Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Angiologia e Cirurgia Vascular
versão impressa ISSN 1646-706X
Angiol Cir Vasc vol.16 no.1 Lisboa mar. 2020
CASOS CLÍNICOS
Parallel graft technique: an alternative procedure to treat the aortic arch
Parallel graft technique: uma alternativa para o tratamento de patologia do arco aórtico
Rita Augusto1,2, Jacinta Campos1,2, Andreia Coelho1,2, Nuno Coelho1,2, Evelise Pinto1, Carolina Semião1,2, João Ribeiro1, João Peixoto1, Daniel Brandão1,2, Alexandra Canedo1,2
1 Serviço de Angiologia e Cirurgia Vascular, Centro Hospitalar de Vila Nova de Gaia/Espinho, Vila Nova de Gaia, Portugal
2 Unidade de Angiologia e Cirurgia Vascular da Faculdade de Medicina da Universidade do Porto, Porto, Portugal
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Treatment of aortic pathologies involving the aortic arch represents a great challenge for vascular surgeons. Endografting techniques, comparing to open surgery, are less invasive approaches. However, an adequate proximal landing zone remains a challenge and, regarding this issue, parallel graft techniques represent a viable endovascular treatment option in patients with challenging aortic arch pathology by extending the proximal landing zone while maintaining aortic side branches perfusion.
Parallel graft techniques required a thorough planning and the clinical and imagiological follow-up are mandatory. They appear to be a safe and minimally invasive alternative techniques in selected fragile patients.
The authors report three clinical cases that required the use for parallel grafts to treat complex pathology of aortic arch.
Keywords: Aorta; Aortic arch; Aneurysm; Chimney technique; Endovascular repair
RESUMO
O tratamento das patologias que envolvem o arco aórtico representam um enorme desafio para os cirurgiões vasculares.
As técnicas de reparação endovascular, em comparação com a cirurgia aberta, são menos invasivas. Contudo, obter uma zona de selagem proximal ideal pode ser complexo. Relativamente a este tópico, as parallel graft techniques representam uma opção viável em doentes com patologia complexa do arco aórtico, ao permitir a extensão da zona de selagem proximal, mantendo a perfusão dos troncos supra-aórticos.
As parallel graft techniques requerem um planeamento apropriado, assim como um follow-up clínico e imagiológico adequados. São técnicas aparentemente seguras e minimamente invasivas, consideradas como alternativas válidas em doentes selecionados.
Os autores têm como objectivo reportar três casos clínicos em que as parallel graft techniques foram utilizadas para tratar patologia complexa do arco aórtico.
Palavras-chave: Aorta; Arco aórtico; Aneurisma; Chimney technique; Reparação endovascular
Introduction
During the last 20 years, a clear shift has been observed towards thoracic endovascular aortic repair (TEVAR) for different aortic pathologies. TEVAR has become the dominant form of repair for descendent thoracic aorta aneurysms, leading to recognized need for thoracic endovascular repairs extending into the aortic arch.(1)
Traditionally, completely open surgery has been the standard method for treating aortic arch diseases. Although the mortality and complication rates are low, open reconstruction of the aortic arch and supra-aortic branches remains a challenge when treating high-risk patients such as those who are elderly and those with complex comorbidities and high American Society of Anesthesiology scores.(2)
Total endovascular aortic arch repair is technically demanding. Simultaneous perfusion of all supra-aortic arteries without longer cerebral ischemia time, whilst trying to avoid cerebral embolization, labels endovascular aortic arch repair with highest level of technical difficulty and surgical expertise. Despite sufficient anticoagulation with heparin, manipulation with endovascular material in the aortic arch can lead to cerebral embolisation, acute arm ischemia and/or paraplegia.(3)
Concerning this topic, the major challenge in TEVAR is the aortic arch and proximal landing zone. Some techniques introduced to preserve the supra-aortic branches and prolong the proximal landing zone include hybrid TEVAR, parallel stent grafts, fenestrated stent grafts and branched stent grafts.
An alternative approach to proximal aortic disease management is use of chimney stent grafts as adjuncts to the treatment. The concept of parallel chimney stents was first described as a "bailout" maneuver after inadvertent visceral vessel coverage during endovascular abdominal aortic repair.(4)
This is an alternative technique that is applicable to elective and nonelective presentations for a variety of aortic diseases. However, chimneys during TEVAR are still an unproven strategy, and concerns about selection of patients, device choice, operative technique, durability, and long-term outcomes remain unresolved.
The authors report three clinical cases that required the use for parallel grafts to treat complex pathology of aortic arch.
Clinical cases
Case 1
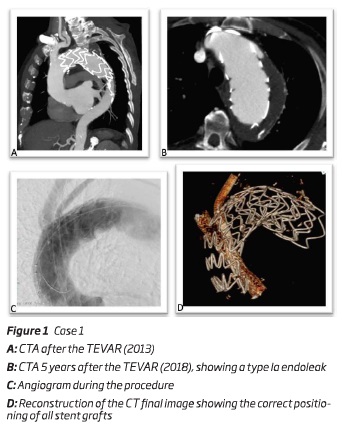
An 82 year-old male, with prior history of a TEVAR (Medtronic Valiant 44*150 mm) and carotid-subclavian transposition five years before, was diagnosed, during the follow-up, with a type 1a endoleak. He also had previous history of an abdominal aortic aneurysm (AAA) and right common iliac artery (CIA) ectasia, corrected by an tubular aortic interposition graft with right outflow to iliac bifurcation and left outflow to CIA. During the follow-up he also developed a left CIA aneurysm corrected by percutaneous endovascular repair - iliac stentgraft 16*16*82 mm (Medtronic Inc, Minneapolis, EUA).
Due to the presence of a type Ia thoracic endoleak, 5 years after the inicial TEVAR, the authors decided to perform a double chimney into the ascendant thoracic aorta (zone 0), being the brachiocephalic trunk (BCT) and left common carotid artery (LCCA) perfused by deployment of self-expandable stent-grafts (between the endoprosthesis and the aortic wall). After an adequate planning, the main body - a "custom made" aortic stent graft
Cook Alpha endograft 50*48*220mm without the proximal open stent (Cook Inc, Bloomington, IN, USA) - was deployed via percutaneous right femoral artery approach; the chimney to BCT - an iliac endograft limb device 16*16*120 mm (W.L. Gore and associates, Flagstaff, Ariz) - was deployed through a surgical right axillar artery approach and the chimney to LCCA - two stent grafts 9*9*100 mm - (Viabahn self-expandable stent grafts (W.L. Gore and associates, Flagstaff, Ariz) - were delivered through a surgical left brachial artery approach. It was also performed a percutaneous access of the left femoral vein to insufflate a balloon in inferior vena cava, to allow a more precise deployment - figure 1. At the end of the procedure a cone beam CT was performed to confirm adequate patency of the parallel grafts.
The patient was discharged uneventfully and the 1-month follow-up AngioCT revealed patency and a correct positioning of the endoprosthesis with no endoleaks.
Case 2
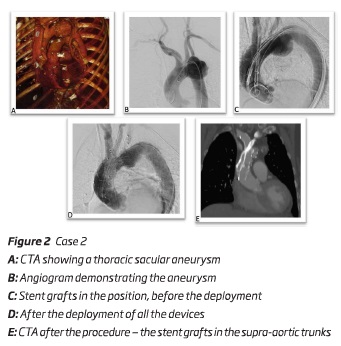
An 83-year-old female presented a 63 mm sacular thoracic aortic arch aneurysm. The authors initiated the treatment with a subclavian-carotid bypass. One month after, it was performed a double chimney technique into the ascendant thoracic aorta. After an adequate planning, the main body - a "custom made" aortic stent graft Cook Alpha endograft 40*30*163mm (Cook Inc, Bloomington, IN, USA) - was deployed via percutaneous right femoral artery approach; the chimney to BCT - an iliac endograft limb device 16*16*120 mm (W.L. Gore and associates, Flagstaff, Ariz) - was deployed through a surgical right axillar artery approach and the chimney to LCCA - 2 stent grafts 9*9*100 mm - Viabahn self-expandable stent grafts (W.L. Gore and associates, Flagstaff, Ariz) - were delivered through a surgical left brachial artery approach. Unfortunately, during the deployment of the first self-expandable stent graft, it became excessively compacted and collapsed and the guide wire accidently removed from the access. Through the femoral approach and the main endograft the authors were able to catheterize the self-expandable stent grafts and to perform a 0,014" through and through guide wire. After this laborious step, it was deployed a second self-expandable stent graft and a balloon-expandable stent between the self-expandable stent grafts - figure 2. It was also performed a percutaneous access of the left femoral vein to insufflate a balloon in inferior vena cava, to allow a more precise deployment. The final angiographic image and cone beam CT showed the correct positioning of all the components with no endoleaks. The patient was discharged uneventfully.
Case 3
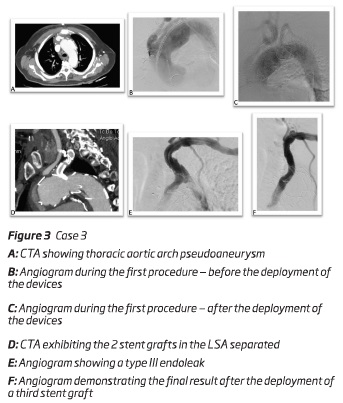
A 78-year-old male presented a 61 mm thoracic aortic arch pseudoaneurysm. He was initially submitted to an open aortic debranching of BCT and LCCA. To exclude the aneurysm, 2 months after the first surgery, it was decided to perform a TEVAR preserving the patency of the LSA through a periscope technique. The main body - a "custom made" aortic stent graft Cook Alpha endograft 50*46*163mm without the proximal open stent (Cook Inc, Bloomington, IN, USA) - was deployed via percutaneous right femoral artery approach and a periscope to LSA - a Viabahn self-expandable stent graft (W.L. Gore and associates, Flagstaff, Ariz) (10*10*100 mm) and a balloon expandable covered stent (12*12*38 mm) - was deployed through left femoral artery after percutaneous approach figure 3. Similarly to the other cases, the procedure was performed with a percutaneous access of the left femoral vein to insufflate a balloon in inferior vena cava.
During the follow-up - 7 months after the procedure - it was diagnosed a type III endoleak - between the balloon expandable covered stent and the Viabahn self-expandable stent graft. The patient was submitted to a new endovascular procedure. Via left brachial artery, a third balloon expandable covered stent (10*10*58 mm) was deployed performing a bridge between the other two, and excluding the endoleak. The patient was discharged uneventfully.
Discussion
TEVAR is the first line approach for the treatment of aortic disease involving the descending thoracic aorta with reduced mortality and morbidity rates compared with conventional surgery.(5)
Concerning the proximal descending aorta and aortic arch disease, open surgical repair is still the gold standard but has historically been reported to have high morbidity (30%-40%) and mortality (2%-20%) rates, depending on the patient’s comorbidities, the indication for repair and the type of the presentation.(6) Neurological event rates of up 18% have been reported for these procedures.(7)
Hybrid TEVAR was first introduced for the treatment of aortic arch disease in 1998.(8) The goal of the hybrid approach is to create a sufficient landing zone in the aortic arch or the ascending aorta. In general, a hybrid approach is defined as debranching of one or more supra-aortic vessel in order to reduce the number of aortic arch vessels, thus simplifying the exclusion of the arch pathology with a tubular stent-graft.(3) According to the literature, hybrid TEVAR with supra-aortic debranching has been proposed in high-risk patients, unfit for conventional surgery, to reduce the invasiveness of the conventional treatment. The peri-operative outcomes reported are encouraging, with reported mortality and neurological event rates of 11.9% and 7.6%, respectively.(9-12) In another meta-analysis of 195 patients, Antoniou et al.(13) demonstrated high rates of morbidity (21%) and mortality (9%), as well an endoleak rate of 9% and stroke rate of 7%.
However, both conventional and hybrid repairs do require at least a median sternotomy and there are no controlled trial comparing these two strategies.
The introduction of fenestrated and branched stent-grafts in the visceral aortic segment in the late 1990’s revolutionized endovascular aortic repair. Total endovascular approach of different aortic arch pathologies with fenestrated or branched stent-grafts became attractive. Chuter et al.(14) were the pioneers using branched stent grafts for endovascular repair of aortic arch aneurysm and dissection.
Fenestrated and branched devices are currently under investigation with promising short-term results. In a recent series, 27 patients with arch aneurysm were treated with an inner branched endograft to maintain blood flow to the BCT and/or LCA. In that series, 100% technical success and no peri-operative mortality was reported. Cumulative neurological events were reported in 5 patients (18.5%; 2 major strokes, 1 minor stroke, and 2 transient spinal cord ischaemia). An early (< 30 days) re-intervention was required in four patients (14.8%). Endoleak incidence was 11.1% (3 type II). During the mean follow-up of 12 months another two re-interventions were required.(15)
Several companies aim for a "universal" off-the-shelf arch branch stent-graft. However, the diversity of anatomical varieties limits the applicability of such a stent-graft. The personalized custom-made production of an aortic arch stent-graft consisting of fenestrations, branches and scallops is currently the most acceptable treatment option for total endovascular aortic arch repair. The major disadvantage of all custom-made branched and fenestrated stent grafts is the long waiting and production time of up to 3 months.(3,16)
Parallel stent grafts (chimney, snorkel, periscope and sandwich grafts) have been additionally proposed as a potential alternative option in patients who are poor surgical candidates for open repair or not suitable for fenestrated endovascular repair. This technique provides inflow to the branch via a stent graft placed alongside the aortic endograft, at least conceptually in a parallel fashion. In case of a single additional branch stent graft alongside the aortic stent/graft, the technique was called "double-barrel," and when two branch stents/grafts were used "triple-barrel".(17)
In a chimney or snorkel stent graft, the inflow segment is proximal to the branch that is covered by an aortic.(1) In the periscope stent graft, the inflow goes to the distal branch distal to the origin of the branch being covered.(18)
Chimney technique in the aortic arch was first used in the treatment of an aortic arch aneurysm to restore the LCCA blood flow in 2005.(19) The use of parallel graft techniques addicionally to the TEVAR has increased in the last decade. They have been proposed as the complete endovascular reconstruction of the supra-aortic branches with minimal trauma. However, the technique requires special consideration of the anatomical characteristics of the aortic arch such as the angulation of the branches, type of arch and local calcification. Moreover, there are no existing guidelines for anatomical-specific applicability and stent size/oversizing selection criteria of chimney in the aortic arch, therefore the indications and contraindications for this procedure remain unknown.
Accurate pre-procedural planning is essential to determine the proposed proximal sealing zone and identify the aortic branches to be covered by the main stent-graft. In addition, imaging and assessment of the upper arm arterial access and aortic arch is essential.
Potential advantages of parallel graft techniques over fenestrated stent grafts (in the thoracic and abdominal sectors) include reduced complexity, wider availability in smaller centers, and an immediate treatment option in the acute setting.
Parallel graft techniques with TEVAR involve the placement of single or multiple uncovered and covered stents parallel to the main aortic stent-graft to extend the proximal or distal sealing zones, while maintaining side branch patency. In experience of Patel et al(18), a 20 mm sealing zone is the absolute minimum for chimney TEVAR and pre-procedural planning should aim for a longer sealing zone where anatomically possible in order to minimise the risk of endoleak.
With the chimney technique, the chimney stent is located in opposition to the aortic neck, creating a gutter between the aortic wall and the thoracic stent graft. Theoretically, this gutter results in an increased risk of a type I endoleak. The use of more conformable aortic stent-grafts and a higher degree of oversizing may reduce the risk of gutters.(20) Concerns about gutter leaks were initially raised by Sugiura et al.(21) They reported midterm outcomes after 11 chimney TEVAR procedures and 2 (18%) patients developed type Ia endoleak.
Planning to minimize this problem involve creating a more longer parallel grafts in the aorta, so that the gutters are more likely to become thrombosed. Most authors suggest that the length of the gutters must be at least 8 cm if the parallel grafts are in the arch.(1)
Other common topic for parallel graft is the issue of how to size or oversize the endografts to best accommodate the parallel branch grafts. Predicting the degree to which the aortic endograft will conform to minimize the gutters is not simple, because of constraining anatomy or multiple parallel grafts, the need to reinforce the parallel graft to prevent it from being crushed or from fracturing as a result of fatigue and differences in aortic and branch diameters. Most writers suggest sizing the endograft in the aorta normally (7%-25% oversizing is typical per manufacturer’s Instruction for Use), allowing adequate circumference for the graft to invaginate into the gutters to an extent.(1)
The ideal sequence of deployment remains a discussion point, although more authors seem to suggest first opening of the main stent-graft followed by the chimney grafts deployment.(18) The parallel graft study with the longest follow-up suggests that there are not dramatic differences in outcomes related to the order of deployment.(1)
The current knowledge on the chimney technique is provided by retrospective case series and systematic reviews.
Moulakakis et al.(9) published a meta-analysis of 136 patients with chimney grafts in the aortic arch, reporting an endoleak rate of 11% and 8% for type Ia and type II endoleak, respectively. A stroke rate of 4% was observed. Similarly, another meta-analysis of 94 patients with 101 chimney grafts in the aortic arch reported a perioperative mortality rate of 3%, stroke rate of 5% and endoleak rate of 18% (of those most frequently type Ia with 6%).(22) The meta-analysis published in 2015 analysing the results of 364 chimney grafts in the aortic arch reported similar endoleak rates of 11%, with reduction of the stroke rate to 1.7%.(23) In this meta-analysis, the ideal radial force should be exerted from the aortic endograft, which is suggested to be oversized up to 30%, so as not to compromise the chimney grafts while maintaining adequate wall apposition. This though is not always possible with the stent grafts that are up to 45 mm large in diameter on the market - the exemple of the clinical cases presented in this paper.
In the presented cases, heparin administration was modulated to maintain an activated clotting time > 280-300s to reduce the risk of thrombus generation and cerebral events while wire, catheters, and stent grafts were parked in the ascending aorta and/or aortic arch. All patients were considered by a multidisciplinary board - anesthesiologist, vascular surgery, cardiothoracic surgery and cardiology - to be unfit for conventional surgery and hybrid repair. In these circumstances a total endovascular solution to address the aortic arch was considered a reasonable alternative. In all cases, at the end of the procedures, a cone beam CT was performed to ensure the correct position of all material, and the absent of kinks in the chimneys. When patients were previously medicated with anticoagulation (due to cardiac pathology), after the procedure, they maintained anticoagulation and mono-antiplatelet (case 1 and 3). If this condition was not observed, they maintained dual anti-platelet theraphy during the first 12 months (case 2).
Conclusion
Treatment of aortic pathologies close to or involving the aortic arch poses a great challenge for the physician facing these patients. Despite the evolution of peri-operative care and the strategies for cerebral protection, the conventional open aortic repair of aortic diseases involving the aortic arch is still associated with considerable postoperative mortality and morbidity rates even in high volume centers.(24)
Recently, endovascular aortic arch reconstruction has been suggested as an attractive alternative or treating aortic arch diseases, especially in treating high-risk patients who would otherwise be unsuitable for open repair.
The parallel graft techniques in the aortic arch used during TEVAR can be performed safely with a high rate of technical success with acceptable perioperative morbidity and mortality rates, even in high-risk patients - as the authors showed in the presented cases - however, long-term results are unknown, and larger series and comparative studies are needed to determine more data.
As the authores demonstrated, the parallel graft techniques could be used in differents scenarios - i.e. thoracic type Ia endoleaks, aortic arch aneurysms. The possibility of re-interventions (case 3) highlights the importance of adequate follow-up of patients in postoperative period.
REFERENCES
1. Cronenwett JL, Johnston W. Rutherford's Vascular Surgery and Endovascular Therapy, 8th Edition. Chapter 91. Philadelphia. Saunders/Elsevier [ Links ]
2. Misfeld M, Leontyev S, Borger MA, Gindensperger O, Lehmann S, Legare JF et al. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann Thorac Surg 2012;93:1502-8 [ Links ]
3. Makaloski V, Tsilimparis N, Rohlffs F, Heidemann F, Debus ES, Kölbel T. Endovascular total arch replacement techniques and early results. Ann Cardiothorac Surg 2018;7(3):380-388 [ Links ]
4. Greenberg RK, Clair D, Srivastava S, Bhandari G, Turc A, Hampton J, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg 2003;38:990-6 [ Links ]
5. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35(41):2873e926 [ Links ]
6. Ouzounian M, LeMaire SA, Coselli JS. Open aortic arch repair: state-of-the-art and future perspectives. Semin Thorac Cardiovasc Surg 2013;25:107-15 [ Links ]
7. Hiraoka A, Chikazawa G, Tamura K, Totsugawa T, Sakaguchi T, Yoshitaka H. Clinical outcomes of different approaches to aortic arch disease. J Vasc Surg 2015;61(1):88e95 [ Links ]
8. Buth J, Penn O, Tielbeek A, Mersman M. Combined approach to stent-graft treatment of an aortic arch aneurysm. J Endovasc Surg 1998;5:329-32 [ Links ]
9. Moulakakis KG, Mylonas SN, Markatis F, Kotsis T, Kakisis J, Liapis CD. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg 2013;2(3): 247e60 [ Links ]
10. Lotfi S, Clough RE, Ali T, Salter R, Young CP, Bell R et al. Hybrid repair of complex thoracic aortic arch pathology: long-term outcomes of extra-anatomic bypass grafting of the supra-aortic trunk. Cardiovasc Intervent Radiol 2013;36:46-55 [ Links ]
11. Iba Y, Minatoya K, Matsuda H, Sasaki H, Tanaka H, Oda T et al. How should aortic arch aneurysms be treated in the endovascular aortic repair era? A risk-adjusted comparison between open and hybrid arch repair using propensity score-matching analysis. Eur J Cardiothorac Surg 2014;46:32-9 [ Links ]
12. De Rango P, Ferrer C, Coscarella C, Musumeci F, Verzini F, Pogany G et al. Contemporary comparison of aortic arch repair by endovascular and open surgical reconstructions. Surg Vasc 2015;61:339-46 [ Links ]
13. Antoniou GA, El Sakka K, Hamady M, et al. Hybrid treatment of complex aortic arch disease with supra-aortic debranching and endovascular stent graft repair. Eur J 33. Vasc Endovasc Surg 2010;39:683-90 [ Links ]
14. Chuter TA, Schneider DB. Endovascular repair of the aortic arch. Perspect Vasc Surg Endovasc Ther 2007;19:188-92 [ Links ]
15. Spear R, Haulon S, Ohki T, Tsilimparis N, Kanaoka Y, Milne CP, et al. Editor’s choice e subsequent results for arch aneurysm repair with inner branched endografts. Eur J Vasc Endovasc Surg 2016;51(3):380e5
16. Anderson JL, Adam DJ, Berce M, Hartley DE. Repair of thoracoabdominal aortic aneurysms with fenestrated and branched endovascular stent grafts. J Vasc Surg 2005;42:600-7 [ Links ]
17. Baldwin ZK, Chuter TAM, Hiramoto JS, et al. Double-barrel technique for preservation of aortic arch branches during thoracic endovascular aortic repair. Ann Vasc Surg 2008; 22: 703-709 [ Links ]
18. Patel RP, Katsargyris A, Verhoeven EL, Adam DJ, Hardman J. Endovascular Aortic Aneurysm Repair with Chimney and Snorkel Grafts: Indications, Techniques and Results. Cardiovasc Intervent Radiol. 2013 Dec;36(6):1443-1451 [ Links ]
19. Larzon T, Gruber G, Friberg O, Geijer H, Norgren L. Experiences of intentional carotid stenting in endovascular repair of aortic arch aneurysms - two case reports. Eur J Vasc Endovasc Surg 2005;30:147-51 [ Links ]
10. Lee JT, Greenberg JI, Dalman RL (2012) Early experience with the snorkel technique for juxtarenal aneurysms. J Vasc Surg 55(4):935-946
21. Sugiura K, Sonesson B, Akesson M et al (2009) The applicability of chimney grafts in the aortic arch. J Cardiovasc Surg (Torino) 50(4):475-481
22. Hogendoorn W, Schlosser FJ, Moll FL, Sumpio BE, Muhs BE. Thoracic endovascular aortic repair with the chimney graft technique. J Vasc Surg 2013;58:502-11. [ Links ]
23. Lindblad B, Bin Jabr A, Holst J, et al. Chimney grafts in aortic stent grafting: hazardous or useful technique? systematic review of current data. Eur J Vasc Endovasc Surg 2015;50:722-31 [ Links ]
24. Estrera AL, Sandhu HK, Afifi RO, et al. Early and late outcomes after complete aortic replacement. Ann Thorac Surg 2015; 100: 528-534 [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: rita.augusto1988@gmail.com (R. Augusto).
Recebido a 14 de junho de 2019
Aceite a 24 de março de 2020