Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Angiologia e Cirurgia Vascular
versión impresa ISSN 1646-706X
Angiol Cir Vasc vol.15 no.4 Lisboa dic. 2019
ARTIGO ORIGINAL
Endovenous management of chronic venous insufficiency
Hall TC1, Braithwaite BD2, O'Neill R1, Habib S1
1 Department of Interventional Radiology. QMC, Nottingham, NG7 2UH
2 Department of Vascular Surgery. QMC, Nottingham, NG7 2UH
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Purpose: The efficacy of endovenous stenting for ileofemoral stenosis or occlusion in post-thrombotic syndrome (PTS) is gaining momentum with studies reporting improved clinical outcome. This study analyses the outcomes of venous stenting in PTS for patients in whom conservative and/or prior surgical treatment modalities had failed.
Materials and Method: Operative and clinical records were retrospectively analysed in all patients who had endovenous stenting for PTS. Baseline demographics, procedural details and symptom severity based on CEAP and Villalta scores were collected. Stent patency was assessed by follow-up duplex ultrasound. Clinical follow-up included Villalta score and a subjective assessment of improvement.
Results: Fifteen patients with a mean Villalta score of 11 were treated. Two were lost to follow-up. Technical success was 100% with no major complication. Two minor complications (self-limiting bleeding) occurred. At follow-up, stent patency, by Duplex ultrasound, was 71.4% at 13 (range 5-54) weeks. 53.8% (n=7) of patients reported subjective improvement in their symptoms, 30.8% (n=4) of patients reporting no improvement and 15.4% (n=2) of patients reporting worsening of their symptoms. The mean Villalta score at follow-up was 8.5; a significant reduction (p=0.049) from the pre-procedural score.
Conclusion: Endovenous stenting for PTS can be performed safely and with a high technical success rate. Patency rates are reasonable and are associated with a significant reduction in Villalta scores.
Keywords: Angioplasty; Deep vein thrombosis; Iliac vein compression syndrome; Stents; Venous insufficiency; Peripheral vascular disease
Introduction
Post thrombotic syndrome (PTS) is a frequent complication of acute deep vein thrombosis (DVT) of the lower limb(1,2). Although prior DVT is the most common aetiology, venous obstruction may also be caused by extrinsic compression from malignancy or anatomical variants (May-Thurner syndrome). Within 2 years of acute DVT one in every 2-3 patients will develop post thrombotic sequelae, which will be severe in approximately 10% of cases(3). This carries significant negative impact on the patients quality of life and has considerable socio-economic consequences(4,5). Indeed, some studies have shown comparability of the quality of life of those with severe PTS to patients suffering from cancer or congestive heart failure(6).
The pathophysiology of PTS is ultimately related to raised venous pressures in the capillary beds that promotes transudation of fluid and large molecules resulting in tissue fibrosis, oedema and tissue hypoxia. As a result of this skin ulceration can occur(7,8). Venous hypertension is due to venous reflux and/or obstruction that is compounded by calf muscle pump dysfunction. Risk factors for developing PTS include popliteal inflow thrombosis, recurrent deep venous thrombosis of the lower limb, iliofemoral(9) and symptomatic DVT(10).
The mainstay of treatment for PTS, once it is diagnosed, is compression therapy. Compression therapy has been conclusively shown to improve venous ulceration(11) and compression pumps can create symptomatic improvement(12) although compliance is a negating factor. Surgical bypass of iliac vein obstruction, such as the Palma femorofemoral bypass procedure, is rarely performed but occasionally reserved for the most severe symptoms. Although durable results have been reported(13) the magnitude of the operation means that only patients with the most severe symptoms would be considered.
Endovascular treatment options for PTS include angioplasty and stenting of the venous obstruction and offer a minimally invasive alternative to a surgical bypass. The procedure can be performed as a day case procedure followed by an immediate return to normal activities upon discharge. Recent meta-analyses of stent placement for ileofemoral obstruction have shown high technical success and an acceptable safety profile(14). Although the potential impact on improved quality of life is cited, the quality of evidence is weak due to lack of RCTs(15). Herein, we review our clinical experience with venous stenting for PTS in patients in whom conservative management strategies have failed to provide symptomatic improvement.
Methods
The principles of the Declaration of Helskinki were adhered to for this study. All patients who had undergone iliocaval venous stenting for chronic venous insufficiency were included in the study. Patients were retrospectively identified using a computerised database during the period from April 2012 to October 2017. Patients who had a venous stent whilst receiving catheter directed thrombolysis for the acute treatment of lower limb deep vein thrombosis were excluded.
Clinical presentation: Patients were referred to the interventional radiology department for venous stenting following assessment by a vascular surgeon in clinic with symptoms of PTS. Such symptoms included leg swelling and/or pain or skin changes/ulceration relating to venous stasis. Only patients refractory to conservative management strategies were eligible for consideration of endovascular management. Patients were referred to the vascular clinic either from the haematology team or general practitioners. Data collected included baseline demographics, Clinical-Etiology-Anatomy-Pathophysiology (CEAP) classification, previous venous surgical procedures (such as superficial venous stripping or Palma procedure) and anticoagulation status. At presentation, the Villalta PTS severity score was calculated and note made of any venous skin ulceration (as either present or absent).
Pre-operative workup: Preparatory imaging for surgical planning and suitability included venous duplex ultrasound, CT (computer tomography) or MR (magnetic resonance) venography and fluoroscopic venography. The imaging findings were recorded to include the extent of any venous disease by way of narrowing/scarring/occlusion, the vessels involved (inferior vena cava, iliac and femoral veins) and for any dynamic evidence of venous reflux (assessed on fluoroscopic venography using the Valsalva manoeuvre).
Procedural details: Two consultant interventional radiologists performed the endovascular procedure under a general anaesthetic following fully informed consent. A general anaesthetic was used in all cases due to the pain encountered during venoplasty. The endovascular treatment was performed as a day case procedure or with a single night's stay depending on the time of the surgery.. Access to the iliac system was obtained via ultrasound-guided puncture of the ipsilateral femoral vein (and/or jugular access for stent placement below the inguinal ligament). IVUS (intravascular ultrasound) was often used to delineate, more accurately, treatable lesions. If there was difficulty in crossing the lesion a rendezvous procedure was used via a right internal jugular vein puncture and the femoral access wire snared to allow for ‘body flossing'. The lesion was predilated with a balloon prior to placement of the venous stent. A suitable stent with a diameter of between 12-16mm (Veniti Vici " or WallStent") was then deployed depending on the caliber of the normal vein. Stents were post dilated matching the diameter of the stent. A sufficient number of stents were used, with overlap, to ensure that there was continuous stenting of the diseased segment. If there was disease (stenosis or occlusion) at the common iliac vein/caval confluence the stent was extended into the cava to prevent early stent stenosis (as has been demonstrated elsewhere(16)). Stents were placed across the inguinal ligament if this was necessary to ensure landing in a healthy vessel. All patients were anticoagulated prior, during and following the procedure. Following the procedure pneumatic compression stockings were used until the patient was mobile. Procedural success was defined as effective crossing of the occlusion and stenting with subsequent in-line flow on venography.
Follow-up examination: Primary patency and venous reflux were assessed with a follow-up duplex study. Patients were assessed for symptomatic improvement in the clinic. The Villalta score was recalculated and note made of the resolution (or not) of any ulcers previously present. Data was also collected for any additional procedures that took place such neovalve construction or treatment of the superficial venous system.
Endpoints and statistics: The primary endpoint was change in the Villalta score following endovenous treatment. The scores pre and post treatment were analysed for any statistical difference using a paired t-test. The secondary endpoints were procedural success and complications (minor and major), stent patency and sonographic evidence of reflux. In cases where the stent had occluded, the procedural fluoroscopic images were re-examined for any structural cause that could have contributed to it.
Results
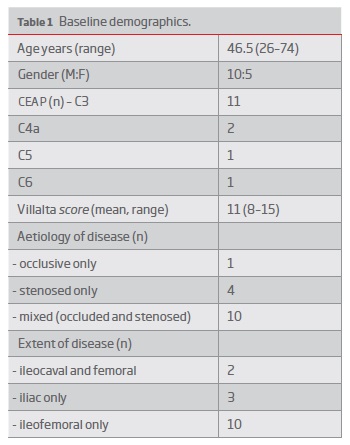
In total 15 patients were treated with a venous stent for PTS following failed conservative management. Baseline demographics are illustrated in Table I. The median age was 46.5 (range 26-74) and 68.8% of the cohort were male. 12 patients had a documented prior history of lower limb deep venous thrombosis a median of 15.5 (range 0.5-36) years prior to the stent procedure. One patient had a history of intravenous drug use. 12 patients had left sided disease and the remaining 3 patients had right sided disease. All patients had a CEAP classification of at least C3 and 3 patients had venous ulcers at the time of venous stent. All patients received oral anticoagulation at the time of treatment and were continued on therapy following the procedure. Two patients were identified as having hypercoagulability: protein C deficiency and antiphospholipid syndrome. 73.3% of patients had obstruction only and 26.7% of patients had both obstruction and reflux. The mean Villalta score was 11 (range 8-17).
Three patients had previously attempted a surgical management of their PTS comprising a neovalve construction in 2 cases and superficial venous ligation in 1 patient. One further patient had a neovalve constructed at the same time as the venous stenting and another had an adjunctive AV fistula created. Two patients also had a venous stent previously placed during the acute phase of the DVT (that was now chronically occluded) with one of these patients also having a failed Palma procedure. All patients had disease affecting the iliofemoral venous system with 2 patients having superadded caval disease. The May-Thurner variant was identified in 2 patients.
The median number of stents used was 2 (range 1-4) with a diameter of 14mm (range 12-18mm). Venous stents were deployed across the inguinal ligament in 93.3% (n=14) of cases. A Veniti Vici "stent was used in 12 patients with 2 patients having just a Wallstent" and 1 patient receiving both Wallstent" and Veniti Vici" stent. Intravenous ultrasound was used in 73% of patients. All patients had predilation with non-compliant high-pressure balloons. This was to a size equal to the stent inserted in 33.3% of cases with the remaining patients having predilation to a size below the stent. Post-stent dilation was used in all patients with a balloon sized at least equal to the stent diameter in all but one case where a 12mm stent was postdilated using a 9mm balloon. Technical success was achieved in 100% of cases. There were two minor complications with post procedural bleeding at the puncture site that were self-limited and did not require blood transfusion or further intervention. No major complications were encountered.
Two patients were lost to clinical follow-up and one patient lost to imagiological follow-up. All remaining patients had both clinical and imagiological follow-up. Median time from procedure to the first follow-up Duplex ultrasound was 13 weeks (range 5-54 weeks). The stents had occluded in 28.6% (n=4) of patients with patency confirmed in the remaining 71.4%. Where possible, stent patency was assessed directly based on direct vision, however, where this was not possible indirect signs, such as phasicity of flow in caudal venous segments, were assessed. The time from procedure to the duplex scan confirming a stent occlusion was 3 days, 3 weeks, 16 weeks and 3 months respectively. Three patients with stent occlusion had either no improvement in symptoms (n=1) or worsening of the symptoms (n=2). The remaining patient reported improved symptoms.
The median duration of clinical follow-up was 26 months (range 3-48 months). At clinical follow-up, 53.8% (n=7) of patients reported subjective improvement in their symptoms, 30.8% (n=4) of patients reporting no improvement and 15.4% (n=2) of patients reporting worsening of their symptoms. One patient with an occluded stent and the creation of a neovalve at the same time of stenting reported improved symptoms. Regarding the two patients that reported worsening of the symptoms, in one patient the stent had occluded and in the other, whilst the stent was patent, he had developed venous reflux into the great saphenous vein causing exacerbation of the pain that also failed to resolve with subsequent great saphenous vein ligation. The mean Villalta score at follow-up was 8.5; a significant reduction (p=0.049) from the preprocedural score.
Discussion
Our analysis has shown that venous stenting can be performed under a general anaesthesia with 100% technical success and with an acceptable complication profile. In the short term follow up there was a significant reduction in the Villalta score corresponding to an improvement in symptoms.
Our data shows that the primary patency (at a median of 13 weeks from the index procedure) was 75%. This compares well to the literature where patency rates range from 32-98.7%(15). The meta-analysis by Razavi et al aimed to propose performance goals for venous stenting primary patency(14). For chronic post thrombotic syndrome, they proposed that the primary patency at 1 year should be 66-71%. We had 1 (6.7%) early thrombotic occlusion (day 3) which matches the early occlusion rate found by Razavi et al in their subgroup analysis of PTS patients (6.8%)(14), higher than in patients having venous stenting for non-occlusive disease or acute thrombosis. They identified the quality of inflow and outflow, rigor of anticoagulation, lesion length and location and stent diameter/length as factors that could relate to early thrombosis. Unfortunately our cohort was too small to perform subgroup analysis for factors contributing to early thrombosis. No structural reasons could be identified retrospectively when reviewing the procedural imaging however.
Interestingly, we encountered one patient, in whom a venous stent was deployed and a neovalve created during the same procedure, where the stent occluded early (3 days from the procedure) but who reported much-improved symptoms at follow-up. Although recent studies have however thrown doubt on the significance of reflux in patients with mixed obstruction/reflux we propose that the obstruction neo-valve construction had possibly treated the reflux for which their symptoms were largely attributed to. Studies have shown no difference in ulcer healing rates in patients treated with stent correction alone between limbs with/without axial reflux(17).
In our hospital it is our current practice to perform a single ultrasound follow-up within 3 months of the procedure with only clinical follow-up beyond this. Other authors suggest early (up to 2 weeks) ultrasound surveillance of venous stents to predict the need for reintervention on those stents at risk of occluding(18). In an abstract presented by Gwozdz and colleagues, the frequency of follow-up surveillance ultrasound was stratified based on the stent diameter reduction with reintervention for those with >50% stenosis. This achieved a stent patency of 86% with a median follow-up of 2.4 years. Gwozdz also found increased rates of reintervention in those patients with stents that extended across the inguinal ligament. A more recent study by the same group however failed to demonstrate any patency or clinical outcome inferiority in patients with stents extending below the inguinal ligament(19). They too reported significant improvement in Villalta scores at 24 months of follow-up (p<0.001) and primary stent patency rates at 12 and 24 months of 59% and 51% respectively. Other authors who reported venous stenting across the inguinal ligament found no increased risk of complications or patency(20). Neglen and colleagues reported reduced patency in patients with occlusive disease versus non-occlusive disease. Our study is too small to deduce any conclusions on this.
Abramowitz and colleagues have suggested that an intravascular ultrasound (IVUS) based scoring system could be used to predict those venous stents that are likely to fail(21). They looked specifically at stenting in May-Thurner syndrome and although the scoring system has yet to be validated it could well prove useful in predicting those patients who would benefit from more a frequent and/or early surveillance ultrasound.
We encountered no major complication and 2 minor self-limiting haemorrhages following venous puncture, both of which settled with conservative management and did not require blood transfusion. Major complication rates in the literature range from 0-8.7%(15).
We mainly used the Veniti Vici" stent for our procedures. Studies have however, failed to show the superiority of any particular stent(22). There is potentially a void in the market for appropriate venous stents that not only exhibit flexibility but also radial force. Frequently the Wallstent" is used with diameters available exceeding 20mm. Whilst the Wallstent" offers strength and flexibility it can foreshorten and is weakest at its end. We believe this can have poor outcomes in narrowings caused by May-Thurner syndrome. There is also however the concern that nitinol stents do not possess sufficient crush resistance to counteract the venous compression syndromes
There are several limitations to our study. The follow-up period is short. Many years of clinical and imaging follow-up are required to assess the efficacy of venous stenting. In our hospital there is only funding available for a single follow-up ultrasound and therefore occlusions after the initial ultrasound cannot be excluded. We instead assess patients for clinical improvement as opposed to surveillance and preemptive reintervention for stenoses over 50%. Such preemptive surveillance could lead to improved clinical results and prolonged stent patency in the long term. We accept that we are therefore unaware of any stent compression from choke points or recurrent postthrombotic stenosis as has been shown to occur in iliac vein stents(23).
As the study was retrospective we have no data on quality-of-life (QoL) measures, either ulcer specific QoL measures or general QoL sores based on venous insufficiency.
In addition, our numbers are small, do not allow for subgroup analysis and include but thrombotic and non-thrombotic aetiologies. With much larger numbers it would be useful to be able to identify by multivariate analysis those patients who fail to gain clinical benefit or those with a stent occlusion. A meta-analysis with accurate data on IVUS findings, adequate inflow/outflow, aetiology (eg May-Thurner syndrome), vessel preparation, stent choice and long-term clinical and imaging follow-up is needed.
In conclusion venous stenting for chronic venous insufficiency is safe with a reasonable rate of patency on short-term follow-up and an associated significant improvement in Villalta scores.
REFERENCES
1. Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. [ Links ]
2. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698-707. [ Links ]
3. Prandoni P, Kahn SR. Post-thrombotic syndrome: Prevalence, prognostication and need for progress. Br J Haematol. 2009;145(3):286-295. [ Links ]
4. Guanella R, Ducruet T, Johri M, et al. Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: A prospective evaluation. J Thromb Haemost. 2011;9(12):2397-2405. [ Links ]
5. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. [ Links ]
6. Kahn SR, Ducruet T, Lamping DL, et al. Prospective evaluation of health-related quality of life in patients with deep venous thrombosis. Arch Intern Med. 2005;165(10):1173-1178. [ Links ]
7. Araki CT, Back TL, Padberg FT, et al. The significance of calf muscle pump function in venous ulceration. J Vasc Surg. 1994;20(6):872-7; discussion 878-9. [ Links ]
8. Nicolaides AN, Hussein MK, Szendro G, Christopoulos D, Vasdekis S, Clarke H. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17(2):414-419. [ Links ]
9. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001;110(7):515-519. [ Links ]
10. Wille-Jorgensen P, Jorgensen LN, Crawford M. Asymptomatic postoperative deep vein thrombosis and the development of postthrombotic syndrome. A systematic review and meta-analysis. Thromb Haemost. 2005;93(2):236-241. [ Links ]
11. O'Donnell MJ, McRae S, Kahn SR, et al. Evaluation of a venous-return assist device to treat severe post-thrombotic syndrome (VENOPTS). A randomized controlled trial. Thromb Haemost. 2008;99(3):623-629. [ Links ]
12. Ginsberg JS, Magier D, Mackinnon B, Gent M, Hirsh J. Intermittent compression units for severe post-phlebitic syndrome: A randomized crossover study. CMAJ. 1999;160(9):1303-1306. [ Links ]
13. Jost CJ, Gloviczki P, Cherry KJ,Jr, et al. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33(2):320-7; discussion 327-8.
14. Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: Systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8(10):e002772. [ Links ]
15. Seager MJ, Busuttil A, Dharmarajah B, Davies AH. Editor's choiceA systematic review of endovenous stenting in chronic venous disease secondary to iliac vein obstruction. Eur J Vasc Endovasc Surg. 2016;51(1):100-120. [ Links ]
16. Sarici IS, Yanar F, Agcaoglu O, et al. Our early experience with iliofemoral vein stenting in patients with post-thrombotic syndrome. Phlebology. 2014;29(5):298-303. [ Links ]
17. Raju S, Kirk OK, Jones TL. Endovenous management of venous leg ulcers. J Vasc Surg Venous Lymphat Disord. 2013;1(2):165-172. [ Links ]
18. Gwozdz A, Saha P, Silickas J, et al. Early duplex surveillance following deep venous stenting for the treatment of post-thrombotic syndrome can predict patients at greatest risk for re-intervention BSET Annual Meeting 2018. 2018. [ Links ]
19. Black S, Gwozdz A, Karunanithy N, et al. Two year outcome after chronic iliac vein occlusion recanalisation using the vici venous stent((R)). Eur J Vasc Endovasc Surg. 2018. [ Links ]
20. Neglen P, Tackett TP,Jr, Raju S. Venous stenting across the inguinal ligament. J Vasc Surg. 2008;48(5):1255-1261.
21. Gustafson A, Chick JFB, Malik RK, et al. VESS06. an intravascular ultrasound-based scoring system may predict future stent failure in the treatment of may-thurner syndrome. Journal of vascular surgery. 2018;67(6):e51. [ Links ]
22. Blanch Alerany M, Izquierdo Lamoca LM, Ramirez Ortega M, Lago Rivas I, Zotta Desboeufs R, Stefanov Kiuri S. Endovascular treatment of iliofemoral chronic post-thrombotic venous flow obstruction. J Vasc Surg Venous Lymphat Disord. 2014;2(1):2-7. [ Links ]
23. Raju S, Davis M. Anomalous features of iliac vein stenosis that affect diagnosis and treatment. J Vasc Surg Venous Lymphat Disord. 2014;2(3):260-267. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: tch2@doctors.org.uk (T. Hall).
Recebido a 28 de maio de 2019
Aceite a 30 de dezembro de 2019