Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Angiologia e Cirurgia Vascular
Print version ISSN 1646-706X
Angiol Cir Vasc vol.16 no.2 Lisboa June 2020
ORIGINAL ARTICLE
Morphologic changes and clinical consequences of wide AAA necks treated with 34-36mm proximal diameter EVAR devices.
Alterações morfológicas e consequências clínicas do tratamento de colos proximais largos requerendo endopróteses com 34-36mm de diâmetro
José Oliveira-Pinto1,2,3, Rita Soares-Ferreira1,4, Nelson F. G. Oliveira1,5, Frederico Bastos Gonçalves4,6, Sanne Hoeks7, Marie Josee Van Rijn1, Sander Ten Raa1, Armando Mansilha2,3, Hence J. M. Verhagen1
1 Department of Vascular Surgery, Erasmus University Medical Centre, Rotterdam, The Netherlands.
2 Department of Angiology and Vascular Surgery, Centro Hospitalar São João, Porto, Portugal.
3 Department of Surgery and Physiology, Faculty of Medicine of Oporto, Porto, Portugal.
4 Department of Angiology and Vascular Surgery, Hospital de Santa Marta, Centro Hospitalar de Lisboa Central, Lisbon, Portugal.
5 Department of Angiology and Vascular Surgery, Hospital do Divino Espírito Santo, Ponta Delgada, Azores, Portugal.
6 NOVA Medical School, Lisbon, Portugal
7 Department of Anesthesiology, Erasmus University Medical Center, Rotterdam, The Netherlands.
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Introduction: Endovascular aneurysm repair (EVAR) became the preferred modality for infrarenal aneurysm (AAA) repair. Several available endografts have main body proximal diameters up to 36mm, allowing for treatment of proximal AAA necks up to 32 mm. However, large neck represents a predictor of proximal complications after EVAR. The purpose of this study is to evaluate mid-term outcomes of patients requiring 34-36mm main body devices.
Methods: Retrospective review of a prospectively maintained database including all patients undergoing elective EVAR for degenerative AAA in a single tertiary referral hospital in The Netherlands were eligible. All measurements were performed on center-lumen line reconstructions obtained on dedicated software. Patients were classified as large diameter (LD) if the implanted device was >32mm wide. The remaining patients were classified as normal diameter (ND). Primary endpoint was neck-related events (a composite of “endoleak” (EL) 1A, neck-related secondary intervention or migration >5mm). Neck morphology changes and survival were also assessed. Differences in groups were adjusted by multivariable analysis.
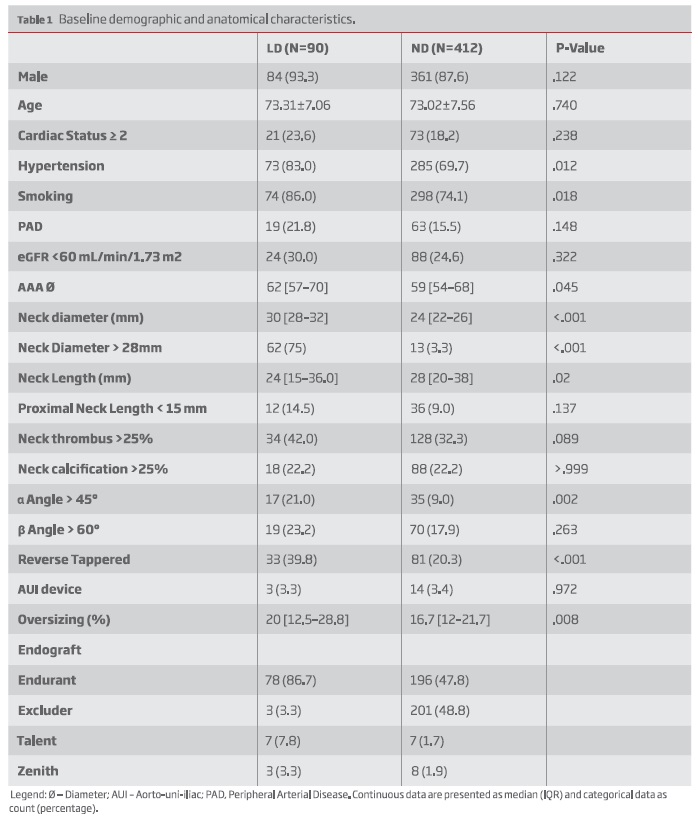
Results: The study included 502 patients (90 in the LD group; 412 in the ND group). Median follow-up was 3.5 years (1.5-6.2) and 4.5 years (2.1-7.3) for the LD and ND groups, respectively (P = .008). Regarding baseline characteristics, hypertension (83% vs 69.7%, P=.012) and smoking (86% vs 74.1%, P=.018) were more frequent in the LD group. Patients in the LD group had wider (Proximal neck Ø > 28 mm: 75% vs 3.3%, P<.001), more angulated (±-angle>45º: 21% vs 9%, P=.002), more conical (39.8% vs 20.3%, P<.001) and a thrombus-laden neck (Neck thrombus >25%: 42% vs 32.3%, P<.089). Oversizing was greater among LD group (20% [12.5-28.8] vs 16.7% [12-21.7], P=.008). All other anatomical risk factors were similar between groups.
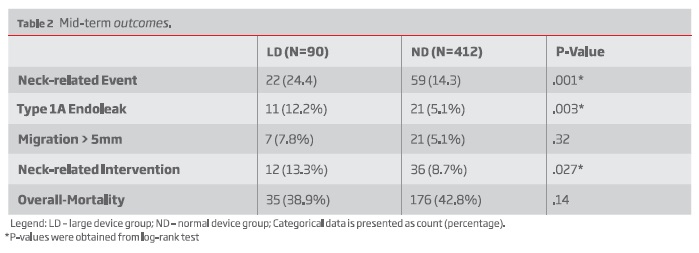
The 5-year freedom from neck-related event was 73% for the LD group and 85% for the ND group, P=.001. Type 1A endoleaks were more common in the LD group (12.2% vs 5.1%, P=.003). Migration > 5mm occurred similarly in both groups (7.8% vs 5.1%, P=.32). Neck-related secondary interventions were also more common among LD patients (13.3% vs 8.7%; P = .027).
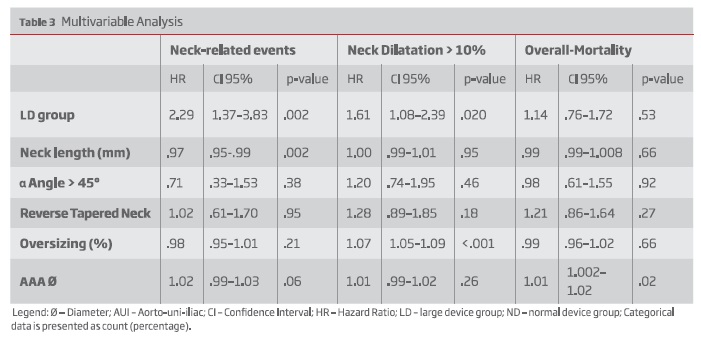
On multivariable regression analysis, LD group was an independent risk factor for neck-related adverse events (Hazard Ratio [HR]: 2.29; 95% confidence interval [CI], 1.37-3.83, P=0.002).
Neck dilatation was greater among LD patients (median, 3 mm [IQR, 0-6] vs 2mm [IQR, 0-4]; P =.034) On multivariable analysis, LD was an independent predictor for neck dilatation > 10 % (HR: 1.61 CI 95% 1.08-2.39, P=.020).
Survival at 5-years was 66.1% for LD and 71.2% for SD groups, P=.14.
Conclusion: Standard EVAR in patients with large infrarenal necks requiring a 34to 36-mm proximal endograft is independently associated to increased rate of neck related events and more neck dilatation. This subgroup of patients could be considered for more proximal seal strategies with fenestrated or branched devices, if unfit for open repair. Tighter surveillance following EVAR in these patients in the long term is also advised.
Keywords: Aortic aneurysm; Abdominal (MeSH); Aneurysm; Aortic neck; Neck Diameter; Neck-related events
RESUMO
Introdução:O tratamento endovascular representa o método de eleição para o tratamento de Aneurismas da Aorta Abdominal (AAA). Existem endopróteses disponíveis com diâmetros do colo proximal até 36mm, que permitem o tratamento de colos proximais até 32 mm. Contudo, a existência de colos largos representa um conhecido preditor de complicações. O objetivo deste estudo é avaliar os resultados a médio-prazo de doentes que requereram endopróteses de 34-36mm.
Métodos: Foi realizada uma análise retrospetiva de uma base de dados prospetiva, incluindo todos os pacientes submetidos a EVAR por AAA degenerativo numa instituição terciária na Holanda. Todas as medições foram realizadas em reconstruções center-lumen line em software dedicado. Os pacientes foram classificados como “diâmetro largo” (LD), se a endoprótese implantada tivesse diâmetro superior a 32 mm.. Os restantes pacientes foram classificados como diâmetro normal (ND). O endpoint primário foi complicações relacionadas com o colo (combinação de endoleak tipo IA, migração>5mm ou qualquer intervenção no colo proximal). Alterações morfológicas no colo e sobrevida foram também analisadas. Diferenças entre grupos foram ajustadas por regressão multivariável.
Resultados: O estudo incluiu 502 pacientes (90 no grupo LD e 412 no grupo ND). O follow-up mediano foi de 3.5 anos IQR (1.5-6.2) e 4.5 anos IQR (2.1-7.3) para os grupos LD e ND, respetivamente, P=.008. Relativamente às características basais, os doentes no grupo LD, apresentavam maior incidência de hipertensão arterial (83% vs 69.7%, P=.012) e tabagismo (86% vs 84.1%, P=.018). Além de colos mais largos (colo Proximal Ø > 28 mm: 75% vs 3.3%, P<.001), os indivíduos do grupo LD apresentavam também colos mais angulados (ângulo-± >45º: 21% vs 9%, P=.002), cónicos (39.8% vs 20.3%, P<.001) e com maior proporção de trombo circunferencial (Trombo no colo >25%: 42% vs 32.3%, P<.089). O oversizing foi maior entre o grupo LD (20% [12.5-28.8] vs 16.7% [12-21.7], P=.008). Todas os restantes detalhes anatómicos eram semelhantes entre grupos.
A ausência de complicações relacionadas com o colo aos 5 anos foi de 73% no grupo LD e de 85% no grupo ND, P=.001. Endoleak tipo 1A foi mais comum no grupo LD (12.2% vs 5.1%, P=.003). Migração>5 mm ocorreu similarmente entre grupos (7.8% vs 5.1%, P=.32). Reintervenções relacionadas com colo o foram também mais frequentes no grupo LD (13.3% vs 8.7%, P=.027).
Na análise multivariável, o grupo LD representou um fator de risco independente para complicações relacionadas com o colo (Hazard Ratio [HR]: 2.29; Intervalo de Confiança [IC] a 95%, 1.37-3.83, P=0.002).
A dilatação do colo foi mais proeminente no grupo LD (3mm IQR [0-6] vs 2mm IQR [0-4], P=.034). Em análise multivariável, o grupo LD representava um preditor independente de dilatação do colo acima de 10% (HR:1.61 IC95% 1.08-2.39, P=.020).
A sobrevida aos 5 anos foi de 66.1% no grupo LD e 71.2% no grupo SD, P=.14.
Conclusão: O EVAR standard em pacientes com colos infra-renais requerendo endopróteses com diâmetro 34-36mm está independentemente associada a maior risco de complicações relacionadas com o colo e maior grau de dilatação do colo. Este subgrupo deverá ser considerado para estratégias de selagem proximal mais eficazes como endopróteses fenestradas, se a correção aberta não constituir opção. Follow-up mais intensivo deve ser adotado se EVAR padrão for a opção preconizada.
Palavras-chave: Aneurisma da Aorta abdominal; Colo aneurismático; Diâmetro do colo; Eventos relacionados com o colo
Introduction
Endovascular aneurysm repair became the preferred modality of AAA repair(1).Progressive technological refinements along with greater operator experience have broadened the range of patients eligible for EVAR. This is particularly important regarding aortic neck anatomy, which is known to influence EVAR durability throughout time(2).
Despite following device IFU, patients with AAA proximal necks > 30mm have been found to be at a greater risk of developing neck-related adverse events, secondary interventions and post-implant ruptures, even if treated with late-generation devices(3,4). In spite of that risk, standard EVAR is still provided to patients with relatively large necks as most manufacturers currently offer devices with 34-36 mm diameter main bodies which may provide seal in neck-diameters up to 32mm.
With the advent of fenestrated/branched EVAR or with snorkel/chimney techniques, the first line endovascular repair technique in these cases has become a matter of debate. Also, fit patients may have better outcomes with open repair.
The aim of our study is to evaluate the mid-term clinical outcomes among standard infrarenal EVAR patients treated with large diameter stent-grafts. Additionally, the morphological changes of the aortic neck were evaluated throughout time.
Methods
Design population
A retrospective case-control study was designed based on a prospectively maintained database from a high-volume center in The Netherlands (Erasmus University Medical Center, Rotterdam). Informed consent was not required according to institutional policy on retrospective research.
All consecutive patients undergoing elective EVAR between January 2000 and December 2016 for infrarenal AAA were included. All endografts with large proximal diameter device options (34-36 mm) were used, including Excluder (W. L. Gore & Associates, Flagstaff, Ariz), Zenith (Cook Medical, Bloomington, Ind), Talent (Medtronic, Minneapolis, Minn) and Endurant I and II (Medtronic).
Anastomotic, infectious or isolated iliac aneurysms were excluded. In addition, patients receiving adjunctive endoanchors during the primary repair, without preoperative computerized tomography (CT) or postoperative CT imaging available for analysis were also excluded. Patients treated with endografts which do not offer 34-36 diameter options were also excluded.
Group Stratification
Patients were stratified into two groups according to the proximal diameter of the implanted endograft. The study group included patients with proximal diameter devices of 34 to 36 mm (large diameter - LD). The control group consisted of those with proximal diameter devices of d32 mm (normal diameter - ND). Endograft characteristics were obtained from the operative reports.
Data management
Baseline demographic and anatomic measurements were collected at the time of the intervention. All subsequent follow-up data were prospectively obtained upon outpatient visits and/or patient record consult at regular, predefined intervals.
Post-operative surveillance
At the beginning of the study period, CTA was performed at 1, 6, and 12 months, and yearly thereafter. However, the 6-month CTA has been progressively reserved for patients with or at an increased risk of developing aneurysm-related complications. If patients were considered to be at low risk of complications or had renal function impairment, colour duplex ultrasound examination or a non-contrast CT scan was preferred during follow-up. Once detected any adverse event on these imaging modalities, such as enlargement of more than 5 mm of diameter or an endoleak other than a type II endoleak, the patient would undergo CTA.
Measurements
All measurements were performed by four experienced observers (FBG; NO; JOP; RF) and obtained using dedicated post-processing software (3mensio vascular 4.2; Medical Imaging B.V., Bilthoven, The Netherlands), following center-line reconstructions. Measurements were performed by different observers thoughout time and not measured by all the four observers. According to institutional criteria and research purposes measurements were always performed homogeneously in an outer to outer fashion, regardless of the endograft used.
Definitions
Neck diameters were measured in two perpendicular axes just distal to the lowermost renal artery ostium, and at every 5 mm distally along the first 15 mm of the infrarenal neck on center-lumen line reconstructed imaging. The reference neck diameter was considered as the average of the two largest neck measurements. In patients with a neck length of <15 mm, the average of the first two measurements was taken as the reference diameter.
Aneurysm neck length was defined as the distance from the lowermost renal artery to the level where the aortic diameter increases by more than 10%. Aneurysm neck thrombus and calcification were categorized according to infrarenal aortic neck circumferential involvement. Neck configuration was classified according to published methodology(5).
Aneurysm volume and neck angulation were also measured according to previously validated methods(6,7).
Patient comorbidities and aneurysm-related outcomes were reported according to the recommendations from the Society for Vascular Surgery/ American Association of Vascular Surgery ad hoc Committee for Standardized Reporting Practices in Vascular Surgery(8).
Oversizing was determined by dividing the difference between the implanted main body diameter and the reference neck diameter.
Endograft migration was calculated as the difference of the distance between the start of the first covered stent and the lowermost renal artery on the last available and the 30-day CT. Neck-related adverse events were considered as a composite of type 1A endoleak, distal endograft migration >5 mm or a secondary intervention related to the infrarenal neck. Neck-related intervention was performed to treat an established complication (type 1A endoleak) migration or pre-emptively performed to avoid one in cases of migration or progressive loss of proximal seal.
Neck dilatation was considered if an increase of more than 10% in the neck diameter was observed at the beginning of the first covered stent of the implanted endograft.
Endpoints
The primary study endpoint was freedom from neck-related adverse events. Secondary endpoints were individual components of the latter composite outcome and survival. Neck morphologic changes throughout time were also assessed.
Statistical analysis
Categorical variables are presented as count and percentage and compared using the Pearson's chi-square test. Continuous variables are presented as mean and standard deviation (SD) or as median, interquartile range (IQR), and range. Differences between groups were analysed using the Mann-Whitney U-test for independent samples with non-normal distributions or with the Student's t-test and significance with the independent samples test for nonrelated variables with normal distributions.
Survival curves for freedom from neck-related adverse events were estimated by Kaplan-Meier methods, and equality was evaluated with the Mantel-Cox log-rank test.
Multivariate regression was performed adjusting for baseline clinical and morphologic features differently distributed among groups at a P value of less than .05 level.
Results
A total of 502 patients met the inclusion criteria previously outlined. Among the study population there were 90 (18%) patients treated with 34-36 main body diameter devices (LD) and 412 (82%) treated with d32mm-diameter devices (ND).
Mean age was 72.7±7.6 years and 88.6% were male. Baseline demographics are presented in Table I. Patients treated with larger devices (LD) were more likely to have hypertension and history of smoking. Regarding anatomical features, neck diameter was 30mm [28-32] in the LD vs 24mm [22-26] in the ND, P<.001. LD group also had shorter (neck length: 24 mm [15-36.0] vs 28 mm [20-38], P=.02) and more angulated necks (± angle > 45º: 17 (21%) vs 35 (9%), P=.002). Additionally, there more reverse tapered necks: 33 (39.8) vs 81 (20.3), P<.001 along with a thrombus-laden necks (Neck thrombus >25%: 42% vs 32.3%, P<.089) in the LD group. Baseline oversizing was also greater in the LD group: 20% [12.5-28.8] vs 16.7 [12-21.7], P=.008 -Table I. AAA diameter was also larger in the LD group (62 [57-70] vs 59 [54-68], P=.045)
(clique para ampliar ! click to enlarge)
Neck-related adverse events
The median clinical follow-up was 4.4 years (IQR, 2.17.3 years): 3.5 years [IQR, 1.5-6.2 years] for LD group and 4.5 years [IQR, 2.2-7.5 years] for ND group; P =.008).
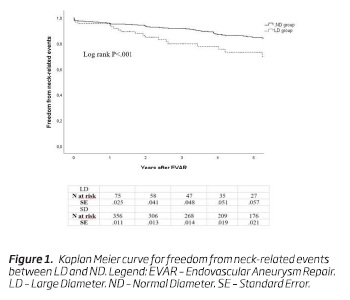
Neck-related adverse events occurred in 22 (24.4%) patients in the LD group and in 59 (14.3%) patients in the ND group. The 5-year freedom from neck-related events was 73% (n = 22; standard error [SE], 0.06) in the LD group and 85% (n = 176; SE, 0.02) in the ND group, respectively (P <.001) (Figure 1).
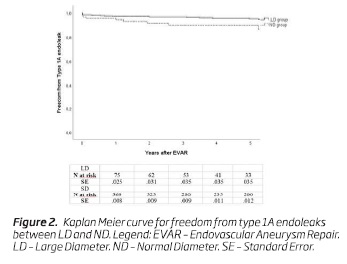
Type 1A endoleaks occurred in 11 (12.2%) patients in the LD group, while in 21 (5.1%) patients in the SD group - Table II. The 5-year freedom from type 1A endoleaks was 89.8% (n = 33; SE, 0.04) in the LD group and 95% (n = 187; SE, .01) in the ND group, respectively, (P = .003) - Figure 2.
(clique para ampliar ! click to enlarge)
Migration > 5 mm was detected in 7 (7.8%) patients in the LD group, while in 21 (5.1%) patients in the SD group, P=.32.
Neck-related interventions were performed in 12 (13.3%) patients in the LD group and in 36 (8.7%) patients in the ND group, P=.027 - Table II.
In an adjusted Cox proportional hazards model, the LD group was at an increased risk for neck-related adverse events (Hazard Ratio (HR), 2.29; 95% CI, 1.37-3.83; P = .002) - table III.
(clique para ampliar ! click to enlarge)
Neck morphologic changes
During follow-up, neck dilatation was greater in the LD group (median, 3 mm [IQR, 0-6] vs 2mm [IQR, 0-4]; P < .034). Thirty-nine patients (44.8%) in the LD group and 154 patients (38.5%) in the SD group had neck dilatation or greater than 10% (P =.27; Table II). When adjusting for baseline anatomical differences, the LD group was at increased risk for greater neck dilatation (HR, 1.61; 95% CI, 1.08-2.39; P = .020) - table III.
After adjusting for smoking, despite not statistically significant, LD group was also at increased risk for neck dilatation (HR: 1.5 CI95% .99-2.23, P=.056).
Survival
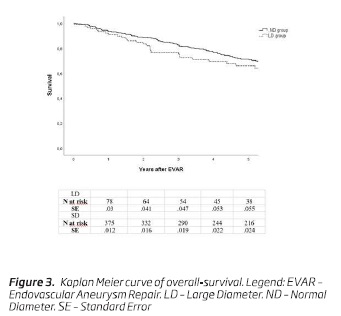
Over the follow-up period, 211 patients (42.1%) died: 35 (38.9%) in the LD and 176 (42.8%) in the ND group. Survival at 5 years was 66.1% (n = 36; SE, 0.06) in the LD group and 71.2% (n = 216; SE, 0.02) in the ND group (P = .14; Fig. 3).
In multivariable analysis, the LD patients were not at increased risk of overall-mortality (HR, 1.14; 95% CI, .76-1.72; P = .53) - Table III.
Discussion
Endograft-related refinements have resulted in better outcomes following EVAR, expanding treatment into progressively more challenging anatomies. Consequently, the EVAR benefits of short-term mortality and morbidity over open repair were extended into a group of patients otherwise not surgical candidates(9-11).
The proximal sealing site is one the most relevant factors for durability after EVAR. Yet, considerable controversy still involves treatment selection of patients with wide necks. Despite the abovementioned findings, large-diameter devices are still frequently used, treating neck-diameters ranging from 29 to 32 mm according to several commercially available device-IFU's(12,13). Whereas initial results in the use of these large-diameter devices to treat these patients were favourable 13 more recent studies, with longer follow-up, have contradicted these initial results(3,14,15).
In our study, patients treated with LD devices were at greater risk of neck adverse events and type IA endoleaks. Importantly, these patients were more likely to have a thrombus-laden neck along with reverse taper configuration. Additionally, there were more angulated necks in the 34-36mm group and AAA diameter was also greater. In a multivariable analysis correcting for these baseline differences, patients treated with 34-36mm devices were still at risk of proximal fixation failure. In an ENGAGE registry-based study from Bastos Gonçalves et al, patients treated with 32or 36-mm Endurant stent-grafts, were not found to be at increased risk of neck-related adverse events (P=.40). Yet, only 438 patients in that cohort (38%) had reached the 2-year follow-up threshold, limiting their conclusions relatively to mid-term outcomes(13). Additionally, as the range of aortic neck diameters of these patients receiving a 32or 36-mm device is not cited nor is the oversizing quantified, this may be a heterogeneous group of patients including patients with smaller neck diameters, adding additional bias to the reported outcomes among this group. In contrast, other reports have associated large infrarenal neck diameter to adverse outcomes following EVAR. Schanzer et al in a large multicentric report (N=10,228) reported that, at a mean follow-up of 31 months, patients with neck diameters >32 mm were at higher risk of aneurysm sac enlargement (HR, 2.1; 95% CI, 1.5-2)(14). This study was limited by lack of clinical or device data and by the nonconsecutive nature of their sample and the fact that old-generation devices were also included. More recently, in a multicentric study from our group including 427 patients treated with the Endurant stent-graft, we found that patients with necks > 30mm were at greater risk for neck related complications(3). In a parallel study investigating the outcomes of EVAR in patients with neck diameters e30 mm included in the Endurant Stent Graft Natural Selection Global Postmarket (ENGAGE) Registry, a greater risk of type 1A endoleak occurrence (HR 3.0; 95% CI, 1.0-9.3; P = .05) and post-implant AAArupture (HR5.1; 95% CI, 1.4-19.2; P = .016) was unveiled(4). Also McFarland et al, have described a greater risk for proximal fixation failure in a population of 500 patients over a follow-up of 34.1 months(16). A greater risk of migration was also noted among large device groups by McFarland et al, which contrasts to our current study, in which using even a stricter 5mm-threshold we did not observe the increased risk of endograft migration. This may be related to differences in endografts included in each study: while the Talent stent-graft accounted for 79% of the implanted grafts in the LD group of McFarland et al's study, in our population the Endurant stent-graft was the most implanted device. Importantly, these devices are quite distinct regarding proximal fixation, which in the Endurant stentgraft is optimised with the use of fixating barbs and hooks located at the suprarenal uncovered stent and at the first main body covered stent, features that the Talent stent-graft lacks(17). In consequence, proximal fixation is greatly enhanced in the Endurant-treated cohort(18).
Acknowledging this greater risk of proximal neck-related adverse events among patients treated with 34/36mm devices, alternative endovascular techniques may be considered for those patients considered unfit for open repair. Naturally, these must be carefully considered since risk for these is not as low as for standard EVAR. As such, it is debatable whether the diameter threshold for repair should be greater in this subgroup of patients and the indication for repair should be clearly discussed in order to improve informed consent since risk of cardiovascular death may overcome risk of AAA rupture. A suprarenal sealing with fenestrated endograft (or chimney technique in case of contraindication to fenestrated EVAR) may be considered, aiming to secure a proximal seal in non-diseased parallel -walled aortic segments above the renal arteries. Long-term data from the Cleveland Clinic, reveals low type 1 endoleak rates (1.1% in with 3 fenestrations and celiac scallop), slightly higher in patients with only renal fenestrations, which may be biased by the learning curve(19). Yet, long-term clinical studies comparing open repair, fenestrated EVAR and standard EVAR is required to objectively elucidate which of the techniques best suit this particular subgroup of patients. Other alternatives include resorting to endoanchors technology, which may be beneficial in patients requiring LD devices. Data from the 4-year results of the ANCHOR registry was presented at the Veith Symposium 2019 (non-published data), showing a 3.4% incidence of type 1A endoleaks. Of note, mean aortic diameter was 26 in the primary cohort. According to Goudeketting et al, endoanchors are at greater risk of poor penetration when performed in a larger aortic neck diameter 10 mm distal to the lowest renal artery (P < .001) and greater proximal neck calcium thickness (P = .004)(20).
Besides, longer-term data on endoanchors results are still expected(21). Finally, if standard EVAR is still the preferred option, a more stringent CT-based follow-up strategy should be followed.
Neck dilatation following EVAR is a well-known event, particularly with oversized self-expanding endografts which exert chronic outward radial force on the infrarenal neck(22). Also, it may be that disease progression and aortic dilatation may also occur at the proximal neck, as the infrarenal neck has been demonstrated to be histologically diseased(23). Aortic neck dilatation has been described both after open repair (OR)(24). Actually, in a study comparing neck dilatation rates between EVAR and OR, revealed similar increases of aneurysmal neck diameter with either of the techniques. This supports the theory of progressive structural deterioration but is of limited clinical importance for patients who have undergone OR(25).
In our cohort, greater neck dilatation was found in the large device group. These differences persisted even after adjusting for differences in oversizing and baseline anatomical features. In line with our findings, Cao et al had previously reported preoperative neck diameter to be predictive of dilatation (HR, 1.21; 95% CI, 1.07-1.35)(26). Yet, long-term clinical consequences of proximal neck dilatation remain to be determined. Even though ESVS guidelines recommend that any clinical consequence attributable to neck dilatation should be considered as so, for a matter of standardization the authors only considered if neck dilatation > 10%(27).
A non-significant greater survival from one-year onward is seen in the ND group, despite no differences after multivariable analysis. While this study does not provide the opportunity to adequately interpret these results due to a lack of power, it may mean that more advanced aortic disease may signal worse overall health status and consequent reduced survival expectancy. This was described by Oliveira, stating that patients with neck > 30mm were at increased risk for cardiovascular mortality, which further supports our hypothesis(28). Data from the EUROSTAR registry also revealed greater mortality in patients with AAA >60 mm and neck >26 mm(29). There are several limitations that need to be considered. First, this is a single center study, limiting generalization of the results. Secondly, its retrospective nature introduces a potential selection bias, which is reflected in the significantly different follow-up time among groups. Additionally, endograft selection was at the discretion of the surgeon and no clear guidelines were established beforehand. Even though, interobserver was not reported in this article, our group has tested and reported inter-observer variability in many previous studies(30,31). Finally, patients were included by a period of 16 years and the more recent patients could have benefited from longer surgical experience.
Conclusion
Patients treated with 34-36 mm devices, are at greater risk for neck-related adverse events. Additionally, patients treated with large devices have higher rates of neck dilatation over time.
Accordingly, open surgery or more complex endovascular strategies, extending proximal sealing, may be considered in patients with dilated necks. If standard EVAR is the preferred modality, close surveillance is warranted.
REFERENCES
1. Schermerhorn ML, Buck DB, Curran T, et al. Long-Term Outcomes and Temporal Trends With Endovascular vs Open Repair of Abdominal Aortic Aneurysms in the Medicare Population . Journal of vascular surgery 2014;60:830. [ Links ]
2. Antoniou GA, Georgiadis GS, Antoniou SA, Kuhan G, Murray D. A meta-analysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. Journal of vascular surgery 2013;57:527-38. [ Links ]
3. Oliveira NFG, Bastos Gonçalves FM, Van Rijn MJ, et al. Standard endovascular aneurysm repair in patients with wide infrarenal aneurysm necks is associated with increased risk of adverse events. Journal of vascular surgery 2017;65:1608-16. [ Links ]
4. Oliveira NFG, Gonçalves FB, Ultee K, et al. Patients with large neck diameter have a higher risk of type IA endoleaks and aneurysm rupture after standard endovascular aneurysm repair. Journal of vascular surgery 2019;69:783-91. [ Links ]
5. Balm R, Stokking R, Kaatee R, Blankensteijn JD, Eikelboom BC, van Leeuwen MS. Computed tomographic angiographic imaging of abdominal aortic aneurysms: implications for transfemoral endovascular aneurysm management. Journal of vascular surgery 1997;26:231-7. [ Links ]
6. van Prehn J, van der Wal MB, Vincken K, Bartels LW, Moll FL, van Herwaarden JA. Intraand interobserver variability of aortic aneurysm volume measurement with fast CTA postprocessing software. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists 2008;15:504-10. [ Links ]
7. van Keulen JW, Moll FL, Tolenaar JL, Verhagen HJ, van Herwaarden JA. Validation of a new standardized method to measure proximal aneurysm neck angulation. Journal of vascular surgery 2010;51:821-8. [ Links ]
8. Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. Journal of vascular surgery 2002;35:1048-60. [ Links ]
9. Tadros RO, Faries PL, Ellozy SH, et al. The impact of stent graft evolution on the results of endovascular abdominal aortic aneurysm repair. Journal of vascular surgery 2014;59:1518-27. [ Links ]
10. Brewster DC, Jones JE, Chung TK, et al. Long-term outcomes after endovascular abdominal aortic aneurysm repair: the first decade. Annals of surgery 2006;244:426-38. [ Links ]
11. Broos PPHL, Stokmans RA, van Sterkenburg SMM, et al. Performance of the Endurant stent graft in challenging anatomy. Journal of vascular surgery 2015;62:312-8. [ Links ]
12. Howard DPJ, Marron CD, Sideso E, Puckridge PJ, Verhoeven ELG, Spark JI. Editor's Choice - Influence of Proximal Aortic Neck Diameter on Durability of Aneurysm Sealing and Overall Survival in Patients Undergoing Endovascular Aneurysm Repair. Real World Data from the Gore Global Registry for Endovascular Aortic Treatment (GREAT). European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 2018;56:189-99. [ Links ]
13. Bastos Goncalves F, Hoeks SE, Teijink JA, et al. Risk factors for proximal neck complications after endovascular aneurysm repair using the endurant stentgraft. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 2015;49:156-62. [ Links ]
14. Schanzer A, Greenberg RK, Hevelone N, et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 2011;123:2848-55. [ Links ]
15. Gargiulo M, Gallitto E, Wattez H, et al. Outcomes of endovascular aneurysm repair performed in abdominal aortic aneurysms with large infrarenal necks. Journal of vascular surgery 2017;66:1065-72. [ Links ]
16. McFarland G, Tran K, Virgin-Downey W, et al. Infrarenal endovascular aneurysm repair with large device (34to 36-mm) diameters is associated with higher risk of proximal fixation failure. Journal of vascular surgery 2019;69:385-93. [ Links ]
17. Verhoeven BA, Waasdorp EJ, Gorrepati ML, et al. Long-term results of Talent endografts for endovascular abdominal aortic aneurysm repair. Journal of vascular surgery 2011;53:293-8. [ Links ]
18. Melas N, Saratzis A, Saratzis N, et al. Aortic and iliac fixation of seven endografts for abdominal-aortic aneurysm repair in an experimental model using human cadaveric aortas. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 2010;40:429-35. [ Links ]
19. Mastracci TM, Eagleton MJ, Kuramochi Y, Bathurst S, Wolski K. Twelve-year results of fenestrated endografts for juxtarenal and group IV thoracoabdominal aneurysms. Journal of vascular surgery 2015;61:355-64. [ Links ]
20. Goudeketting SR, van Noort K, Ouriel K, et al. Influence of aortic neck characteristics on successful aortic wall penetration of EndoAnchors in therapeutic use during endovascular aneurysm repair. Journal of vascular surgery 2018;68:1007-16. [ Links ]
21. Tassiopoulos AK, Monastiriotis S, Jordan WD, Muhs BE, Ouriel K, De Vries JP. Predictors of early aortic neck dilatation after endovascular aneurysm repair with EndoAnchors. Journal of vascular surgery 2017;66:45-52. [ Links ]
22. Sampaio SM, Panneton JM, Mozes G, et al. Aortic Neck Dilation after Endovascular Abdominal Aortic Aneurysm Repair: Should Oversizing Be Blamed? Annals of vascular surgery 2006;20:338-45. [ Links ]
23. Diehm N, Di Santo S, Schaffner T, et al. Severe structural damage of the seemingly non-diseased infrarenal aortic aneurysm neck. Journal of vascular surgery 2008;48:425-34. [ Links ]
24. Falkensammer J, Oldenburg WA, Biebl M, et al. Abdominal aortic aneurysm neck remodeling after open aneurysm repair. Journal of vascular surgery 2007;45:900-5. [ Links ]
25. Oberhuber A, Buecken M, Hoffmann M, Orend K-H, Mühling BM. Comparison of aortic neck dilatation after open and endovascular repair of abdominal aortic aneurysm. Journal of vascular surgery 2012;55:929-34. [ Links ]
26. Cao P, Verzini F, Parlani G, et al. Predictive factors and clinical consequences of proximal aortic neck dilatation in 230 patients undergoing abdominal aorta aneurysm repair with self-expandable stent-grafts1 1Competition of interest: none. Journal of vascular surgery 2003;37:1200-5. [ Links ]
27. Wanhainen A, Verzini F, Van Herzeele I, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery 2019;57:8-93. [ Links ]
28. Oliveira NFG, Ultee K, van Rijn MJ, et al. Anatomic predictors for late mortality after standard endovascular aneurysm repair. Journal of vascular surgery 2019;69:1444-51. [ Links ]
29. Waasdorp EJ, de Vries JP, Hobo R, Leurs LJ, Buth J, Moll FL. Aneurysm diameter and proximal aortic neck diameter influence clinical outcome of endovascular abdominal aortic repair: a 4-year EUROSTAR experience. Annals of vascular surgery 2005;19:755-61. [ Links ]
30. Bastos Goncalves F, van de Luijtgaarden KM, Hoeks SE, et al. Adequate seal and no endoleak on the first postoperative computed tomography angiography as criteria for no additional imaging up to 5 years after endovascular aneurysm repair. Journal of vascular surgery 2013;57:1503-11. [ Links ]
31. Oliveira-Pinto J, Ferreira RS, Oliveira NFG, et al. Total Luminal Volume Predicts Risk after Endovascular Aneurysm Repair. European Journal of Vascular and Endovascular Surgery 2020. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: oliveirapintoj89@gmail.com (J. Oliveira-Pinto).
Recebido a 27 de março de 2020. Aceite a 29 de maio de 2020