Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Angiologia e Cirurgia Vascular
versão impressa ISSN 1646-706X
Angiol Cir Vasc vol.15 no.2 Lisboa jun. 2019
ARTIGO DE REVISÃO
The role of the skeletal muscle in atherosclerosis
Papel do músculo esquelético na aterosclerose
Joana Ferreira1,2, Pedro Cunha1,3,4, Armando Mansilha5,6,7, Cristina Cunha3, Cristina Silva3, Isabel Vila3, Jorge Cotter1,3, Amílcar Mesquita2
1 Life and Health Science Research Institute (ICVS), School of Medicine, University of Minho, Guimarães/Braga, Portugal;
2 Vascular Surgery Department- Hospital da Senhora da Oliveira, Guimarães.3 Center for the Research and Treatment of Arterial Hypertension and Cardiovascular Risk, Internal Medicine Department- Hospital da Senhora da Oliveira, Guimarães.
4 Vascular Surgery Department- Hospital de São João
5 Faculty of Medicine, Porto University
6 Vascular Surgery Department, Hospital CUF Porto
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
The role of visceral obesity in atherosclerosis is well recognized by the medical community. On the contrary, the importance of skeletal muscle is almost unknown. Muscle is nowadays understood as an endocrine organ producing myokines with direct action in several physiological and pathological pathways, including atherosclerosis. The myokines reduce the formation of neointima, expression of inflammatory mediators, the recruitment of inflammatory cells and the formation of foam cells. Epidemiological studies are demonstrating the association between reduced muscle mass and cardiovascular atherosclerotic disease. Low muscle mass is associated with an increased prevalence of coronary heart disease, aortic calcification, carotid atherosclerosis, carotid intima-media thickness, intracranial artery stenosis and endothelial dysfunction. In this way resistance training, which increases the muscle size and strength may have a key role in atherosclerosis while endurance training alone might not. The time and type of protein intake by older adults may be critical to the maintenance of muscle mass and to the increase survival. The objective of this paper was to perform a review about the published literature in the last 13 years about the role of skeletal muscle in atherosclerosis.
Keywords: Skeletal muscle; Atherosclerosis; Sarcopenia; Peripheral artery disease; coronary artery disease; Carotid artery disease
RESUMO
O papel da obesidade visceral na doença aterosclerótica está bem definido. Pelo contrário, a importância do músculo esquelético não está completamente esclarecido. O músculo é entendido hoje, como um órgão endócrino produtor de mioquinas com uma acção direta em vários mecanismos fisiológicos e fisiopatológicos, incluindo a aterosclerose. As mioquinas reduzem a produção da neo-íntima, a expressão de mediadores inflamatórios, o recrutamento de células inflamatórias e a formação de “foam cells”. Os estudos epidemiológicos demonstram uma associação entre a redução da massa muscular e a doença cardiovascular. A baixa massa muscular está associada a aumento da prevalência da doença coronária, calcificação aórtica, aterosclerose carotídea, espessamento média-íntima, estenose das artérias cerebrais e disfunção endotelial. Deste modo, os exercícios de resistência, que causam hipertrofia muscular e aumentam a foça muscular, podem ter um papel na aterosclerose. A ingestão proteica, em particular pelos idosos parece ser crítica para a manutenção da massa muscular e para aumentar a sobrevivência. O objetivo deste artigo é proceder a uma revisão da literatura relativamente ao papel musculo esquelético na aterosclerose.
Palavras-chave: Músculo esquelético; Aterosclerose; Sarcopenia; Doença arterial periférica; Doença coronária; Doença carotídea
Introduction
The role of skeletal muscle as an endocrine organ with a direct role in atherosclerosis is starting to be defined(1,2,3,4,5,6). Understanding the skeletal muscle has an extraordinary importance in these days, due to the increased prevalence of sarcopenia(7). Sarcopenia is the loss of muscle mass and function, and one of its causes is aging(7). Sarcopenia and sarcopenia associated with obesity are both linked with cardiovascular disease(1,4). Define the primary care intervention, such as the tailored nutrition and exercise could help in the prevention of cardiovascular disease.
Methodology
A systematic review was performed. Were included all kind of articles published in english and Portuguese, from January 2005 till December 2018 in which the full text was available. The words used in research were: Peripheral Arterial Disease AND Skeletal Muscle, Peripheral Arterial Disease AND Sarcopenia, Atherosclerosis AND Skeletal Muscle, Atherosclerosis AND Sarcopenia, Carotid Artery Disease AND Skeletal Muscle, Carotid Artery Disease e AND Sarcopenia, Coronary Artery Disease AND Skeletal Muscle, Coronary Artery Disease AND Sarcopenia. The research was conducted between December 2017 to January 2019.
Resultados
1580 articles were found. After reading the title and abstracts. 45 articles were selected. These articles were read and 32 were selected to write this paper.
Discussion
Skeletal muscle as an endocrine organ
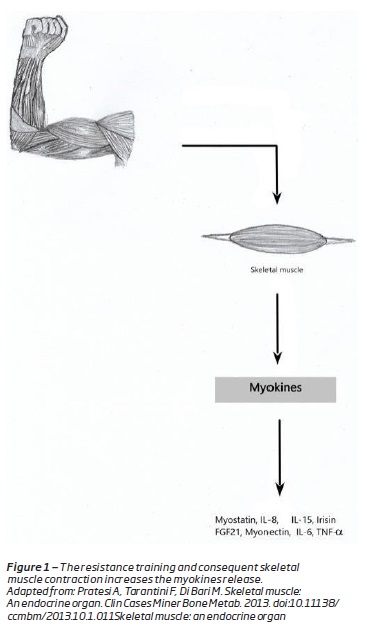
Skeletal muscle is nowadays understood as an endocrine organ(8,9). It has the capacity to produce hormone-like factor, called myokines, that influence metabolism and organs(8). Myokines have an autocrine, paracrine and endocrine actions(8). They have major implications on metabolic and other properties of muscle as well as distal organs(9). They are responsible for the protective actions of exercise(8) (Figure 1). The myokines release is the biological explanation for the metabolic and anti-inflammatory effect of exercise(1).
Are examples of myokines: irisin, myostatin, myonectin, interleukin (IL)-6, IL-15 and IL-8(3). The first muscle-derived secreted protein to be described was IL-6(9). IL-6 induces glucose uptake and fatty acid ²-oxidation in muscle, stimulates hepatic gluconeogenesis and induces lipolysis(9).w Therefore the skeletal muscle is responsible for body’s glucose and lipid metabolism(10). Decreased muscle mass causes deterioration of insulin sensitivity, which is an underlying risk factor for atherosclerosis(10).
IL-15 is a myokine induced by resistance training that negatively regulates the visceral fat mass and increases the insulin sensitivity(3,8,9,11,12). Irisin is also released by skeletal muscle in response to exercise and regulates the glucose and lipids homeostasis(13). Irisin, induces glucose uptake, causes increased in energy expenditure and oxidative metabolism in skeletal muscle, and modulates the expression of metabolic enzymes and intermediates(3). In obese subjects the seric levels of irisin are reduced and are related with insulin sensitivity(3).
Sarcopenia
Sarcopenia is a progressive decline of skeletal muscle mass and strength that is associated with age and is related to physiological, metabolic and functional compromise(4,9,14,15,16,17). From a structural point of view, there is mitochondrial disfunction, reduction in size and number of muscle fibbers, particularly type II and decrease in protein synthesis(5).
The number of patients with sarcopenia has been increasing worldwide as a result of the aging population, being a major healthcare concern for older adults(7,10).
A progressive loss of muscle mass occurs from approximately 40 years of age(5,9). This loss has been estimated at about 8% per decade until the age of 70 years, after which the loss increases to 15% per decade(5,9,10).
Sarcopenia is usually accompanied by physical inactivity, decreased mobility, slow gait, functional decline, decrease in quality of life and mortality(1,7,14,15,16,17). As the muscle is the major “reserve” of ready available amino acids(7). Inadequate muscle mass prior to surgery or to the onset of a disease may impair the recovery or increase the risk of mortality(7).
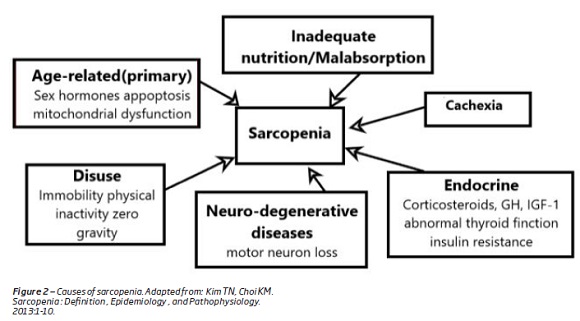
The causes of sarcopenia with age are (Figure 2):
1. A progressive and irreversible neuron loss that is associated with age and contributes to the reduction in the fiber number and fiber mass(1).
2. Modification of hormones production and sensitivity which occur with age, in particular growth hormone, corticosteroids, androgen and estrogens(1,7,14). These hormones influence the anabolic and the catabolic state of the muscle protein metabolism(1). For instance the reduction, in testosterone level, associated with age causes a decrease in muscle mass and bone strength, with an increased risk of fractures(1,18).
3.With aging the synthesis of protein is resistant to the anabolic action of insulin(1). Inversely, loss of skeletal muscle, which is the largest insulin-responsive target tissue, may produce insulin resistance that promotes cardiovascular disease(1).
Other factors, such as nutritional deficiencies, insulin resistance, cigarette smoking, chronic lung disease, oxidative stress, inflammation and physical inactivity also contributes to sarcopenia (Figure 2)(5,14).
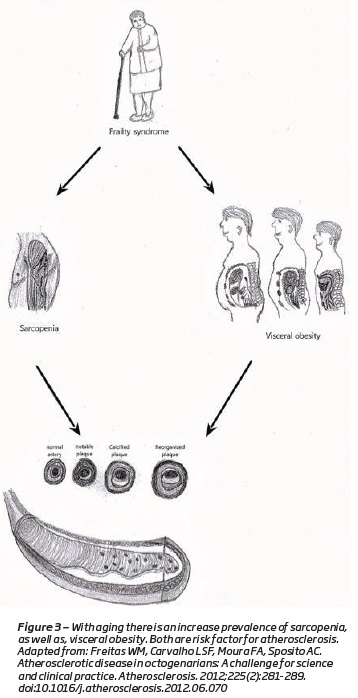
The loss of muscle mass and strength causes a reduction in physical activity(1). Sedentary life style plays a major role on the loss of the muscle mass (Figure 2)(14,18). They cause a decrease in total of energy expenditures, which results in the accumulation of fat mass(1,4,19,20)(Figure 3). Along with visceral fat accumulation, loss of skeletal muscle, which is the largest insulin-responsive target tissue, produces insulin resistance promoting metabolic syndrome(1). These imbalances are extreme in some individuals, producing a condition that is a combination of obesity and sarcopenia termed “sarcopenic obesity”(1,20,21).
Sarcopenia, “sarcopenic obesity” and atherosclerosis
Some studies support the association between the decrease in skeletal muscle mass and cardiovascular disease(4,5,6,14,16,22,23).
The sarcopenic subjects have a higher incidence of cardiovascular risk factors. The total cholesterol, LDL-cholesterol, systolic and diastolic blood pressures are higher in patients with sarcopenia than in those without sarcopenia(19). Sarcopenia often co-exists with insulin resistance(19,20). The mass and quality of skeletal muscle are associated with glucose uptake and consequently sarcopenia can induce the development of type 2 diabetes(20).
It was found that individuals with sarcopenia have higher seric level of high sensitivity c reactive protein than those without sarcopenia(19).
“Sarcopenic obesity” exacerbates even more the cardiovascular risk(20). A reduction in muscle mass results in lower total energy expenditure, leading to the development of visceral obesity(19,20). Visceral fat accumulation, rises the levels of inflammatory cytokines, increasing the skeletal muscle catabolism(7,20). The skeletal muscle mass decline, diminish the myokine secretion, increasing the inflammation and the insulin resistance(20). Combinations of these alterations in humoral factors might induce or increase oxidative stress(20). Oxidative stress is the key pathway in vascular endothelial damage, leading to the development of atherosclerosis(20).
Dislipidemia, hypertension, insulin resistance, sedentarism, obesity and high sensitivity c reactive protein are well known risk factor for arteriosclerosis(19).
Clinical studies demonstrated an association between reduced muscle mass and cardiovascular atherosclerotic disease(6,14,22,23). Low muscle mass is associated with an increased prevalence of coronary heart disease, aortic calcification, carotid atherosclerosis, carotid intima-media thickness, intracranial artery stenosis and endothelial dysfunction(4,5,6,14,16,22). Other studies, also demonstrated that low urinary creatinine excretion (an indirect measure of muscle mass) is related to major cardiovascular events in general population(14,24).
In sarcopenic patients, the muscle function - strength - is even more important than muscle mass. The strength reduction is associated with subclinical atherosclerosis, endothelial dysfunction, carotid intima-media thickness, high morbidity and mortality, in particular cardiovascular mortality(14,16,25,26,27).
Sarcopenia is also strongly associated with peripheral arterial disease(4,7). Patients with peripheral arterial disease (PAD) have a lower psoas muscle area and muscle size compared to non-PAD patients(4). Older patients with peripheral arterial disease have 10-40% less muscle mass compared to age-matched control(7). Particularly, in PAD the dysfunction of skeletal muscle is increased by two mechanisms: the muscle hypoxia consequently to atherosclerotic occlusive disease induces muscle atrophy impairing its function(7,22); the intermittent claudication pain and critical limb ischemia inactivity decreases the physical movement increasing the risk of “sarcopenic obesity”(7). Critical limb ischemia patients with reduced skeletal muscle mass have also a worse survival when compared to critical limb ischemia patients without sarcopenia(28).
As an endocrine organ the skeletal muscle secretes several myokines, some of them with a direct role in atherosclerosis.
Irisin is an example of a myokine (a soluble transmembrane protein I) described in 2012, released by the skeletal muscle in response to exercise(3, 13,22). It induces glucose uptake and regulates the lipids homeostasis(3,13,22). Some authors considered that irisin can prevent obesity and obesity-associated type 2 diabetes(3).
Irisin reduces the formation of neointima, expression of inflammatory mediators (ICAM-1, VCAM- 1, MCP-1), the recruitment of inflammatory cells and the formation of foam cells(13). It preserves the endothelial cells integrity(13). In animal models the irisin reduces the atherosclerosis in aorta(13). It has also been proved that serum irisin is a significant independent predictor for carotid atherosclerosis(24).
IL-15 is a myokine induced by resistance training that negatively regulates visceral fat mass, increases insulin sensitivity and interferes with the lipid metabolism(2,3,9,10,29,30).
Follistatin-like 1 is another myokine that protects against vascular and cardiac injury(9). It controls vascular smooth muscle cell proliferation endothelial cell apoptosis and neointimal formation(9).
Muscle mass is important for prevention of metabolic disease and atherosclerosis(25). Therefore, the treatment of sarcopenia may play an important role in the prevention of arteriosclerotic diseases(4,14). Resistance training and nutritional therapies used to treat sarcopenia will prevent cardiovascular events(5).
Exercise training AND Food
Regular exercise, particularly resistance training, contributes to the maintenance of muscle mass, reducing the sarcopenia and the rate of functional decline(4,6,22).
It is known that endurance exercise reduces the cardiovascular mortality and morbidity(6,9,18). More interesting is that resistance training (which causes muscle hypertrophy and strengthening) also benefits the cardiovascular system(9). It has been proposed that the well-known protective actions of exercise are due to increased secretion of myokines(6,9,18). Contracting skeletal muscles release myokines with endocrine actions, mediating anti-inflammatory effects, reducing the visceral fat and improving the metabolism(6,9,29). The benefits of exercise go far beyond the molecular adaptations of working skeletal muscle(29).
The neuromuscular system have the ability to respond to heavy loading, even in sedentary older adults(4). Sedentary people into their 80s demonstrate an increase in skeletal muscle mass and strength after short-term resistance exercise(4).
Another interesting data, is that, resistance training have a key role in sarcopenia, compared to the endurance exercise, because it increases the muscle size and strength, while endurance training alone might not(16,31).
Besides physical activity, nutrition have also a role in sarcopenia. The timing and type of protein intake by older adults may be critical to the maintenance of muscle mass. Patient should have an adequate high-quality protein intake(32). Maintenance of muscle mass requires relatively high protein intake on the order of 1.2 to 1.5 g/kg(22). There is evidence that the current recommended dietary for protein intake may not be adequate to maintain optimal skeletal muscle health in older adults(4). Over the course of three years, the people who consumed more than the recommended dietary allowance for protein of 0.8 g/kg/day experienced the smallest losses of skeletal mass(4). In contrast, individuals who had the most significant muscle atrophy consumed less quantities of proteins(4). Moreover, protein synthesis declines in the elderly, when meals contain less protein or are ingested in conjunction with carbohydrates(4).
Loss of appetite, anorexia, economic limitations, gastrointestinal, taste, smell and social changes, contribute to a decrease in protein intake in the elderly people(4). Caloric insufficiency can results the loss of muscle mass, but excess of caloric intake results in obesity and accelerates sarcopenia(4).
Older adults have inadequate diet and do not practice regular exercise. The US Department of Agriculture’s Healthy Eating Index indicates that 80% of adults over the age of 65 years do not perform any in regular physical activity(4). 88% do not practice muscle strengthening exercises, which provide many beneficial effects in skeletal muscle(4). 80% of older must decrease the consumption of saturated fats and increase the protein and fibber intake(4).
Conclusion
Skeletal muscle is understood as an endocrine organ, producing myokines with direct action in several disease pathways, including atherosclerosis. Due to the population aging, the number of sarcopenic patients, particularly “sarcopenic obese” is increasing, with a negative impact on atherosclerosis. Resistance training, diets rich in proteins and poor in carbohydrates can have a role in atherosclerosis prevention.
REFERENCES
1. Pratesi A, Tarantini F, Di Bari M. Skeletal muscle: An endocrine organ. Clin Cases Miner Bone Metab. 2013. DOI: 10.11138/ccmbm/2013.10.1.011. [ Links ]
2. Pedersen BK, Brandt C. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010. DOI: 10.1155/2010/520258. [ Links ]
3. Li F, Li Y, Duan Y, Hu CAA, Tang Y, Yin Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017;33:73-82. DOI: 10.1016/j.cytogfr.2016.10.003. [ Links ]
4. Ko BJ, Chang Y, Jung HS, et al. Relationship between low relative muscle mass and coronary artery calcification in healthy adults. Arterioscler Thromb Vasc Biol. 2016. DOI: 10.1161/ATVBAHA.116.307156. [ Links ]
5. Freitas WM, Carvalho LSF, Moura FA, Sposito AC. Atherosclerotic disease in octogenarians: A challenge for science and clinical practice. Atherosclerosis. 2012;225(2):281-289. DOI: 10.1016/j.atherosclerosis.2012.06.070. [ Links ]
6. Alexandersen P, Christiansen C. Associations between Aortic Calcification and Components of Body Composition in Elderly Men. 2006;14(9):1571-1578.
7. Buford TW, Anton SD, Judge AR, et al. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010. DOI: 10.1016/j.arr.2010.04.004. [ Links ]
8. Ouchi N, Ohashi K, Shibata R, Murohara T. Protective Roles of Adipocytokines and Myokines in Cardiovascular Disease. Circ J. 2016;80(10):2073-2080. DOI: 10.1253/circj.CJ-16-0663. [ Links ]
9. Kim TN, Choi KM. Sarcopenia : Definition , Epidemiology , and Pathophysiology. 2013:1-10.
10. Hida T, Imagama S, Ando K, et al. Sarcopenia and physical function are associated with inflammation and arteriosclerosis in community-dwelling people: The Yakumo study. Mod Rheumatol. 2018. DOI: 10.1080/14397595.2017.1349058. [ Links ]
11. Dionne IJ, Dionne IJ. Risk Factors and Chronic Disease Effect of Sarcopenia on Cardiovascular Disease Risk Factors in Obese Postmenopausal Women. 2006;14(12):2277-2283.
12. Li F, Li Y, Duan Y, Hu CA, Tang Y, Yin Y. Cytokine & Growth Factor Reviews Myokines and adipokines : Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017;33:73-82. DOI: 10.1016/j.cytogfr.2016.10.003. [ Links ]
13. Askari H, Rajani SF, Poorebrahim M, Haghi-Aminjan H, Raeis-Abdollahi E, Abdollahi M. A glance at the therapeutic potential of irisin against diseases involving inflammation, oxidative stress, and apoptosis: An introductory review. Pharmacol Res. 2018;129:44-55. DOI: 10.1016/j.phrs.2018.01.012. [ Links ]
14. Ochi M, Kohara K, Tabara Y, et al. Arterial stiffness is associated with low thigh muscle mass in middle-aged to elderly men. Atherosclerosis. 2010;212(1):327-332. DOI: 10.1016/j.atherosclerosis.2010.05.026. [ Links ]
15. Morley JE, Kim MJ, Haren MT, Kevorkian R, Banks WA. Frailty and the aging male. Aging Male. 2005. DOI: 10.1080/13685530500277232. [ Links ]
16. Campos AM, Moura FA, Santos SN, Freitas WM, Sposito AC. Sarcopenia, but not excess weight or increased caloric intake, is associated with coronary subclinical atherosclerosis in the very elderly on behalf of Brasilia Study on Healthy Aging and Brasilia Heart Study. Atherosclerosis. 2017:1-7. DOI: 10.1016/j.atherosclerosis.2017.01.005. [ Links ]
17. Bano G, Trevisan C, Carraro S, et al. Maturitas Inflammation and sarcopenia : A systematic review and meta -analysis. Maturitas. 2017;96:10-15. DOI: 10.1016/j.maturitas.2016.11.006. [ Links ]
18. Hida T, Imagama S, Ando K, et al. Sarcopenia and physical function are associated with inflammation and arteriosclerosis in community-dwelling people : The Yakumo study. Mod Rheumatol. 2017;0(0):1-6. DOI: 10.1080/14397595.2017.1349058. [ Links ]
19. Nakano R, Takebe N, Ono M, et al. Involvement of oxidative stress in atherosclerosis development in subjects with sarcopenic obesity Obesity Science & Practice. 2017;(5). DOI: 10.1002/osp4.97. [ Links ]
20. Hoffmann C, Weigert C. Skeletal muscle as an endocrine organ: The role of myokines in exercise adaptations. Cold Spring Harb Perspect Med. 2017. DOI: 10.1101/cshperspect.a029793. [ Links ]
21. Ferro G, Basile C, Liguori I, et al. NU SC. EXG. 2016. DOI: 10.1016/j.exger.2016.09.007.
22. Jung HJ, Jung H, Lee T, et al. Decreased muscle mass in Korean subjects with intracranial arterial stenosis: The Kangbuk Samsung Health Study. Atherosclerosis. 2017. DOI: 10.1016/j.atherosclerosis.2016.05.003. [ Links ]
23. Lee JSW, Auyeung TW, Kwok T, Lau EMC, Leung PC, Woo J. Associated factors and health impact of sarcopenia in older Chinese men and women: A cross-sectional study. Gerontology. 2008. DOI: 10.1159/000107355. [ Links ]
24. Jensky NE, Allison MA, Loomba R, et al. Null association between abdominal muscle and calcified atherosclerosis in community-living persons without clinical cardiovascular disease: The multi-ethnic study of atherosclerosis. Metabolism. 2013. DOI: 10.1016/j.metabol.2013.06.001. [ Links ]
25. Mazza C, Barbe C, Perrier M, Botsen D, Renard Y. Feasibility of Systematic Handgrip Strength Testing in Digestive Cancer Patients Treated With Chemotherapy : The FIGHTDIGO Study. 2017:1-6. DOI: 10.1002/cncr.31207.
26. Shimizu Y, Sato S, Koyamatsu J, et al. Handgrip strength and subclinical carotid atherosclerosis in relation to platelet levels among hypertensive elderly Japanese. Oncotarget. 2017. DOI: 10.18632/oncotarget.20618. [ Links ]
27. Matsubara Y, Matsumoto T, Aoyagi Y, et al. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J Vasc Surg. 2015;61(4):945-950. DOI: 10.1016/j.jvs.2014.10.094. [ Links ]
28. Matsubara Y, Matsumoto T, Aoyagi Y, Tanaka S. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J Vasc Surg. 2015;61(4):945-950. DOI: 10.1016/j.jvs.2014.10.094. [ Links ]
29. Morley JE. Diabetes , Sarcopenia , and Frailty. 2008;24:455-469. DOI: 10.1016/j.cger.2008.03.004.
30. Pedersen BK. Muscles and their myokines. J Exp Biol. 2011. DOI: 10.1242/jeb.048074. [ Links ]
31. Barazzoni R, Bischoff SC, Boirie Y, Busetto L, Yumuk V, Vettor R. Sarcopenic obesity : Time to meet the challenge. Clin Nutr. 2018;(May):1-7. DOI: 10.1016/j.clnu.2018.04.018. [ Links ]
32. Lee MJ, Lee SA, Nam BY, et al. Irisin, a novel myokine is an independent predictor for sarcopenia and carotid atherosclerosis in dialysis patients. Atherosclerosis. 2015;242(2):476-482. DOI: 10.1016/j.atherosclerosis.2015.08.002. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: joana222@gmail.com (J. Ferreira).
Acknowledgements
I want to thank Joana Raquel Ferreira for helping in the article formation and language correction; Tania Ferreira for the drawing adaptation and Andreia Ferreira for literature research.
Recebido a 28 de abril de 2019
Aceite a 23 de agosto de 2019