Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Angiologia e Cirurgia Vascular
versão impressa ISSN 1646-706X
Angiol Cir Vasc vol.14 no.4 Lisboa dez. 2018
ARTIGO DE REVISÃO
Catheter-directed Thrombolysis for Deep Venous Thrombosis in pregnant women and patients with active cancer (A Comprehensive Review)
Trombólise dirigida por cateter no tratamento da trombose venosa profunda em grávidas e doentes com neoplasia ativa (Revisão Compreensiva)
Duarte Rego1, Gabriela Teixeira1, Daniel Mendes1, Paulo Almeida1, Rui Almeida1
1Serviço de Angiologia e Cirurgia Vascular, Centro Hospitalar Universitário do Porto
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Introduction: Post-thrombotic syndrome is associated with severely decreased quality of life and develops in up to 50% of patients with iliofemoral deep vein thrombosis (DVT) despite effective anticoagulation. Catheter-directed thrombolysis (CDT) use has become widespread and is supported by a growing body of scientific evidence (including randomized controlled trials). However, almost all of these trials have excluded two groups of patients in which DVT has a particularly increased burden: pregnant women and patients with active cancer.
Aims: This non-systematic review of literature aims to provide a comprehensive analysis of the existing evidence on the safety and efficacy of CDT for iliofemoral DVT in these subgroups of patients.
Results: Endovascular treatment of iliofemoral DVT during pregnancy and puerperium seems safe and effective both for the pregnant woman and the fetus. The risks of radiation (especially in the first trimester) must be discussed and taken in consideration.
CDT and pharmacomechanical thrombolysis (PMT) are both safe and effective in patients with active cancer, as long as metastatic brain lesions are excluded. However, effective anticoagulation (with low-molecular weight heparin or, in selected patients, direct oral anticoagulants) should be prescribed after the intervention to maintain patency in these patients with continued thrombophilia.
Conclusions: CDT, with or without PMT, should be offered to pregnant patients and patients with active cancer provided that a careful risk-benefit assessment is made for each individual patient.
Keywords: Deep Vein Thrombosis; Thrombolytic therapy; Catheter-directed thrombolysis; Post-thrombotic syndrome; Cancer; Pregnancy
RESUMO
Introdução: A síndrome pós-trombótico está associada a uma diminuição significativa da qualidade de vida dos doentes e ocorre em até 50% dos casos de trombose venosa profunda (TVP) iliofemoral apesar de adequada anticoagulação. O recurso à trombólise dirigida por cateter (TDC) tem-se generalizado e o seu uso sustenta-se num crescente volume de evidência científica que inclui estudos randomizados controlados. No entanto, a quase totalidade dos estudos excluíram dois grupos de doentes nos quais a TVP tem uma incidência e gravidade particularmente aumentadas: grávidas e doentes com neoplasia ativa.
Objetivos: Esta revisão não-sistemática da literatura pretende realizar uma análise compreensiva da evidência existente no que concerne à segurança e eficácia da TDC nos subgrupos de doentes acima descritos.
Resultados: O tratamento endovascular da TVP iliofemoral durante a gravidez e puerpério parece ser seguro e eficaz, tanto para a grávida como para o feto/recém-nascido. Os riscos associados à radiação (especialmente durante o primeiro trimestre) devem ser discutidos e tidos em consideração.
A TDC e a trombólise fármaco-mecânica são eficazes e seguras no tratamento de doentes com neoplasia ativa, desde que sejam previamente excluídas lesões cerebrais. No entanto, após a intervenção, a prescrição de anticoagulação eficaz (com heparinas de baixo peso molecular ou, em doentes selecionados, anticoagulantes orais diretos) é essencial para a manutenção da patência venosa neste subgrupo de doentes com trombofilia sustentada.
Conclusões: A TDC, com ou sem trombólise fármaco-mecânica, pode ser oferecida a grávidas ou doentes com neoplasia ativa desde que seja assegurada uma cuidada avaliação do risco-benefício em cada caso particular.
Palavras-chave: Trombose venosa profunda; Terapêutica trombolítica; Trombólise dirigida por cateter; Síndrome pós-trombótico; Cancro, Gravidez
Introduction
The post-thrombotic syndrome (PTS) is a severe clinical entity, associated with reduced quality of life, comprising chronic limb pain and swelling, venous claudication and, ultimately, leg ulceration(1,2). Despite adequate treatment of lower limb deep vein thrombosis (DVT) with anticoagulation, up to 50% of patients may manifest PTS symptoms in the long term(3). The risk of evolution to PTS after lower limb DVT varies according to the veins involved with iliofemoral veins thrombosis being associated with the highest risk(2,4). Additionally, thrombosis of this venous segment carries a significant risk of recurrent DVT.
Given the poor prognosis of this disease an effort was made by vascular surgeons to offer their patients a better treatment. Open surgical thrombectomy became popularized in the 1950 to 1960s and early results showed a decreased incidence of PTS, especially in the operated patients who had symptoms for less than 10 days(5). However, long-term follow-up results were poor due to decreased venous patency(6). In the 80s the technical evolution led to the use of surgical adjuncts after venous thrombectomy, such as the creation the of a temporary arteriovenous fistula (AVF) or the construction of a venous bypass (with or without an AVF); however, the outcome was still rather unpredictable, the procedures complex and, in some cases, associated with severe complications such as death or major amputation(7).
In parallel with the evolution of such surgical techniques, in the 80s and early 90’s a growing interest in the use of fibrinolytic therapy was observed based on the experiences in other thrombotic events such as myocardial infarction and stroke. Early experiences with streptokinase(8 - 10) and later with, the safer, recombinant tissue plasminogen activator (tPA) infused systemically by intravenous (IV) administration showed increased restoration of venous patency (in comparison with the standard treatment with heparin) and a significantly decreased incidence of PTS(11-13). The enthusiasm with this treatment was however hampered by the increased incidence of bleeding complications, namely intracranial hemorrhage [incidence of up to 3-6% with IV tPA(14)](13). Protocols using low-dose tPA, either systemic or loco-regional, were tested in order to reduce the incidence of bleeding but the therapeutic effect was significantly affected(15).
Modern day fibrinolysis
The technical evolution, and increased interventional skills, brought a true revolution to the way patients with symptomatic iliofemoral DVT were treated. Catheter-directed thrombolysis (CDT), by directly exposing the fresh thrombus to the tPA, exponentially increased its efficacy and allowed a dramatic tPA dose reduction: about 0.01 mg/kg/h, usually ranging between 0.5 and 1 mg/h instead of a single dose of 50-100 mg (0.9 mg/kg)(16). This dose reduction, and the in-clot administration, greatly reduces the systemic exposure to the drug; in fact, the risk of intracranial hemorrhage with CDT is quite low and probably comparable to the one seen with anticoagulation alone(17).
With increased experience small, but important, clinical and technical details further increased the safety profile of this treatment, such as: regular biochemical marker monitoring [hemoglobin, platelet count and activated partial thromboplastin time (APTT); there still is debate about the clinical value of fibrinogen monitoring(18)], better patient selection (particularly patients with symptoms for less than 14 to 21 days) and ultrasound-guided puncture of the access vein. The feared complication of pulmonary embolism has a low reported incidence(19).
Quality data
Despite the increasing usage of CDT at the turn of the millennia most of all published data comprised low quality retrospective case series reports. It wasn’t until 2002 that the first randomized controlled trial was published confirming increased venous patency and valve competence in patients with iliofemoral DVT treated with CDT(20).
This study was, however, flawed by a short follow-up (6 months) and lack of health-related quality of life (HRQOL) assessment.
The CaVenT Trial was a well-designed RCT, with a long follow-up and HRQOL assessment, it showed a significant increase in venous patency with CDT vs anticoagulation alone (p=0.012) and decreased incidence of PTS (absolute risk reduction of 14.4%, p=0.047)(21). The 5-year results of this RCT reported a further benefit in terms of PTS prevention (absolute risk reduction of 28%, p<0.0001)(22). Despite these positive findings, HRQOL didn’t differ between treatment and control groups in both publications(21,22). A low rate of adjunctive iliac vein stenting (with maintained residual venous outflow obstruction) may, at least partially, explain this poorer outcome(23).
The recently published RCT, the Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis (ATTRACT) Trial was a highly awaited multicenter NIH-funded study(24). It was the largest RCT to date and its results had the potential to reflect the technical evolution observed in the latest years, particularly the novel devices for pharmacomechanical thrombolysis (PMT). These devices recur to different physical methods to disrupt the thrombus increasing its contact with the thrombolytic drug therefore increasing its efficacy (this translates into shorter treatment sessions and a reduced total dose of thrombolytic)(25).
In ATTRACT 66% of the treatment patients were treated with PMT, most of them (61%) with AngioJet Rheolytic Thrombectomy System® (Boston Scientific). Despite its promises, ATTRACT failed to reveal any significant difference between treatment (CDT ± PMT + anticoagulation and compression therapies) and control (anticoagulation and compression alone) groups in terms of PTS prevention or improved QOL. In fact, the only difference encountered was an increased incidence of bleeding events in the treatment group(24). These disconcerting results didn’t reflect the previous Case Series or CaVenT Trial results nor the daily practice impression that patients indeed benefited from this additional intervention. However, this RCT actually validates today’s practice based on the Society of Vascular Surgery’s Guidelines that recommend early thrombus removal only in selected patients with iliofemoral DVT(26). Due to patient enrollment pressures in the ATTRACT Trial only 58% of the patients had DVT extending into the common femoral vein, iliac vein or both and this reflected on a rather low overall incidence of PTS both in the treatment and control groups(24) and an underestimation of CDT and/or PMT benefits. Another RCT, the Dutch CAVA Trial is still enrolling patients and may further delineate the role of CDT in iliofemoral DVT(27).
As in all fields of Medicine, our practice towards a particular patient will always be based on high quality data from RCT (if available), Clinical Practice Guidelines from recognized Scientific Societies and our own personal clinical experience. Further validation of this practice comes from retrospective data such as a recently published nonrandomized retrospective study in which the large majority of patients (93%) had extensive iliofemoral DVT and where the authors achieved a low incidence of PTS (21.3%) with a low risk of bleeding complications(28).
Special Populations
All the evolution described above in the treatment of iliofemoral DVT is based in a significant number of studies including well designed RCTs, however, there are two special populations of patients, in which DVT has an increased incidence, that are commonly excluded from those trials: pregnant women and patients with active malignancy. This was the case for both the CaVenT and ATTRACT trials(21,24).
Given the high incidence of DVT in these patients and the fact that many of these patients are active, young, patients that would highly benefit from early thrombus removal it is essential that an effort should be made to offer them the best treatment possible.
Pregnancy
Pregnancy has significant effects on the lower extremity venous system. The cardiovascular system undergoes dynamic physiologic changes throughout the course of pregnancy to meet the demands of both the mother and the fetus. These changes predispose pregnant women to an increased incidence of venous thromboembolism (VTE), including DVT and pulmonary embolism (PE), nearly five times more often than nonpregnant women, this risk increases even further during the postpartum period(29,30). A combination of altered venous flow hemodynamics [due to iliac veins and inferior vena cava compression by the gravid uterus(31)] and the establishment of a hypercoagulable state with a significant rise in the concentrations of coagulation factors V, VII, VIII, IX, X, and XII and the adhesive protein von Willebrand factor as well as increased fibrinogen levels. The anticoagulation system is also impaired with decreased protein S and increased resistance to activated protein C. In addition, fibrinolysis is affected because of increased plasminogen activator inhibitor, decreased tissue plasminogen activator, and acquired antithrombin deficiency(32); all these changes lead to an increased risk of DVT. Certain factors further increase this risk: inherited or acquired thrombophilias, a previous DVT, antiphospholipid syndrome, lupus, heart disease and sickle cell disease, age 35 and older, null parity, multiple gestations, obesity and immobility(33). In the puerperium, post-partum infection increases the risk of thrombosis by 4-fold and cesarean delivery increases the risk 2-fold(33).
The benefit of treatment in pregnant women must be weighed against the risks of miscarriage or fetal malformations and the risks to the woman itself.
Concerns on fetal malformations derive only from the radiation exposure, that CDT demands, as tPA does not cross the human placenta due to its large molecular size (7200 kDa)(34). The International Commission on Radiological Protection states that no deterministic effects of practical significance would be expected in the developing human at doses lower than 100 mGy(35) and the fetus is particularly sensitive to radiation between 8 and 15 weeks, during which there is rapid neuronal development and migration(36). Besides, fetal radiation exposure increases the risk of childhood cancer with a relative risk of approximately 3.19 in the first trimester, 1.29 in the second trimester, and 1.30 in the third trimester(36). If the procedure is to be performed the fetus should have a minimal exposure to radiation, if possible by avoiding imaging the uterus and placing a lead shield to protect the uterus from external scattered radiation(37). If this is unavoidable, minimal fluoroscopy acquisitions should be made, with the lowest frame rate possible, maximizing collimation and resorting to digital zooming instead of magnification. Additionally, use of intravascular ultrasound (IVUS) should be considered in order to minimize the use of fluoroscopy(38).
The problematic of radiation shouldn’t only focus on the procedure itself. Duplex ultrasound has excellent sensitivity and specificity for proximal DVT diagnosis, 97% and 94% respectively(39), however, adequate common iliac vein evaluation may not be possible in up to 53% of patients(40). For this reason, the SVS guidelines recommend, in order to adequately establish the diagnosis and plan the procedures, the use of adjunct imaging modalities such as computed tomographic venography (CTV) or magnetic resonance venography (MRV)(26). Although CTV is generally the chosen imaging method, in pregnant patients, because of the fetal and maternal radiation exposure associated with CTV, MRV should be the preferred method.
In relation to thrombolysis itself, although the available literature is scarce, it doesn’t seem to carry an increased bleeding risk for the pregnant women in comparison to the general population. Regarding IV administration of tPA during pregnancy for stroke, both a review of case reports (16 cases)(41) and a retrospective case series (with 15 cases)(42) show an incidence of intracranial hemorrhage comparable to non-pregnant patients and no specific obstetrical complications.
The results of CDT and PMT for iliofemoral DVT during pregnancy are, as previously stated, based on a few retrospective studies, the largest two are from Bloom et al(43) and Herrera et al(44). The first one reported on 11 patients and was essentially a report on post-partum treatment (2 patients who presented in the first trimester terminated their pregnancies after CDT, 2 patients who presented in the third trimester delayed CDT until after delivery, and 7 patients who presented with postpartum DVT underwent immediate CDT). PMT was performed using AngioJet followed by overnight CDT and a repeat venogram was done the next morning with adjunct procedures (balloon angioplasty and/or stenting) if necessary. No major bleeding events were observed and a greater than 90% clot lysis was achieved in 82% of patients.
The retrospective series from Herrera et al describes the treatment of 13 pregnant patients (gestational age ranged from 8 to 34 weeks), of these, 2 declined endovascular treatment and underwent surgical thrombectomy. There were two major bleeding events: one hematuria (secondary to trauma by a Foley catheter) and a popliteal artery pseudoaneurysm (treated with ultrasound compression); there were also 3 minor bleeding events (puncture site hematomas). Mean follow-up was 1.3 years with one registered DVT recurrence (a patient that didn’t comply with anticoagulation). There were no pregnancy or postpartum complications.
Another potential concern treating these patients is the behavior of stents compressed by the gravid uterus and the inherent risk of structural damage. Two retrospective analysis of a total of 372 women of reproductive age who received iliocaval stenting; 19 were identified to have had at least one pregnancy after stenting(45,46). During pregnancy and follow-up all but one patient [treated with a Palmaz XXL balloon-expandable stent that became completely crushed during pregnancy(45)] had patent stents with no ultrasound-identified structural damage or thrombosis.
In conclusion, endovascular treatment of iliofemoral DVT during pregnancy and puerperium seems safe and effective both for the pregnant woman and the fetus. The risks and potential benefits of the procedure should be carefully discussed and, although thrombolysis itself doesn’t seem to present an additional risk in this population, the risks of radiation (especially in the first trimester) must be taken in consideration. Given the fact that the safety and efficacy of these interventions in this particular group of patients are, as discussed above, based on few retrospective series the decision to proceed with them should probably be reserved to the few patients that are severely symptomatic and that show a poor clinical response to a trial of adequate anticoagulation and compression therapy.
Cancer
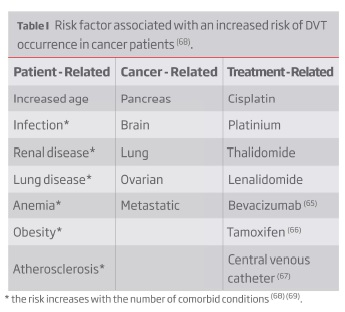
Cancer is one of the most significant risk factors for the occurrence of DVT: cancer patients have a 4.1-fold increased risk of thrombosis, this value increases to 6.5-fold during chemotherapy(47). Worsening this scenario is the fact that cancer patients often have greater initial clot burdens in proximal veins and achieve less venographic improvement on anticoagulation than patients without cancer who develop DVT thereby increasing their chances of developing PTS(48). The increased risk of DVT in cancer patients is multifactorial (Table I).
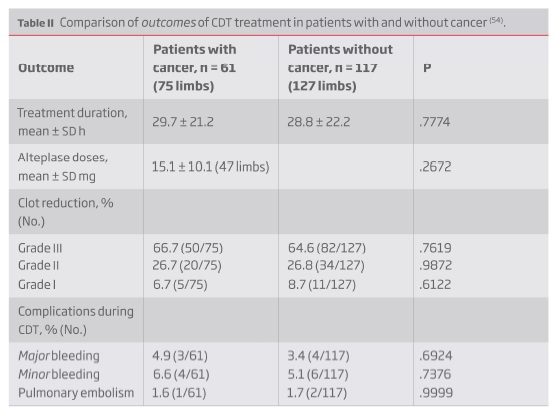
Despite the expected benefit from thrombus-removal treatments in cancer patients, practitioners more than often opt out for a conservative approach due to fear of bleeding complications. This fear is based on an increased (two to sixfold) bleeding risk that cancer patients present on anticoagulation(49,50). This fear is reflected on the exclusion criteria from the RCTs comparing CDT and anticoagulation alone for proximal DVT: both CaVenT and ATTRACT trials excluded patients with active malignancy(21,24). However, limited evidence derived from systemic thrombolytic treatment in cancer patients with ischemic stroke or pulmonary embolism (PE) indicates that this is a safe treatment although the reported rate or reperfusion in PE was slower compared to non-cancer patients(51-53); it should be, however, noted that all this studies excluded patients with known metastatic brain lesions. In light of this results, one would expect CDT, given its lower risk profile, to be even safer and that was the conclusion of a retrospective study published in 2008 comparing CDT for brachiosubclavian and iliofemoral DVT in patients with (61 patients, 75 limbs) and without (117 patients, 127 limbs) cancer(54). The outcomes in cancer patients were non-inferior, both in efficacy and safety, to those without cancer (Table II).
The addition of PMT techniques to CDT may provide a further reduction of bleeding risk (lower lytic doses and shorter procedures) and allow cancer patients to rapidly resume their antineoplastic treatments (if applicable). Two small-size retrospective reports, comprising 7 limbs treated with AngioJet® and 14 limbs treated with Trellis-8® [Bacchus Vascular, Santa Clara, California, USA; this device was later removed from the market(55)] show promising results regarding the use of these techniques in this subset of patients(56,57).
One, interesting, common finding to the above described retrospective series is the increased rates of stenting after CDT or PMT in comparison to patients without cancer. This may be related to sequelae of radiation-associated stenosis or undiagnosed prior chronic DVT and, in some instances, to the need to treat an underlying venous tumor compression.
Patients with cancer who undergo CDT±PMT for iliofemoral DVT are still at increased risk for recurrent DVT (hazard ratio of 6.75) and medium to long-term failure after interventional treatment(58) stemming from an ongoing hypercoagulable state. Therefore, strict adherence to an optimal secondary prevention strategy for DVT is of paramount importance. On this matter, the review International clinical practice guidelines (ITAC-CME)(59) state that: LMWH is recommended for the initial treatment of VTE in patients with cancer (Grade 1B). Fondaparinux and UFH can also be used (Grade 2D);
For early maintenance (10 days to 3 months) and long-term (beyond 3 months) LMWH is preferred over VKA (Grade 1A) and should be used for a minimum of 3 months to treat established VTE in patients with cancer (Grade 1A). Evidence for 6 months is low due to inconsistency of the data. Direct oral anticoagulants (DOAC) can be considered for VTE treatment of patients with stable cancer not receiving systemic anticancer therapy (Guidance);
After 3-6 months, termination or continuation of anticoagulation should be based on individual assessment of the benefit to risk ratio, tolerability, drug availability, patient preference, and cancer activity (Guidance).
Although the treatment of acute DVT with DOACs is attractive due to its ease of use (no need of INR-monitoring), non-inferior efficacy and overall superior safety profile(60) there is still limited supportive evidence for its use in cancer patients. Two recently published RCTs, the Select-D(61) and the Hokusai VTE Cancer(62) trials, comparing treatment with DOAC (rivaroxaban and edoxaban, respectively) for acute DVT in cancer patients, both showed a low rate of recurrent VTE with DOAC (non-inferior to low-molecular weight heparin) but at the expense of a significantly increased risk of bleeding events (mostly gastrointestinal bleeding events); therefore, on the basis of the available evidence the use of DOACs in these patients can be supported in well-selected, low-risk, patients(63).
The data here provided, although scarce and retrospective, supports an interventional strategy in cancer patients with iliofemoral DVT that are physically active and present with debilitating symptoms (the same as for the non-cancer population). The DVT episode is more than often the first manifestation and coincides with the diagnosis of a neoplastic disorder; this means that an accurate survival prognosis can’t be established and one should proceed (bearing in mind that the best results are obtained if treatment is initiated in the first 14 days after the DVT’s onset) with the treatment that will provide the best quality of life, on the long-term, for the patient.
Conclusion
Interventional treatment, with CDT (with or without associated PMT), in patients with iliofemoral DVT is supported by a robust amount of data obtained from retrospective series and prospective randomized, and nonrandomized, trials. RCT’s often don’t reflect the real-world practice: a study from the Mayo Clinic noted that a total of 75% of the patients they treated with CDT±PMT would have been excluded from CaVenT or ATTRACT(64). Despite this, based on the above reviewed literature, and according to the local expertise and experience, CDT±PMT may be offered to pregnant patients and patients with active cancer (that are physically active and have an otherwise good surgical risk), that remain severely symptomatic after a short trial of adequate anticoagulation and compression therapy, provided that a careful risk-benefit assessment is made for each individual patient.
Additionally, the use of PMT with thrombolysis should be considered to reduce the total dosage of thrombolytics (and hemorrhagic risks) and the availability of the required institutional conditions (high-dependency ward with adequate nurse and physician-to-patient ratios, quality fluoroscopy in the operating room or interventional suite as well as the clinical support of medical specialties such as hematology, oncology and obstetrics/gynecology to assess eventual specific complications).
REFERENCES
1. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008, 6, pp. 1105-12. [ Links ]
2. Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004, 239, pp. 118-26. [ Links ]
3. Kahn, SR. The post-thrombotic syndrome. Hematology Am Soc Hematol Educ Program. 2010, pp. 216-220. [ Links ]
4. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008, Vol. 149, pp. 698-707. [ Links ]
5. Haller JA, Abrams BL. Use of thrombectomy in the treatment of acute iliofemoral venous thrombosis in forty-five patients. Ann Surg. 1963, Vol. 158 (4), pp. 561-6. [ Links ]
6. Lansing AM, Davis WM. Five-year follow-up study of iliofemoral venous thrombectomy. Ann Surg. 1968, Vol. 168(4), pp. 620-8. [ Links ]
7. Plate G, Einarsson E, Eklof B, et al. Iliac vein obstruction associated with acute iliofemoral venous thrombosis. Results of early reconstruction using polytetrafluoroethylene grafts. Acta Chir Scand. 1985, Vol. 151, 7, pp. 607-11. [ Links ]
8. Common HH, Seaman AJ, Rosch J, Porter JM, Dotter CT. Deep vein thrombosis treated with streptokinase or heparin: follow-up of a randomized study. Angiology. 1976, Vol. 27, pp. 645-54. [ Links ]
9. lliot MS, Immelman EJ, Jeffrey P, et al. A comparative randomized trial of heparin versus streptokinase in the treatment of acute proximal venous thrombosis: an interim report of a prospective trial. Br J Surg. 1979, Vol. 66, pp. 838-43. [ Links ]
10.Arneson H, Hoiseth A, Ly B. Streptokinase or heparin in the treatment of deep vein thrombosis: follow-up results of a prospective study. Acta Med Scand. 1992, Vol. 211, pp. 65- 8. [ Links ]
11. Goldhaber SZ, Meyerovitz MF, Green D, et al. Randomized controlled trial of tissue plasminogen activator in proximal deep venous thrombosis. Am J Med. 1990, Vol. 88, pp. 235-40. [ Links ]
12. Turpie AG, Levine MN, Hirsh J, et al. Tissue plasminogen activator (rt-PA) vs heparin in deep vein thrombosis. Results of a randomized trial. Chest. 1990, Vol. 97, pp. 172s-5s. [ Links ]
13. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2014, Vol. 1, CD002783. [ Links ]
14. Albers GW, Bates VE, Clark WM, et al. Intravenous tissue-type plasminogen activator for treatment of acute stroke: the Standard Treatment with Alteplase to Reverse Stroke (STARS) study. JAMA. 2000, Vol. 283, pp. 1145-50. [ Links ]
15. Schweizer J, Kirch W, Koch R, et al. Short- and Long-Term Results After Thrombolytic Treatment of Deep Venous Thrombosis. J Am Coll Cardiol. 2000, Vol. 36, 4, pp. 1336-43. [ Links ]
16. Oklu R, Wicky S. Catheter-directed thrombolysis of deep venous thrombosis. Semin Thromb Hemost. 2013, Vol. 39, pp. 446-51. [ Links ]
17. Vedantham S, Sista AK, Klein SJ, et al. Quality improvement guidelines for the treatment of lower-extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2014, Vol. 25, pp. 1317-25. [ Links ]
18. Elbasty A, Metcalf J. Safety and Efficacy of Catheter Direct Thrombolysis in Management of Acute Iliofemoral Deep Vein Thrombosis: A Systematic Review. Vasc Specialist Int. 2017, Vol. 33, pp. 121-134. [ Links ]
19. Wang L, Zhang C, Mu S, et al. Safety of catheter-directed thrombolysis for the treatment of acute lower extremity deep vein thrombosis: A systematic review and meta-analysis. Medicine. 2017, Vol. 96, 35, p. e7922. [ Links ]
20. Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002, Vol. 24, pp. 209-14. [ Links ]
21. Enden T, Haig Y, Kløw NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012, Vol. 379, pp. 31-38. [ Links ]
22. Haig Y, Enden T, Grøtta O, et al. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016, Vol. 3, 2, pp. e64 - e71. [ Links ]
23. Haig Y, Enden T, Slagsvold CE, et al. Determinants of early and long-term efficacy of catheter-directed thrombolysis in proximal deep vein thrombosis. J Vasc Interv Radiol. 2013, Vol. 24, 1, pp. 17-24. [ Links ]
24. Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. N Engl J Med. 2017, Vol. 377, 23, pp. 2240-2252. [ Links ]
25. Sharifi M, Bay C, Mehdipour M, Sharifi J. Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial: midterm results. J Endovasc Ther. 2012, Vol. 19, 2, pp. 273-80. [ Links ]
26. Meissner MH, Gloviczki P, Comerota AJ, et al. Early thrombus removal strategies for acute deep venous thrombosis: Clinical Practice Guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012, Vol. 55, 5, pp. 1449-62. [ Links ]
27. DUTCH CAVA-trial: CAtheter Versus Anticoagulation Alone for Acute Primary (Ilio)Femoral DVT. 2009. [ Links ]
28. Lin M, Hsieh JCF, Hanif M, et al. Evaluation of thrombolysis using tissue plasminogen activator in lower extremity deep venous thrombosis with concomitant femoral-popliteal venous segment involvement. J Vasc Surg Venous Lymphat Disord. 2017, Vol. 5, 5, pp. 613-620. [ Links ]
29. Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005, Vol. 143, pp. 697-706. [ Links ]
30. Pomp ER, Lenselink AM, Rosendaal FR, et al. The postpartum period and prothrombotic defects:risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008, Vol. 6, pp. 632-7. [ Links ]
31. Gherman RB, Goodwin TM, Leung B, et al. Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999, Vol. 94, 1, pp. 730-4. [ Links ]
32. B, Brenner. Haemostatic changes in pregnancy. Thromb Res. 2004, Vol. 114, pp. 409-14. [ Links ]
33. James AH, Jamison MG, Brancazio LR, et al. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors and mortality. Am J Obstet Gynecol. 2006, Vol. 194, p. 1311. [ Links ]
34. Tassi R, Acampa M, Marotta G, et al. Systemic thrombolysis for stroke in pregnancy. Am J Emerg Med. 2013, Vol. 31, 448, pp. 1-3. [ Links ]
35. The 2007 Recommendations of the International Commission on Radiological Protection. Ann ICRP. 2007, Vol. 37, pp. 1-332. [ Links ]
36. Tirada N, Dreizin D, Khati NJ, et al. Imaging Pregnant and Lactating Patients. Radiographics. 2015, Vol. 35, pp. 1751-65. [ Links ]
37. BA, Atwell TD, et al. Radiation Exposure and Pregnancy: When Should We Be Concerned? Radiographics. 2007, Vol. 27, pp. 909-17.
38. Siah TH, Chapman A. Should catheter-directed thrombolysis be the standard of care for pregnancy-related iliofemoral deep vein thrombosis? BMJ Case Reports. 2018. [ Links ]
39. Kearon C, Julian JA, Math M, et al. Noninvasive diagnosis of deep venous thrombosis. McMaster diagnostic imaging practice guidelines initiative. Ann Intern Med. 1998, Vol. 128, pp. 663-77. [ Links ]
40. Messina LM, Sarpa MS, Smith MA, et al. Clinical significance of routine imaging of iliac and calf veins by color flow duplex scanning in patients suspected of having acute lower extremity deep venous thrombosis. Surgery. 1993, Vol. 114, pp. 921-7. [ Links ]
41. Tversky S, Libman RB, Reppucci ML, et al. Thrombolysis for Ischemic Stroke during Pregnancy: A Case Report and Review of the Literature. J Stroke Cerebrovasc Dis. 2016, Vol. 25, 10, pp. 167-70. [ Links ]
42. Leffert LR, Clancy CR, Bateman BT, et al. Treatment patterns and short-term outcomes in ischemic stroke in pregnancy or postpartum period. Am J Obstet Gynecol. 2016, Vol. 214, 723, pp. 1-11. [ Links ]
43. Bloom AI, Farkas A,Kalish Y, et al. Pharmacomechanical Catheter-Directed Thrombolysis for Pregnancy-Related Iliofemoral Deep Vein Thrombosis. J Vasc Interv Radiol. 2015, Vol. 26, 7, pp. 992-1000. [ Links ]
44. Herrera S, Comerota AJ, Thakur S, et al. Managing iliofemoral deep venous thrombosis of pregnancy with a strategy of thrombus removal is safe and avoids post-thrombotic morbidity. J Vasc Surg. 2014, Vol. 59, 2, pp. 456-64. [ Links ]
45. Hartung O, Barthelemy P, Arnoux D, et al. Management of pregnancy in women with previous left ilio-caval stenting. J Vasc Surg. 2009, Vol. 50, pp. 355-9. [ Links ]
46. Dasari M, Avgerinos E, Raju S, et al. Outcomes of iliac vein stents after pregnancy. J Vasc Surg Venous Lymphat Disord. 2017, Vol. 5, 3, pp. 353-357. [ Links ]
47. Heit JA, Silverstein MD, Mohr DN, etal. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000, Vol. 160, 6, pp. 809-815. [ Links ]
48. SR, Deitcher. Cancer-related deep venous thrombosis: clinical importance, treatment challenges, and management strategies. Semin Thromb Hemost. 2003, Vol. 29, pp. 247-58. [ Links ]
49. Hutten BA, Prins MH, Gent M, et al. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. J Clin Oncol. 2000, Vol. 18, pp. 3078-83. [ Links ]
50. Prandoni P, Lensing AWA, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002, Vol. 100, pp. 3484-8. [ Links ]
51. Mikkola KM, Patel SR, Parker JA, et al. Attenuation over 24 hours of the efficacy of thrombolysis of pulmonary embolism among patients with cancer. Am Heart J. 1997, Vol. 134, pp. 603-7. [ Links ]
52. Cappellari M, Carletti M, Micheletti N, et al. Intravenous Alteplase for acute ischemic stroke in patients with current malignant neoplasm. J Neurol Sci. 2013, Vol. 325, pp. 100-2. [ Links ]
53. Masrur S, Abdullah AR, Smith EE, et al. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovasc Dis. 2011, Vol. 20, 2, pp. 124-30. [ Links ]
54. Kim HS, Preece SR, Black JH, et al. Safety of catheter-directed thrombolysis for deep venous thrombosis in cancer patients. J Vasc Surg. 2008, Vol. 47, 2, pp. 388-94. [ Links ]
55. Administration, U.S. Food and Drug. [Online] http://www.ie.ufrj.br/datacenterie/pdfs/seminarios/pesquisa/texto1904.pdf. [ Links ]
56. O'Sullivan GJ, Lohan DG, Gough N, Cronin CG, Kee ST. Pharmacomechanical thrombectomy of acute deep vein thrombosis with the Trellis-8 isolated thrombolysis catheter. J Vasc Interv Radiol. 2007, Vol. 18, 6, pp. 715-24.
57. Yoon WJ, Halandras P, Aulivola B, Crisostomo P. Malignancy Does not Affect Outcomes of Pharmacomechanical Thrombolysis in Acute Symptomatic Iliofemoral Deep Vein Thrombosis. Ann Vasc Surg. 2018. [ Links ]
58. Avgerinos ED, Hager ES, Naddaf A, Dillavou E, Singh M, Chaer RA. Outcomes and predictors of failure of thrombolysis for iliofemoral deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2015, Vol. 3, 1, pp. 35-41. [ Links ]
59. Farge D, Bounameaux H, Brenner B, et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016, Vol. 17, 10, pp. e452-e466. [ Links ]
60. Makam RCP, Hoaglin DC, McManus DD, et al. Efficacy and safety of direct oral anticoagulants approved for cardiovascular indications: Systematic review and meta-analysis. PLoS One. 2018, Vol. 13, e0197583. [ Links ]
61. Young AM, Marshall A, Thirlwall J, et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol. 2018, Vol. 10. [ Links ]
62. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018, Vol. 378, 7, pp. 615-624. [ Links ]
63. AYY, Lee. Overview of VTE treatment in cancer according to clinical guidelines. Thromb Res. 2018, Vol. 164, pp. S162-S167.
64. Wysokinska EM, Sobande F, Wysokinski WE, Bjarnason H, McBane Ii RD. Iliac vein thrombosis: feasibility assessment of randomized controlled trials of endovascular pharmacomechanical thrombolysis. J Thromb Haemost. 2010, Vol. 8, 9, pp. 1943-9.
65. Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients:a meta-analysis. JAMA. 2008, Vol. 300, pp. 2277-85. [ Links ]
66. Pritchard KI, Paterson AH, Paul NA, et al. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. J Clin Oncol. 1996, Vol. 14, pp. 2731-7. [ Links ]
67. Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013, Vol. 11, 1, pp. 71-80. [ Links ]
68. Khorana AA, ConnollyGC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009, Vol. 27, pp. 4839-47. [ Links ]
69. Kyriazi V, Theodoulou E. Assessing the risk and prognosis of thrombotic complications in cancer patients. Arch Pathol Lab Med. 2013, Vol. 137, pp. 1286-95. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Correio eletrónico: duarterego@hotmail.com (D. Rego).
Recebido a 30 de junho de 2018
Aceite a 21 de janeiro de 2019