Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Angiologia e Cirurgia Vascular
versión impresa ISSN 1646-706X
Angiol Cir Vasc vol.15 no.4 Lisboa dic. 2019
ARTIGO ORIGINAL
Novel anti-inflammatory and immunomodulation effects of Rose on the endothelium in normal and hypoxic invitro conditions
Mark Christopher Arokiaraj1, Eric Menesson2
1 Cardiology, Pondicherry institute of Medical Sciences, Pondicherry, India. 605014
2 Tebu Bio, France
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Aims: The study was performed in search of a novel agent useful for inflammation modulation, and cytokine and related biomarkers levels, which would result in the treatment of cardiovascular disorders and various other clinical scenarios.
Materials and methods: A crushed red rose extract was prepared from the petals, and it was processed for analysis. The extract was tested on HUVEC cells at various concentrations. By microscopic examination of cells, a safe concentration was identified, and the levels below the safe limit were tested at 72 hours and seven days for selected cytokines secretion. After hypoxia treatment, the experiment was performed at various concentrations and varying degrees of hypoxia (12%, 5%, and 1% oxygen) during treatment, and the results were compared with the controls.
Results: The majority of the Inflammatory cytokine's secretion was reduced by the treatment of red rose extract on the endothelial cells. VEGF and angiogenic cytokine levels were reduced, but VEGF-R2 levels were maintained after the cell treatment. Below the safe concentration limit (0.5%), there were only minimal changes in the cytokine levels tested at various dilutions. IL 1, TNF ±, ADAM ST13, Angiopoietin levels and other inflammatory markers were reduced. In further experiments, the rose extract also induced Fas ligand, ERB4, integrin A5, Insulin R, IGF1 R, and XIAP; and reduces Trail R1, ICAM 1, and BMPR2. LDL R receptors were elevated in the endothelial cells.
Conclusion There is potential for a red rose extract for the reduction of in vascular inflammatory biomarkers and certain other cytokine levels. Further studies need to be performed to evaluate the benefits and pharmacokinetics.
Keywords: Inflammation; Molecular biology; Rose
Introduction
The inflammatory response to cardiovascular injury or infections is common in clinical practice. Predominantly this is a protective response in the process of healing. The inflammatory response is required in control for sepsis and the inflammation induced by autoimmune disorders. However, in many instances, this could be overwhelming, and the products of the inflammatory process initiate a negative vicious cycle that needs to be controlled. The anti-inflammatory agents are frequently toxic and induce multiorgan injuries and eventually resulting in their dysfunction. The overwhelming response to inflammation or infection could result in multiorgan malfunctions(1,2). The current anti-inflammatory agents are steroids or non-steroidal anti-inflammatory agents (NSAIDS). Both these agents are effective, but they have potential side effects, and injudicious use of these agents could result in severe and life-threatening side effects due to these agents. Hence, a novel anti-inflammatory agent is necessary without side effects to mitigate the illness as well as maintaining safety. Also, in organ-transplant scenarios, a simple inflammatory agent without side effects is much desirable. The study was performed in search of a novel anti-inflammatory and immune regulation agent, which could be used for various clinical conditions. In this study, the red rose extract was used to evaluate the beneficial effects in controlling inflammation as well as their effects on angiogenesis. Traditionally rose is known as a symbol of love, and it is also known to have positive psychological effects(3-5). It is admired time immemorially by various artists for its aura, and it is vividly described in various literature. In some studies, it has been shown to have anti-inflammatory effects(6, 7), and antibacterial properties(8-10). It also modulates the immune system(11). It has mild antidiabetic properties(12), increases heart rate in Langendroff preparations(13), and it is known to inhibit angiotensin-converting enzyme(14). It increases the levels of reproductive hormones(15), and it is also used as an ophthalmic herbal medication(16). In this study, we evaluated the potential of the red rose extract on inflammatory and angiogenic markers. This is the first study in which red rose extract was evaluated for its vascular anti-inflammatory effects and its angiogenic potentials.
Methods
Preparation of red rose extract
A red rose was cut from a plant, and 2091 mg of freshly collected petals were distributed in 6 Precellys CK14 lysing tubes (Bertin Technologies, ref. 03961-1-003) for homogenization of soft tissue. One ml of PBS (ThermoFisher, ref. 14190) was added into each tube. The tubes were run on Precellys 24 homogenizer at 5000 rpm for 2×30 seconds. Then, the supernatant from all tubes was gathered and spun down at 200g, 4°C for 5 min. The supernatant was spun down twice at 4°C for 5 min, the first at 2000g, and the last at 10000g. The final supernatant was 0.22 ¼m filtered (Sartorius, ref. 16534-K) and stored until use at -20°C.
Cell treatment
HUVEC were seeded at passage 5 in 96-well plates at 3300 cells/well in 100 ¼l of endothelial cell growth medium (Cell Applications, ref. 211-500). 24h after plating, the cells were treated in duplicate with 150 ¼l of red rose extract diluted at 10%, 5%, 1%, 0.5%, 0.1%, 0.05%, 0.01% and 0.005% (v/v) in endothelial cell growth medium (Cell Applications, ref. 210-500) or with growth medium only as control for 72h and 7 days. One replicate cell culture medium of each different condition was collected after 72h and after seven days. The cell viability was evaluated under the microscope.
Profiling of secreted cytokines
The Human angiogenesis antibody array G-1000 is a multiplexed sandwich ELISA-based array enables to detect multiple cytokines simultaneously. It combines the advantages of the high detection sensitivity and the high throughput of arrays. Like a traditional sandwich-based ELISA, it uses a pair of cytokine specific antibodies for detection. A capture antibody is first bound to the glass surface. After incubation with the sample, the target cytokine is trapped on the solid surface. A second biotin-labelled detection antibody is then added, which can recognize a different epitope of the target cytokine. The cytokine-antibody-biotin complex can then be visualized through the addition of the streptavidin-conjugated Cy3 equivalent dye, using a laser scanner.
In detail, one standard glass slide is divided into eight wells of identical cytokine antibody arrays. Each antibody, together with the positive controls is arrayed in duplicate. The slide comes with an 8-well removable gasket, which allows for the process of 8 samples on one slide. By comparing signals from different arrays, the cytokine fold change between a sample and a control can be determined.
During the incubation time, the volume of medium varied differently in each well, and from the 150 ¼l added at the beginning of incubation, the volume collected was reduced. To normalize the profiling results, sample diluent from antibody array kit was added to the different collected media to reach 150 ¼l before performing the profiling assay. Then, the medium samples were tested undiluted on arrays.
The slide was scanned by using a microarray scanner (Innopsys, model InnoScan 710). The analysis of the image was done with Mapix 7.0 software (Innopsys) as follows: To determine the position of each spot, a grid containing the parameters (spot spacing, diameter, etc.) must be assigned. To assign a grid, a GAL file was used, which is a standard ATF file generated by the spotter itself. The grid was automatically positioned on the image. Once the grid is correctly positioned, the image is quantified. Each spot was framed in a gridding circle. This circle defines the border between spots and background. Background value is calculated as a local value estimated within a circular area, centered on the spot and excluding the spot itself. Photometric quantification was exported in a spreadsheet (GPR file).
Hypoxia treatment
In the next phase of the study, other inflammatory markers as targets and insulin-related biomarkers on the endothelium were studied. The red rose extract used in this study was prepared for project POMC-032018 and stored until use at -20°C.
HUVEC were seeded at passage 8 in 48-well plates at 9500 cells/well (10000 cells/cm2) in 250 ¼l of endothelial cell growth medium (Cell Applications, ref. 211-500). 24h after plating, the cells were treated in duplicate with 250 ¼l of red rose extract diluted at 0.5%, 0.05% and 0.005% (v/v) in endothelial cell growth medium (Cell Applications, ref. 211500) or with growth medium only as control for 72h at 21%, 12%, 5% and 1% O2.
The hypoxia INVIVO2 workstation (Baker Ruskinn) was used to simulate hypoxic conditions at 12%, 5%, and 1% O2. At each of these lower oxygen levels, the medium incubated on cells was previously preconditioned with the HypoxyCOOL device (Baker Ruskinn). The duplicate cell culture medium of each different condition was collected and pooled after 72h. The cell viability was evaluated under the microscope.
Results
The red rose extract was evaluated on HUVEC cells at various concentrations. The cells were incubated, and the effects of the extract concentration were studied 24 hours and 48 hours after incubation under the microscope. The results are summarised in Figures 1 to 3. At concentration 0.5 percent and less, the cell lysis was minimal, and the cell morphology was well preserved. At levels >0.5 percent cell lysis was predominant. Hence, further extended duration analysis in the study, a concentration of less than 0.5 percent, i.e., 0.1 percent or less, was chosen.
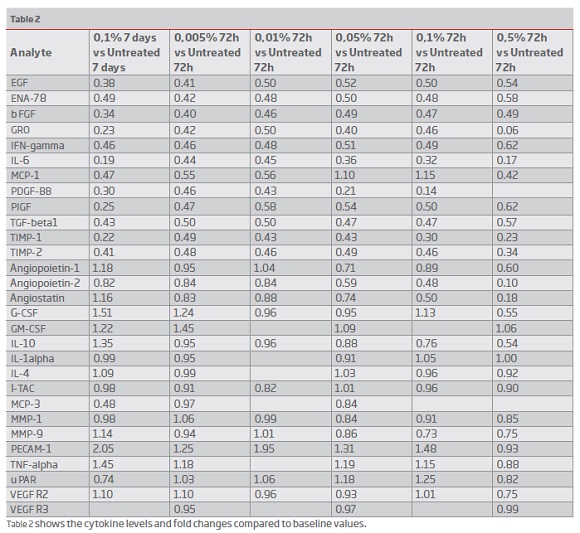
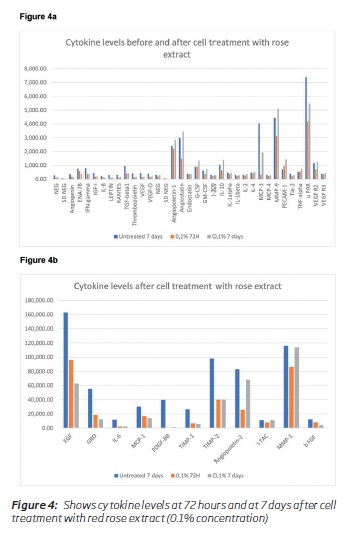
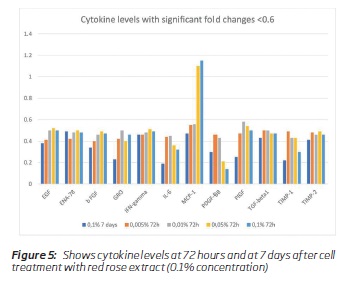
The results of the cytokines secretion are summarised in table 1. Table 2 shows the fold changes between treatment vs. untreated cells for each incubation time as final results. Many inflammatory cytokines expression was reduced, and the angiogenic factors secretion was reduced to a certain extent. VEGF secretion was reduced, and VEGF receptor expression was normal. Between concentrations 0.1 to 0.005%, there were only minimal changes in the levels of cytokines observed (Table 1). Many cytokines levels were reduced at 72 hours of cell treatment (0.1 percent concentration), and the levels rise after seven days (Figure 4a and 4b). MCP 3 (CCL7) and PDGF BB levels were less than 10% of the baseline values. Based on the fold changes, i.e., compared to the baseline values, a significant shift in specific cytokines like GRO, MCP-1, IL-6, INF-gamma was noticed (Figure 5).
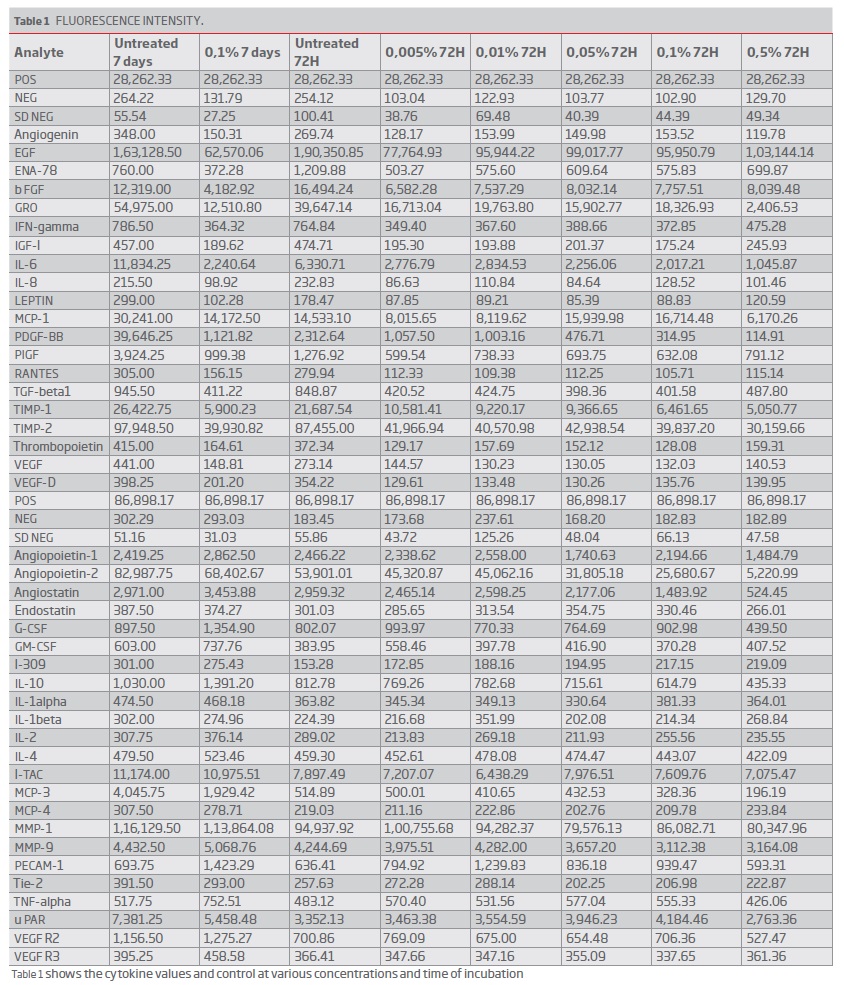
(clique para ampliar ! click to enlarge)
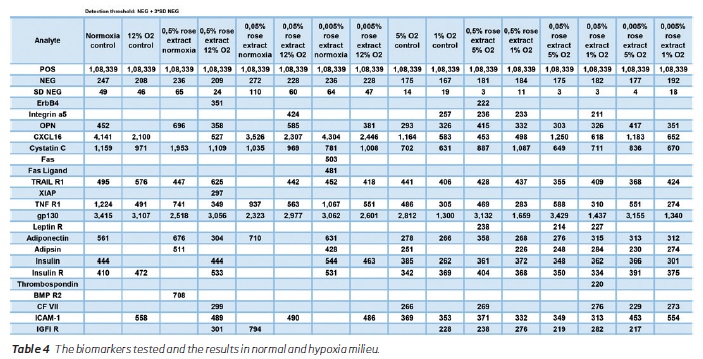
In the next phase of the study, inflammatory markers were reduced, and adiponectin levels were mildly elevated. Antiapoptotic XIAP was elevated, and there was also a mild increase in apoptotic markers like Fas, Fas ligand, and TRAIL R1 (Table 3). There were no significant changes in factor VII and thrombospondin levels. BMP R2 levels were reduced; Cystatin C levels were increased at 0.5% concentration, but in lower concentrations, it was reduced. Integrin A5 levels were maximally increased with 0.05% concentration treatment. ERB4 from endothelium increases during treatment with 0.005% concentration of the extract only. Osteopontin levels showed decreasing trend at lower concentrations of the extract. Insulin, Insulin R, and IGF1 R signal intensities were increased at 0.005% concentration extract treatment. Table 4 shows the values above the detection threshold wherein the detection threshold's cut-off was set at a higher value inclusive of 3 standard deviations. XIAP, Fas, Fas ligand, BMP R2 were not detected above the this higher threshold levels.
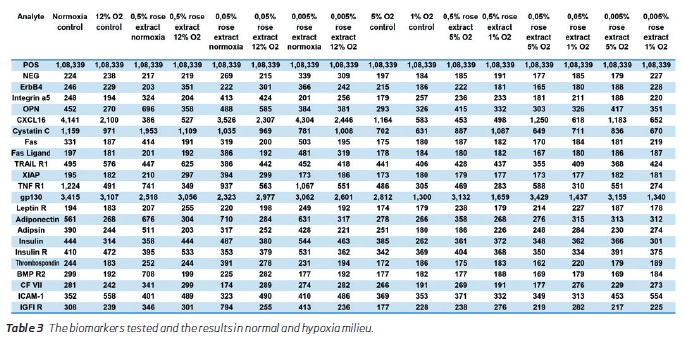
(clique para ampliar ! click to enlarge)
(clique para ampliar ! click to enlarge)
Phase 3 results
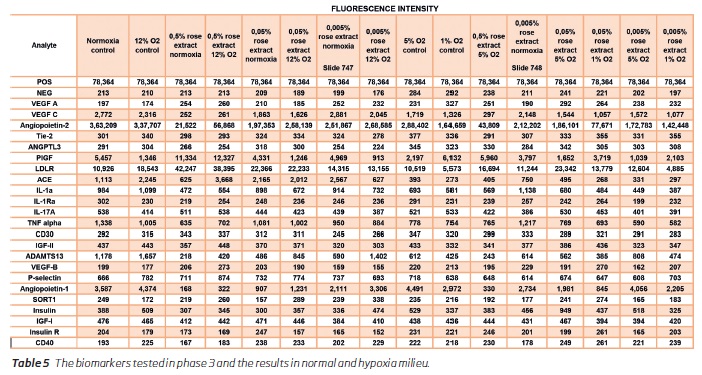
In the third phase of the study further investigations of the target markers were performed. The results in the phase 1 and 2 were observed in this phase also (Table 5 and 6). There was a marked reduction in the inflammatory markers IL 1A, IL-17A, TNF alpha. Among the angiogenesis markers the VEGF C was markedly reduced. VEGF A, VEGF B and Tie-2 levels were maintained in the near-baseline levels. Angiopoietin 1 and 2 levels were reduced and LDL R receptor showed an elevation with increasing concentrations of the rose concentration. ANGPLT3 levels showed an increasing trend at 0.05% concentration only. There were no significant changes in the endothelial CD 30 and CD 40 counts expressed on endothelial cells.
(clique para ampliar ! click to enlarge)
(clique para ampliar ! click to enlarge)
Hypoxia effects
Hypoxia reduced the secretion of gp130 in untreated cells, and the rose extract tended to minimize the effect of hypoxia and even increase the secretion at mid-hypoxia (12% and 5% O2) compared to 21% O2 for highest concentrations of rose extract (0.5% and 0.05%). Similarly, hypoxia reduced the secretion of TNF R1 in untreated cells with a drop at 1% O2 but probably due to the lower cell growth at this level. The same effect was observed at each concentration of rose extract. Rose extract decreased the secretion of TNF R1, but this effect was erased at lower oxygen levels (5% and 1%).
Discussion
Inflammation and angiogenesis
The study results show a significant reduction in the secretion of anti-inflammatory cytokines by endothelial cells treated with the red rose extract (Table 1-3). The effects of red-rose extract inhibited major inflammatory cytokines like TGF-beta, TNF-alpha, GRO, IL-6, INF-gamma, etc. These inflammatory proteins are known for their inflammatory response wherein renal, and pulmonary tissues are primarily affected, resulting in acute renal failure and acute respiratory distress syndromes, respectively. In severe conditions, the accumulation of these cytokines can lead to multiorgan dysfunction.
Steroids are known for their anti-inflammatory action. The role of steroids in the adjuvant treatment of septicaemia is always under debate, and the beneficial effects against the risk involved are not yet proved decisively(17-19). Steroids are useful in the treatment of bronchial asthma and COPD exacerbations. However, in these conditions also, if there is underlying sepsis or urinary tract infection, the infections can be exacerbated, especially in large dosages. Also, in post-transplantation scenarios, infections due to steroids are common. Non-steroidal anti-inflammatory agents are effective as an anti-inflammatory but with side effects, especially in the long term use, and most of them are nephrotoxic(20),and gastrointestinal effects are often seen(21).
In this study, VEGF levels were reduced, and it was not almost not detected in the medium under all conditions, and VEGF-R2 levels were near normal, which are markers for angiogenesis(22-24). VEGF R3 was not well detected, and it was too close to background. This action on VEGFR2 could, however, indicate a direct effect of the red rose extract on angiogenesis, which needs to be evaluated further.
The leptin levels, which are a marker of inflammation, was below the line of detection, and the leptin levels are associated with obesity(25). Reduced leptin levels are observed after mammoplasty in obese patients, and it correlates with increased insulin sensitivity(26).
Atherosclerosis
Some of the cytokines tested also have effects on apoptosis(27) (TNF beta, IGF-1) and atherosclerotic processes (RANTES, MCP-1, TGF beta 1, and INF-gamma and IL-1 beta(28). Hence, by regulating the cytokine actions, long term benefits and control of these pathways could be achieved. The significance of the marked reduction in specific cytokines like MCP-3, TIMP 1, and PDGF BB needs to be evaluated by future studies.
Other inflammatory markers
In the second phase, the results were consistent with the first phase of the study i.e., a reduction in inflammatory markers. In the second phase, other inflammatory-related markers involved in immune regulation like ICAM 1 were reduced, and Integrin A5 was elevated. Integrin A5 rise may be beneficial in myocarditis(29). ICAM modulates the neutrophil migration and adhesion to the endothelial cells(30). Adiponectin is associated with anti-inflammatory effects, and its levels are low in obesity and diabetes or patients with insulin resistance(31,32). Hence, a rise in adiponectin could have favourable outcomes. Adipsin was increased at 0.05% concentration only, and adipsin has been shown insulin secretion activity in the Beta cells33. There was a tendency for an increase in the leptin R receptors (table 3). Leptin R deficiency is associated with obesity and diabetes(34). Again about leptin R, the signal is below the threshold or really close to it, so the protein is not detected for me.
A reduction in inflammatory markers and the cystatin c levels would indicate a decrease in multiorgan failures seen in various infective and inflammatory conditions(35). Elevated cystatin c levels are an early marker of diabetes, and it is elevated in renal failures, and it is also a marker of impending renal failure in the future. Chronic reduction of these markers could result in a reduction of chronic inflammatory conditions and renal failures. Osteopontin is a well recognized vascular inflammatory marker associated with insulin resistance and diabetic complications(36), which showed a decreasing trend in the study.
BMPR2 and ERB4
BMP R2 is strongly associated with primary pulmonary hypertension, where the current treatment methods are not satisfactory(37). BMP R2 inhibitory effects could be useful in primary pulmonary hypertension. ERB4 is reduced in neuronal tissues in psychiatric disorders like schizophrenia. The endothelium assessment of ERB4 need not reflect on the neuronal levels. ERB4 induction could reduce the incidence of atrial fibrillation and other arrhythmias(38).
Apoptosis
Fas ligand inductions were seen in lower concentrations of the extract (0.05 and 0.005%), and the Fas induction was observed at 0.05% concentration only. Fas ligand induction in experimental studies has shown benefits to regulate leukocyte extravasation and cell adhesion mechanisms(39, 40). Fas pathway apoptotic induction could be a part of normal homeostasis mechanism, and the exact effect has to be studied in detail(41). Antiapoptotic XIAP increase, though not detected at higher thresholds, and reduction in the TRAIL R1 have favourable effects in apoptosis regulations(42,43).
Insulin related markers
The majority of inflammatory proteins and steroids result in the decrease in insulin levels or hyperglycaemia, and the impact of rose extract seems to increase insulin R levels, and IGF1 R. Increase in IGF1 R is associated with lower concentrations of the extract (0.05 and 0.005%). These would have favourable effects on insulin sensitivity, and it enhances endothelial regeneration(44). This the first study showing the benefits of the red-rose extract on the vascular endothelial response.
Toll (IL 1) interception, LDL R, Angiopoietin levels and phase 3 results
IL1 is a major marker of inflammation and belongs to the toll receptor superfamily and participates in the early host defence (45,46). There is marked reduction at 0.5% concentration and mild reduction in 0.05 and 0.05% concentration. When subjected to hypoxia there is marked reduction in the levels of IL1 concentration irrespective of the rose extract concentration. IL 1 RA (receptor antagonist) levels were mildly reduced. IL1Ra is a competitive inhibitor of IL 1a and it is actively involved in the regulation of inflammation(47). IL(17) levels were also reduced by all concentrations. IL 17 is a potent mediator of inflammatory pathways(48). TNF ± is known for inflammation and immune regulation properties. Reduction in TNF ± could result in reduction of inflammation mediated disorders.
Also, there was a marked reduction in the angiopoietin 1 and 2 levels compared to the untreated baseline values. In pre-ecclampsia the levels of VEGF and angiopoietin values are increased, whereas the placental inhibitory growth factor is reduced which can offset the balance to increase the placental growth factor and this can reduce pre-ecclampsia(50,51). There is a tendency for a mild reduction in the levels of the PIGF when treated at lower concentrations (0.05 and 0.005%). PIGF inhibition can increase the placental growth factor levels(52).
There was a marked increase in LDL R on the endothelial cells when treated with 0.5% and 0.05% concentrations, and at 0.005 concentration treatment the LDL receptors were mildly elevated. The LDL R play a major role in the metabolic modulation of LDL(53). Hence induction of this receptor is useful in atherosclerosis control. In analysing the diabetes related markers the IGF1 levels were not changed, whereas there was a mild reduction in the IGF2 levels. There is a tendency for SORT1 to be reduced and SORT1 is associated with calcification of the vessels(54). The ACE levels increase at 0.5% concentration treatment and it reduces when treated with 0.005 and 0.05% levels.
ADAMST13 is strongly inhibited by all concentration, and this cytokine is closely associated with microangiopathic disorders and its perpetuation including disseminated intravascular coagulation(55,56). The CD 40 count on the endothelial cells increases with 0.05% concentration treatment, whereas in other concentrations it is normal. The CD 30 on the cells mildly increase at 0.5% treatment but in other concentrations the levels are maintained.
Limitations and future perspectives
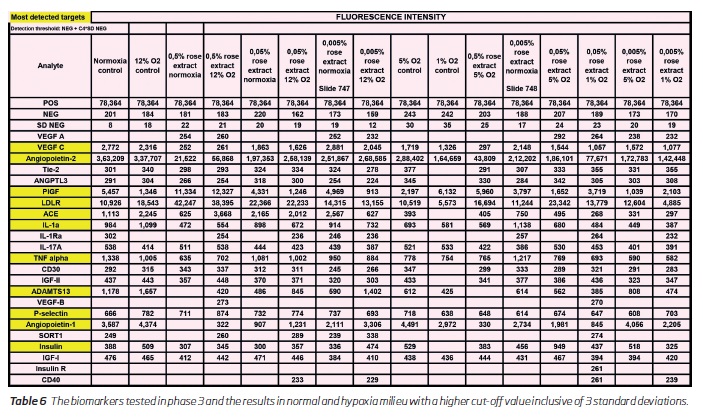
Further studies need to be performed to analyse the effects of the extract on inflammation and angiogenesis. Also, the side effect profile and active ingredients need to be studied. The observations in this study could be of use in the potential treatment of a wide range of disorders. When the threshold levels were increased to higher by including 3 standard deviations some markers were not detected (tables 4 and 6). Hence further experiments need to be performed with a large sample volume for more validation. This is a bench study, and the observations in animal models need to be studied.
The anti-inflammatory actions of the rose extract needs to be investigated in special conditions like corona virus and Ebola virus infections, etc.
Conclusion
There is potential for a red rose extract for the reduction in vascular inflammatory biomarkers and related cytokine levels on endothelial cell treatment. Further studies need to be performed to evaluate the benefits and pharmacokinetics.
Disclosures: None
Competing Interests: None
Source of funding: None
Competing financial interests: None to any of the authors
Footnotes: The study is dedicated to Dr Robert A. Levine, Massachusetts General Hospital, Boston.
Author Contributions: MCA conceived the idea and method, designed the study, analysed the results, and wrote the paper. EM performed the analysis, derived the results and contributed to its details.
BIBLIOGRAPHY
1. Aird W. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101(10):3765-3777. [ Links ]
2. Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. The American Journal of Emergency Medicine. 2008;26(6):711-715. [ Links ]
3. Ikei H, Komatsu M, Song C, Himoro E, Miyazaki Y. The physiological and psychological relaxing effects of viewing rose flowers in office workers. J Physiol Anthropol. 2014;33(1):6. Published 2014 Mar 8. DOI: 10.1186/1880-6805-33-6. [ Links ]
4. Komatsu M, Matsunaga K, Lee J, Ikei H, Song C, Himoro E, Miyazaki Y. The physiological and psychological relaxing effects of viewing rose flowers in medical staff. Jpn J Physiol Anthropol. 2013;18:1-7. [ Links ]
5. Song C, Igarashi M, Ikei H, Miyazaki Y. Physiological effects of viewing fresh red roses. Complementary Therapies in Medicine. 2017;35:78-84. [ Links ]
6. Boskabady MH, Shafei MN, Saberi Z, Amini S. Pharmacological effects of rosa damascena. Iran J Basic Med Sci. 2011;14(4):295-307. [ Links ]
7. Maleev A, Neshtev G, Stoianov S, Sheikov N. The ulcer protective and antiinflamatory effect of Bulgarian rose oil. Eksp Med Morfol. 1972;11:55-60. [ Links ]
8. Andoan BC, Baydar H, Kaya S, Demirci M, Özba_ar D, Mumcu E. Antimicrobial activity and chemical composition of some essential oils. Arch Pharm Res. 2008;25:860-864. [ Links ]
9. Adwan G, Mhanna M. Synergistic effects of plant extracts and antibiotics on Staphylococcus aureus strains isolated from clinical specimens. Middle East j sci res. 2008;3:134-139. [ Links ]
10. Hamidpour R, Hamidpour S, Hamidpour M, et al. Pelargonium graveolens (Rose Geranium). A Novel Therapeutic Agent for Antibacterial, Antioxidant, Antifungal and Diabetics. Arch Can Res. 2017, 5:1. DOI: 10.21767/2254-6081.1000134. [ Links ]
11. Mahmood N, Piacente S, Pizza C, Burke A, Khan AL, Hay AJ. The anti-HIV activity and mechanisms of action of pure com-pounds isolated from Rosa damascena. Biochem Biophys Res Commun. 1996;229:73-79. [ Links ]
12. Gholamhoseinian A, Fallah H, sharifi-far F, Mirtajaddini M. The inhibitory effect of some Iranian plantstracts on the alpha glucosidase. Iran J Basic Med Sci. 2008;11:1-9. [ Links ]
13. Boskabady MH, Vatanprast A, Parsee H, Ghasemzadeh M. Effect of aqueous-ethanolic extract from Rosa damascena on guinea pig isolated heart. Iran J Basic Med Sci. 2011;14:116-121. [ Links ]
14. Kwon EK, Lee DY, Lee H, Kim DO, Baek NI, Kim YE, et al. Flavonoids from the Buds of Rosa damascena inhibit the Activity of 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase and Angiotensin I-Converting Enzyme. J Agric Food Chem. 2010;58:882-886. [ Links ]
15. Jahromi HK, Jashni hk and Dialemeh S. Effect of Damask Rose Extract on FSH, LH and Testosterone Hormones in Rats. Int J Med Res Health Sci. 2016, 5, 5(S):267-271. [ Links ]
16. Biswas NR, Gupta SK, Das GK, Kumar N, Mongre PK, Haldar D, et al. Evaluation of ophthacare® eye drops - a herbal formulation in the management of various ophthalmic disorders. Phytother Res. 2001;15:618-620. [ Links ]
17. Gibbison B, López-López JA, Higgins JP, et al. Corticosteroids in septic shock: a systematic review and network meta-analysis. Crit Care. 2017;21(1):78. Published 2017 Mar 28. DOI: 10.1186/s13054-017-1659-4. [ Links ]
18. Patel G, Balk R. Systemic Steroids in Severe Sepsis and Septic Shock. American Journal of Respiratory and Critical Care Medicine. 2012;185(2):133-139. [ Links ]
19. Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. New England Journal of Medicine. 2018;378(9):797-808. [ Links ]
20. Hörl W. Nonsteroidal Anti-Inflammatory Drugs and the Kidney. Pharmaceuticals. 2010;3(7):2291-2321. [ Links ]
21. Russell R. Non-steroidal anti-inflammatory drugs and gastrointestinal damage--problems and solutions. Postgraduate Medical Journal. 2001;77(904):82-88. [ Links ]
22. Hoeben A. Vascular Endothelial Growth Factor and Angiogenesis. Pharmacological Reviews. 2004;56(4):549-580. [ Links ]
23. Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Antiand Pro-Angiogenic Therapies. Genes Cancer. 2011;2(12):1097-105. [ Links ]
24. Cébe-Suarez S, Zehnder-Fjällman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63(5):601-15. [ Links ]
25. Iikuni N, Lam QL, Lu L, Matarese G, La Cava A. Leptin and Inflammation. Curr Immunol Rev. 2008;4(2):70-79. [ Links ]
26. Hakan Uzun, Ozan Bitik, Yahya Baltu, Çidem Sönmez, and Ay_egül Öztürk Kaymak, “The Effects of Reduction Mammaplasty on Serum Leptin Levels and Insulin Resistance,” International Journal of Endocrinology, vol. 2015, Article ID 719824, 5 pages, 2015. https://doi.org/10.1155/2015/719824.
27. Grunnet LG, Aikin R, Tonnesen MF, et al. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes. 2009;58(8):1807-15. [ Links ]
28. Sulkava M, Raitoharju E, Levula M, Seppälä I, Lyytikäinen L, Mennander A et al. Differentially expressed genes and canonical pathway expression in human atherosclerotic plaques - Tampere Vascular Study. Scientific Reports. 2017;7(1). [ Links ]
29. Porter JC, Hogg N (1999). Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 8 (10): 390-6. DOI: 10.1016/S0962-8924(98)01344-0.PMID9789327. [ Links ]
30. Hubbard A, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radical Biology and Medicine. 2000;28(9):1379-1386. [ Links ]
31. Thundyil, J., Pavlovski, D., Sobey, C. G., and Arumugam, T. V. (2012). Adiponectin receptor signalling in the brain. Br. J. Pharmacol. 165, 313-327. DOI: 10.1111/j.1476-5381.2011.01560.x. [ Links ]
32. Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005; 6(1):13-21. [ Links ]
33. Lo J, Ljubicic S, Leibiger B, Kern M, Leibiger I, Moede T et al. Adipsin Is an Adipokine that Improves ² Cell Function in Diabetes. Cell. 2014;158(1):41-53. [ Links ]
34. Sharma K, McCue P, Dunn S. Diabetic kidney disease in the db/dbmouse. American Journal of Physiology-Renal Physiology. 2003;284(6):F1138-F1144. [ Links ]
35. Donahue R, Stranges S, Rejman K, Rafalson L, Dmochowski J, Trevisan M. Elevated Cystatin C Concentration and Progression to Pre-Diabetes: The Western New York Study. Diabetes Care. 2007;30(7):1724-1729. [ Links ]
36. Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014;3(4):384-393. Published 2014 Mar 22. DOI: 10.1016/j.molmet.2014.03.004. [ Links ]
37. Hopper R, Moonen J, Diebold I, Cao A, Rhodes C, Tojais N et al. In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug. Circulation. 2016;133(18):1783-1794. [ Links ]
38. Zhou X, Wang Z, Huang B, Yuan S, Sheng X, Yu L et al. Regulation of the NRG1/ErbB4 Pathway in the Intrinsic Cardiac Nervous System Is a Potential Treatment for Atrial Fibrillation. Frontiers in Physiology. 2018;9. [ Links ]
39. Sata M, Walsh K. TNF± regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nature Medicine. 1998;4(4):415-420. [ Links ]
40. Takada Y, Strominger JL, Hemler ME: The very late antigen family of heterodimers is part of a superfamily of molecules involved in adhesion and embryogenesis. Proc Natl Acad Sci U S A. 1987 May;84(10):3239-43. [ Links ]
41. Wang L, Azad N, Kongkaneramit L, et al. The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J Immunol. 2008;180(5):3072-3080. DOI: 10.4049/jimmunol.180.5.3072. [ Links ]
42. Latour S, Aguilar C. XIAP deficiency syndrome in humans. Seminars in Cell & Developmental Biology. 2015;39:115-123. [ Links ]
43. Li J, Kirkiles-Smith N, McNiff J, Pober J. TRAIL Induces Apoptosis and Inflammatory Gene Expression in Human Endothelial Cells. The Journal of Immunology. 2003;171(3):1526-1533. [ Links ]
44. Imrie H, Viswambharan H, Sukumar P, et al. Novel role of the IGF-1 receptor in endothelial function and repair: studies in endothelium-targeted IGF-1 receptor transgenic mice. Diabetes. 2012;61(9):2359-2368. DOI: 10.2337/db11-1494. [ Links ]
45. Di Paolo NC, Shayakhmetov DM. Interleukin 1± and the inflammatory process. Nat Immunol. 2016;17(8):906-913. DOI: 10.1038/ni.3503. [ Links ]
46. Dunne A, O'Neill L. The Interleukin-1 Receptor/Toll-Like Receptor Superfamily: Signal Transduction During Inflammation and Host Defense. Science Signaling. 2003;2003(171):re3-re3. [ Links ]
47. Arend WP, Guthridge CJ. Biological role of interleukin 1 receptor antagonist isoforms. Annals of the Rheumatic Diseases 2000;59:i60-i64. [ Links ]
48. Kuwabara T, Ishikawa F, Kondo M, Kakiuchi T. The Role of IL-17 and Related Cytokines in Inflammatory Autoimmune Diseases. Mediators of Inflammation. 2017;2017:1-11. [ Links ]
49. Idriss H, Naismith J. TNF? and the TNF receptor superfamily: Structure-function relationship(s). Microscopy Research and Technique. 2000;50(3):184-195. [ Links ]
50. Tandon V, Hiwale S, Amle D, Nagaria T, Patra P. Assessment of Serum Vascular Endothelial Growth Factor Levels in Pregnancy-Induced Hypertension Patients. Journal of Pregnancy. 2017;2017:1-5. [ Links ]
51. Boztosun A. Angiopoietin-related growth factor level in preeclampsia. Cumhuriyet Medical Journal. 2012;34(1): [ Links ]
52. Duhig K, Myers J, Seed P, Sparkes J, Lowe J, Hunter R et al. Placental growth factor testing to assess women with suspected pre-eclampsia: a multicentre, pragmatic, stepped-wedge cluster-randomised controlled trial. The Lancet. 2019;393(10183):1807-1818. [ Links ]
53. Goldstein J, Brown M. The LDL Receptor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(4):431-438. [ Links ]
54. Kjolby M, Nielsen M, Petersen C. Sortilin, Encoded by the Cardiovascular Risk Gene SORT1, and Its Suggested Functions in Cardiovascular Disease. Current Atherosclerosis Reports. 2015;17(4). [ Links ]
55. Rurali E, Noris M, Chianca A, Donadelli R, Banterla F, Galbusera M et al. ADAMTS13 Predicts Renal and Cardiovascular Events in Type 2 Diabetic Patients and Response to Therapy. Diabetes. 2013;62(10):3599-3609. [ Links ]
56. Sarig G. ADAMTS-13 in the Diagnosis and Management of Thrombotic Microangiopathies. Rambam Maimonides Med J. 2014;5(4):e0026. Published 2014 Oct 29. DOI: 10.5041/RMMJ.10160. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: christomark@gmail.com (MC. Arokiaraj).
Recebido a 03 de maio de 2019
Aceite a 20 de novembro de 2019