi:10.1007/BF00301691 [ Links ]
Dumbauld, B.R.; Armstrong, D.A.; Feldman, K.L. (1996) - Life history characteristics of two sympatric thalassinidean shrimps, Neotrypaea californiensis and Upogebia pugettensis, with implications for oyster culture. Journal of Crustacean Biology (ISSN: 0278-0372), 16:689-708, New Braunfels, TX, USA. Available at http://decapoda.nhm.org/pdfs/15596/15596.pdf [ Links ]
Dworschak, P.C. (1988) - The biology of Upogebia pusilla (Petagna)(Decapoda, Thalassinidea) Growth and production. Marine Ecology, 9(1):51-77. doi:10.1111/j.1439-0485.1988.tb00198.x [ Links ]
Felder, D.L.; Lovett, D.L. (1989) - Relative growth and sexual maturation in the estuarine ghost shrimp Callianassa louisianensis Schmitt, 1935. Journal of Crustacean Biology (ISSN: 0278-0372), 9:540-553, New Braunfels, TX, USA. Available at http://www.jstor.org/discover/10.2307/1548586?uid=2134&uid=2&uid=70&uid=4&sid=21103303217313 [ Links ]
Felder, D.L.; Griffis, R.B. (1994) - Dominant infaunal communities at risk in shoreline habitats: burrowing thalassinid Crustacea. 87p., New Orleans, U.S. Department of the Interior, Minerals Management Service. Available at http://www.data.boem.gov/PI/PDFImages/ESPIS/3/3439.pdf [ Links ]
Forbes, A.T. (1973) - An unusual abbreviated larval life history in the estuarine burrowing prawn Callianassa kraussi (Crustacea: Decapoda: Thalassinidea). Marine Biology (ISSN: 0022-0981), 22:361–365. DOI: 10.1007/BF00391395.
Fingerman, M. (1995) - Endocrine mechanisms in crayfish, with emphasis on reproduction and neurotransmitter regulation of hormone release. American Zoology (ISSN: 0003-1569), 35:68-78, McLean, VA, USA. Available at http://icb.oxfordjournals.org/content/35/1/68.full.pdf [ Links ]
Frankenberg, D.; Coles, S.L.; Johannes, R.E. (1967) - The potencial trophic significance of Callianassa major fecal pellets. Limnology and Oceanography (ISSN: 0024-3590), 12(1): 113-120, Waco, TE, USA. Available at http://wap.aslo.org/lo/toc/vol_12/issue_1/0113.pdf [ Links ]
Griffis, R.B.; Chavez, F.L. (1988) - Effects of Sediment Type on Burrows of Callianassa californiensis Dana, 1854 and Callianassa gigas Dana, 1852. Journal of Experimental Marine Biology and Ecology, 117(3):239-253. DOI: 10.1016/0022-0981(88)90060-3 [ Links ]
Hailstone, T.S.; Stephenson, W. (1961) - The biology of Callianassa (Trypaea) australiensis Dana, 1852 (Crustacea, Thalassinidea).University of Queensland papers, Department of Zoology (ISSN: 0079-8835), 1(12): 259-285. Available at http://espace.library.uq.edu.au/view/UQ:222651 [ Links ]
Hanekom, N.; Baird, D. (1992) - Growth, production and consumption of the thalassinid prawn Upogebia africana (Ortmann, 1894) in the Swart kops estuary. South African Journal of Zoology (ISSN: 0379-4369), 27: 130-139. Available at http://content.ajarchive.org [ Links ]
Hernáez, P.; Wehrtmann, I.S. (2007) - Population biology of the burrowing shrimp Callichirus seilacheri (Decapoda: Callianassidae) in northern Chile. Revista de Biología Tropical (ISSN: 0034-7744), 55:141-152, San José, Costa Rica. Available at http://www.redalyc.org/pdf/449/44909918.pdf [ Links ]
Hill, B.J. (1977) - The effect of heated effluent on egg production in the estuarine prawn Upogebia africana (Ortmann, 1894). Journal of Experimental Marine Biology and Ecology, 29:291-302. DOI: 10.1016/0022-0981(77)90072-7 [ Links ]
Keough, M.J.; Downes, B. J. (1982) - Recruitment of marine invertebrates: The roles of active larval choice and early mortality. Oecologia Brasiliensis, 54:348-352. DOI: 10.1007/BF00380003. [ Links ]
Köeppen, W. (1948) - Climatologia: con un estudio de los climas de la Tierra. 478p., Fondo de Cultura Economica, México. [ Links ]
Laufer, H.; Landau, M. (1991) - Endocrine control of reproduction in shrimp and other Crustacea. In: Loach, P.F.; Dougherty, W.J. and Davidson, M.A. (org.), Frontiers of shrimp research, pp.65-81, Elsevier, Amsterdam, the Netherlands. ISBN: 978-0-444-52850-6 [ Links ]
Manning, R.B.; Felder, D.L. (1986) - The status of the callianassid genus Callichirus Stimpson, 1866 (Crustacea: Decapoda: Thalassinidea). Proceedings of the Biological Society of Washington (ISSN: 0006-324X), 99: 437-443. [ Links ]
Melo, G.A.S. (1999) - Manual de identificação dos crustáceos decapodos do litoral brasileiro: Anomura, Thalassinidea, Palinuridea, Astacidea. 551p., São Paulo, Plêiade/FAPESP, São Paulo, SP, Brasil. ISBN: 85-85795-08-5 [ Links ]
Nates, S.F.; Felder, D.L. (1999) - Growth and maturation of the ghost shrimp Lepidophthalmus sinuensis Lemaitre and Rodrigues, 1991 (Crustacea, Decapoda, Callianassidae), a burrowing pest in penaeid shrimp culture ponds. Fishery Bulletin (ISSN: 0090-0656), 97:541-562. Available at http://fishbull.noaa.gov/10natesf.pdf [ Links ]
Noro, C.K.; Buckup, L. (2008) - Estrutura populacional e biologia reprodutiva de Parastacus defossus (Crustacea: Decapoda: Parastacidae). Revista Brasileira de Zoologia (ISSN: 1806-969X), 25:624-629. Available at http://www.scielo.br/pdf/rbzool/v25n4/07.pdf [ Links ]
Pezzuto, P.R. (1993) - Ecologia populacional de Neocallichirus mirim (Rodrigues, 1971) (Decápoda, Callianassidae) na praia do Cassino, RS, Brasil. 172p., Dissertação de Mestrado, Universidade do Rio Grande, Rio Grande do Sul, RS, Brasil. Não publicado. [ Links ]
Pinheiro, M.A.A.; Fransozo, A. (2002) - Reproduction of the speckled swimming crab Arenaeus cribrarius (Brachyura: Portunidae) on the Brazilian coast near 23°30´S. Journal Crustacean Biology (ISSN: 0278-0372), 22(2):416-428. Available at http://www.jstor.org/stable/1549966 [ Links ]
Posey, M.H. (1986) - Predation on a burrowing shrimp, distribution and community consequence. Journal of Experimental Marine Biology and Ecology, 103(1):143-162. DOI: 10.1016/0022-0981(86)90138-3 [ Links ]
Quackenbush, L.S. (1994) - Lobster reproduction: a review. Crustaceana (ISSN: 0011-216X), 67 (1): 82-94. Available at http://www.jstor.org/discover/10.2307/20104969?uid=3738880&uid=2&uid=4&sid=21103260528427 [ Links ]
Rodrigues, S.A. (1976) - Sobre a reprodução, embriologia e desenvolvimento larval de Callichirus major Say, 1818 (Crustacea, Decapoda, Thalassinidea). Boletim de Zoologia da Universidade de São Paulo (ISSN: 0031-1049), 1:85-104, São Paulo, SP, Brasil. [ Links ]
Rodrigues, S.A. (1985) - Sobre o crescimento relativo de Callichirus major (Say, 1818) (Crustacea, Decapoda, Thalassinidea). Boletim de Zoologia da Universidade de São Paulo (ISSN: 0031-1049), 9: 195-211, São Paulo, SP, Brasil. [ Links ]
Rodrigues, S.A., Shimizu, R.M. (1997) - Autoecologia de Callichirus major (Say, 1818). In: Absalão, R. and Esteves, A.M. (org.), Ecologia de praias arenosas do litoral brasileiro,Volume 3. p. 155-170. Oecologia Brasiliensis (ISSN:1980-6442), Rio de Janeiro,Brasil. [ Links ]
Rowden, A.A., Jones, M.B. (1994) - A contribution to the biology of the burrowing mud shrimp, Callianassa subterranea (Decapoda: Thalassinidea). Journal of the Marine Biological Association of the United Kingdom (ISSN: 0025-3154), 74:623-635. DOI:10.1017/S0025315400047706 [ Links ]
Shimizu, R.M. (1997) - Ecologia populacional de Scolelepis squamata (Muller, 1806) (Polychaeta: Spinodae) e Callichirus major (Say 1818) (Crustacea: Decapoda: Thalassinidae) da praia de Barequeçaba (São Sebastião, SP). 49p.,Teses de Doutorado, Universidade de São Paulo, São Paulo, Brasil. Não publicado. [ Links ]
Shimoda, K.; Wardiatno, Y.; Kubo, K.; Tamaki, A. (2005) - Intraspecific behaviors and major cheliped sexual dimorphism in three congeneric callianassid shrimps. Marine Biology, 146:543-557. DOI: 10.1007/s00227-004-1453-0. [ Links ]
Souza, J.R.B.; Borzone, C.A.; Brey, T. (1998) - Population dynamics and secondary production of Callichirus major (Crustacea: Thalassinidea) on a southern Brazilian sandy beach. Archives of Fisheries and Marine Research, 46(2):151-164. Available at http://epic.awi.de/417/ [ Links ]
Souza, J.R.B.; Borzone, C.A. (2003) - A extração do corrupto, Callichirus major (Say) (Crustacea, Thalassinidea), para uso como isca em praias do litoral do Paraná: as populações exploradas. Revista Brasileira de Zoologia (ISSN: 1806-969X), 20(4):625-630. Available at http://www.readcube.com/articles/10.1590/S0101-81752003000400011?locale=en [ Links ]
Strasser, K.M., Felder, D.L. (1999) - Larval Development in Two Populations of the Ghost Shrimp Callichirus major (Decapoda: Thalassinidea) under Laboratory Conditions. Journal of Crustacean Biology (ISSN: 0278-0372), 19(4):844-878. Available at http://www.jstor.org/discover/10.2307/1549305?uid=2&uid=4&sid=21103311600993 [ Links ]
Teixeira, R.L.; Sá, H.S. (1998) - Abundância de macrocrustáceos decápodas nas áreas rasas do complexo lagunar Mundaú/Manguaba, AL. Revista Brasileira de Biologia (ISSN: 0034-7108), 58(3):339-404, São Carlos, SP, Brazil. Available at http://www.scielo.br/pdf/rbbio/v58n3/4567.pdf [ Links ]
Tamaki, A.; Ingole, B. (1993) - Distribution of juvenile and adult ghost shrimps Callianassa japonica Ortmann (Thalassinidea), on an intertidal flat: intraspecific facilitation as a possible pattern-generating factor. Journal of Crustacean Biology (ISSN: 0278-0372), 13:83-175. Available at http://www.jstor.org/discover/10.2307/1549132?uid=2&uid=4&sid=21103311600993 [ Links ]
Tamaki, A.; Tanoue, H.; Itoch, J.; Fukuda, Y. (1996) - Brooding and larval developmental periods of the callianassid ghost shrimp, Callianassa japonica (Decapoda: Thalassinidea). Journal of the Marine Biological Association of the United Kingdom (ISSN: 0025-3154), 76:675-689. doi: http://dx.doi.org/10.1017/S0025315400031386 Available at http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=4369508 [ Links ]
Tamaki, A.; Ingole, B.; Ikebe, K.; Muramatsu, K.; Taka, M.; Tanaka, M. (1997) - Life history of the ghost shrimp Callianassa japonica Ortmann, 1891 (Decapoda: Thalassinidea), on an intertidal sandflat in western Kyushu. Japan. Journal of Experimental Marine Biology and Ecology (ISSN: 0022-0981), 210:223-250. doi:10.1016/S0022-0981(96)02709-8 [ Links ]
Tunberg, B. (1986) - Studies on the population ecology of Upogebia deltaura (Leach) (Crustacea, Thalassinidea). Estuarine, Costal and Shelf Science (ISSN: 0272-7714), 22(6):753-765. DOI: http://dx.doi.org/10.1016/0272-7714(86)90097-1 [ Links ]
Vazzoler, A.E.A.M. (1996) - Biologia da reprodução de peixes teleósteos: teoria e pratica. 169p., EDUEM, Maringá. ISBN: 85-85545-16-x [ Links ]
Weimer, R.J.; Hoyt, J.H. (1964) - Burrows of Callianassa major Say 1818, geologic indicators of littoral and shallow neritic environments. Journal of Paleontology (ISSN: 0022-3360), 38(4):761-767. Available at http://raznoe.photo29.ru/Burrows_Callianassa1967.pdf [ Links ]
Wenner A.M., Fusaro, C.; Oaten, A. (1974) - Size at onset of sexual maturity and growth rate in crustacean populations. Canadian Journal of Zoology (ISSN: 1480-3283), 52:1095-1106. Available at http://www.nrcresearchpress.com/doi/abs/10.1139/z74-147#.Us2MUPRDspo [ Links ]
Witbaard, R.; Duineveld, G.C.A. (1989) - Some aspects of the biology and ecology of the burrowing shrimp Callianassa subterranea (Montagu, 1808) (Thalassinidea) from the southern North Sea. Sarsia North Atlantic Marine Science (ISSN: 0036-4827), 74:145-222. [ Links ]
Wynberg, R.P.; Branch, G.M. (1991) - An assessment of bait-collecting for Callianassa Kraussi Stebbing in Langebaan Lagoon, Western Cape, and of associated avian predation. South African Journal of Marine Sciences, 11: 141-152. doi:10.2989/025776191784287592 [ Links ]
*Submission: 20 August 2013; Evaluation: 26 September 2013; Reception of revised manuscript: 20 August 2013; Accepted: 13 October 2013 Available on-line: 14 January 2014














