Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Obstétrica e Ginecológica Portuguesa
Print version ISSN 1646-5830
Acta Obstet Ginecol Port vol.13 no.4 Coimbra Dec. 2019
ORIGINAL STUDY/ESTUDO ORIGINAL
Predictive factors of pathological complete response in a Portuguese population with breast cancer treated with neoadjuvant chemotherapy
Factores preditivos de resposta patológica completa numa população portuguesa com cancro da mama submetida a quimioterapia neoadjuvante
Ana Raquel Neves1,2,3, Rafaela Pires1, Olga Caramelo4, Isabel Henriques5, Joana Belo5
Serviço de Ginecologia do Centro Hospitalar e Universitário de Coimbra
1 Interna de Formação Específica de Ginecologia e Obstetrícia, Serviço de Ginecologia e Serviço de Obstetrícia B do Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal;
2 Clínica Universitária de Ginecologia, Faculdade de Medicina da Universidade de Coimbra, Coimbra, Portugal
3 Centro Académico Clínico de Coimbra
4 Assistente em Ginecologia e Obstetrícia, Serviço de Ginecologia do Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
5 Assistente Graduada em Ginecologia e Obstetrícia, Serviço de Ginecologia do Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Overview and Aims: Neoadjuvant chemotherapy is increasingly being used in the treatment of operable breast cancer. Our aim was to evaluate the factors that predict pathological complete response (pCR) after neoadjuvant chemotherapy in a Portuguese population of breast cancer patients.
Study design: Retrospective, cross-sectional study.
Population: Women diagnosed with breast cancer stage I-III treated with neoadjuvant chemotherapy and subsequent surgery in our institution during a 6-year period.
Methods: Clinical records were reviewed. Patient demographics and tumour characteristics were evaluated. Univariate and multivariate analysis were performed to evaluate tumour’s clinical and immunohistochemical characteristics associated with pCR.
Results: A total of 164 patients enrolled the sudy. The overall rate of pCR was 17.7%. The rate was significantly higher in HER2-positive (non-luminal) tumours (66.7%) and lower in luminal A tumours (0%). The nuclear grade, estrogen receptor (ER), progesterone receptor (PR), HER2 status and Ki67 proliferation index significantly influenced the rate of pCR. However, multivariate analysis revealed that only negative ER and positive HER2 are significant predictors of pCR [respectively, ORadj 0.187 (95% CI 0.038-0.908; p=0.038) and ORadj 4.186 (95% CI 1.401-12.508; p=0.010)].
Conclusion: ER status and HER2 status are the most important predictors of pathological complete response. These findings should be considered in patient counselling, providing a more customized therapeutic approach..
Keywords: Breast cancer; Neoadjuvant therapy; Pathology; Immunohistochemistry.
Introduction
Neoadjuvant chemotherapy (NAC), initially considered the standard of care for inoperable locally advanced breast cancer, has become more common for patients with operable disease1. The results of the NSABP B-18 trial showed that preoperative chemotherapy allows for a higher rate of breast conserving surgery, especially in patients with tumours larger than 5 cm2,3. Also, it provides predictive information regarding the response of the primary tumour and involved lymph nodes to treatment2-5.
The interpretation of data from NAC trials is challenging due to the lack of a uniform definition for pathological complete response (pCR). According to the CTNeoBC consortium, pCR may be defined as the absence of any residual invasive cancer (ypT0/is ypN0) or any invasive and non-invasive tumour (ypT0 ypN0) following neoadjuvant therapy6. Eradication of the tumour from both the breast and lymph nodes was associated with improved event free survival (EFS) and overall survival (OS) compared to eradication of the tumour from the breast alone6.
Studies have shown that pCR is significantly correlated with improved survival. Patients achieving a pCR after NAC show a significantly improved disease free survival (DFS) and OS compared to those without pCR3,4,7-9. The strongest association between pCR and long-term outcome seems to be in patients with triple negative; hormone-receptor-positive, high-grade, and HER2-negative; and HER2-positive and hormone-receptor-negative tumours6,7,10. This association is more controverse for luminal B (HER2-negative or positive) and hormone-receptor-positive low grade tumours6,9-12.
The prognostic impact of pCR is well established. Defining clinical and immunohistological factors that better predict the achievement of pCR improves patient counseling and allows for a more tailored and individualized therapeutic approach.
We reviewed our single-institution experience of obtaining a pCR after NAC over a 6-year period and report for the first time the factors that predict pCR in a Portuguese population treated with NAC.
Methods
An observational retrospective single-centre study was conducted between January 2011 and December 2016 with patients diagnosed with breast cancer clinical stage I-III at Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal.
As eligibility criteria we included patients who had a histological diagnosis of breast cancer based on a core biopsy; had received neoadjuvant chemotherapy (NAC) and had undergone surgery after NAC. Inflammatory carcinomas, stage IV patients and patients whose preor post-NAC specimens were unavailable were excluded.
Tumour stage and T and N factors were stratified based on the American Joint Committee on Cancer (AJCC) Cancer Staging System, Eighth Edition13.
Patient characteristics were obtained through retrospective clinical records analysis and included: age at diagnosis, primary tumour classification (cT), clinical nodal status (cN), histological subtype, nuclear grade, immunohistochemical (IHC) evaluation of estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2) status, Ki67 proliferation marker, NAC regimen, type of surgery and pCR status. Clinicopathological classification was concluded based on ER, PR and HER2 status, as well as on Ki67 index: Luminal A-like (ER-positive, HER2-negative, Ki67 <20%, PR >20%); Luminal B-like HER2 positive (ER-positive, HER2-positive, any Ki67, any PR); Luminal B-like HER2 negative (ER-positive, HER2-negative and either Ki67>20% or PR<20%); HER2-positive (non-luminal) (ER and PR absent, HER2-positive); triple negative (ER and PR <1%, HER2-negative)14,15. Tumours were considered ER-positive when ER ≥ 1% of tumour cell nuclei were immunoreactive as defined by the American Society of Clinical Oncology/ College of American Pathologists Guideline Recommendations15.
ER, PR, HER2 and Ki67 were evaluated by IHC analyses, performed on formalin-fixed paraffin-embedded tissue sections14,16. Tumours were considered HER2-positive when a score of 3+ was obtained on IHC and/or a ratio of greater than 2.0 was obtained on FISH analysis14.
All patients were submitted to a sequential regimen of anthracyclines and taxanes. In HER2-positive tumours, trastuzumab was started in association with the taxane part of the chemotherapy regimen and maintained for one year.
pCR was defined as absence of invasive cancer in the breast and axillary nodes (ypT0/is ypN0), as previously described6,7.
Statistical analysis was performed using SPSS v.23.0 (IBM Corp.). Univariate analysis was carried out to describe and compare the baseline characteristics tumours with or without pCR and to explore correlations between variables. Categorical variables were compared using the ÷2 test or the Fisher’s exact test, according to the Cochran rules. Normality of continuous variables was assessed with Kolmogorov-Smirnov test, determining the use of independent samples Student’s t-test when normality was assumed or independent samples Mann-Whitney U test as the non-parametric alternative. Multivariate logistic regression was performed to analyse the factors that were predictive of pCR in univariate analysis.
Results
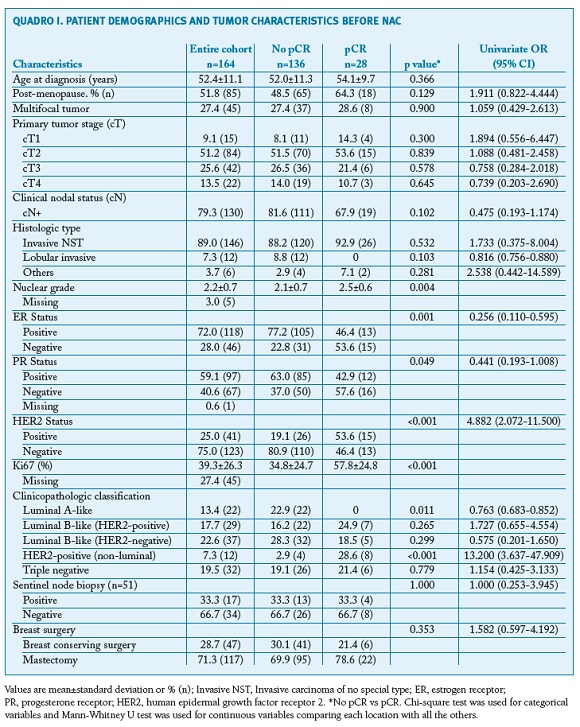
A total of 164 patients were enrolled in the study. Patient demographics and tumour characteristics are described in Table 1. The mean age at diagnosis was 52.4±11.1 years. The histological type invasive NST was the most frequent (89.0%, n=146) and most tumours were unifocal (72.6%, n=119). Tumour size classification before and after NAC is displayed in Figure 1. Most patients were cT2 or higher before NAC (90.8%, n=148), while the majority was yT1 or lower after NAC (69.2%, n=114).
Most patients (79.3%, n=130) had cN+ nodal status before NAC. Sentinel node biopsy was performed in 31.1% (n=51), of which 44.9% (n=20) were positive for tumour deposits. Axillary lymphadenectomy was performed in 84.1% (n=138), of which 54.3% (n=75) presented metastasis. Nodal pCR was 43.1%.
The overall pCR was 17.7% (n=28). pCR by intrinsic subtype was as follows: Luminal A, 0%; Luminal B like HER2-negative, 13.5% (n=5); Luminal B like HER2-positive, 24.1% (n=7); HER2-positive (non luminal), 66.7% (n=8); Triple negative, 18.8% (n=6).
Univariate analysis (Table 1) revealed that nuclear grade, ER, PR and HER2 status and Ki67 significantly influenced the rate of pCR. However, regarding the clinicopathological classification, the rate of pCR was only significantly different for Luminal A-like and HER2-positive (non-luminal) tumours [respectively, OR 0.763 (95% CI 0.683-0.852; p=0.011) and OR 13.200 (95% CI 3.637-47.909;p<0.001)]. In our analysis, neither tumour histology, cT nor cN influenced the rate of pCR. The rate of pCR was also similar between patients submitted to mastectomy and breast conservative surgery [respectively, 18.8% (n=22) vs. 12.8% (n=6), OR= 1.582 (95%CI 0.597-4.192); p=0.353].
Multivariate analysis is displayed in Figure 2. Positive ER and HER2 status are significant predictors of pCR [respectively, ORadj 0.187 (95% CI 0.038-0.908; p=0.038) and ORadj 4.186 (95% CI 1.401-12.508; p=0.010)].
Discussion
The prognostic impact of the achievement of pCR is well recognised. Therefore, selecting patients who are most likely to benefit from NAC would be an important clinical step in their therapeutic approach. To our knowledge, this is the first study to perform such an analysis in a Portuguese population.
Our overall pCR rate was 17.7%. This result is similar to previous studies using the same pCR definition (ypT0/is N0). In a retrospective study by Choi et al (n=353) the pCR rate was 17.6%9. A pooled analysis from the Japan Breast Cancer Research Group (n=353) reported a pCR rate of 18.4%17. The meta-analysis published by the CTNeoBC group (n=13.125) revealed a pCR rate of 18%.6 The Cochrane meta-analysis published in 2012 (n=1972) reported a pCR varying between 4.0% and 29.2%3. The German Breast Group and Arbeitsgemeinschaft Gynäkologische Onkologie-Breast Group (n=6377) reported a pCR rate of 19.8%11.
Similarly to the results of McFarland et al and Galvez et al, the only variables associated with pCR were the tumour’s IHC features and not the patient’s clinical characteristics (eg. age at diagnosis, menopause status, primary tumour size, tumour histology, tumour focality, clinical nodal status and sentinel node biopsy)8,18. Despite not reaching statistical significance, pCR was more prevalent in smaller tumours, which is in line with previous reports2,8. Some studies have reported non-lobular histology has a predictor of pCR. Besides, pCR patients with a lobular carcinoma do not seem to have a better long-term outcome when compared to non-pCR patients2,10. In our sample, none of the lobular tumours achieved pCR. However, our sample was underpowered to evaluate this difference.
As previously reported, univariate analysis revealed that nuclear grade, ER, PR and HER2 status and Ki67 significantly influenced the rate of pCR1,2,8,19. However, multivariate analysis revealed that only negative ER and positive HER2 are significant predictors of pCR1,2,5,10. This is in line with the finding of a statistically significant difference in the pCR rate of subtypes Luminal A [OR 0.763 (95% CI 0.683-0.852); p=0.011] and HER 2-positive (non luminal) [OR 13.200 (3.637-47.909); p<0,001]. Similar findings have been reported previously2,18. ER-positive tumours (luminal A) are less responsive to chemotherapy and the prognostic significance of the achievement of pCR in these patients is yet to be cleared7. Highlighting the importance of targeted therapy, patients with HER2-positive tumours have experienced a significant prognostic improvement since the introduction of trastuzumab as a part of the primary treatment, especially when achieving pCR after NAC10. Similar to the results published by Asano et al but unlike previous studies, our study did not reveal significant differences regarding the pCR rate of triple negative tumours2,10,20. We hypothesize that this may be due to the small sample size of patients with triple negative tumours.
The finding of such a high rate mastectomy rate in the pCR group (78.6%, n=22) was surprising. However, in 4 patients, mastectomy was performed on patient demand. In the remaining 18 patients mastectomy was performed based on tumour size (large T2, T3 and T4 tumours), which was our Institutional approach during the study period.
The potential limitations of this study include its retrospective design and the limited number of patients and pCR events on the subgroups according to breast cancer subtypes.
Conclusions
ER status and HER2 status were the most important predictors of pCR in our sample of Portuguese breast cancer patients treated with NAC. Patients with HER 2-positive (non luminal) breast cancer are most likely to achieve pCR while the benefit of NAC in Luminal A patients is yet to be established. In our sample triple negative tumours were not associated with pCR.
REFERENCES
1. Kaufmann M, Von minckwitz G, Bear HD, Buzdar A, Mcgale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: New perspectives 2006. Ann Oncol. 2007;18(12):1927-1934. [ Links ]
2. Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast. 2014;23(5):526-537. [ Links ]
3. Mieog JS, van der Hage JA van de VC. Preoperative chemotherapy for women with operable breast cancer ( Review ). Cochrane Database Syst Rev. 2012;CD005002(2):48. [ Links ]
4. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: Updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778-785. [ Links ]
5. Rapoport BL, Demetriou GS, Moodley SD, Benn CA. When and how do i use neoadjuvant chemotherapy for breast cancer? Curr Treat Options Oncol. 2014;15(1):86-98. [ Links ]
6. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. [ Links ]
7. Pennisi A, Kieber-emmons T, Makhoul I, Hutchins L. Relevance of Pathological Complete Response after Neoadjuvant Therapy for Breast Cancer. Breast Cancer (Auckl). 2016;10:103-106. [ Links ]
8. Galvez M, Castaneda CA, Sanchez J, Castillo M, Rebaza LP, Calderon G, et al. Clinicopathological predictors of long-term benefit in breast cancer treated with neoadjuvant chemotherapy Marco. World J Clin Oncol. 2018;9(2):33-42. [ Links ]
9. Choi M, Park YH, Ahn JS, Im Y-H, Nam SJ, Cho SY, et al. Evaluation of Pathologic Complete Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: Experience in a Single Institution over a 10-Year Period. J Pathol Transl Med. 2017;51 (1):69-78. [ Links ]
10. Loibl S. Neoadjuvant treatment of breast cancer: Maximizing pathologic complete response rates to improve prognosis. Curr Opin Obstet Gynecol. 2015;27(1):85-91. [ Links ]
11. Von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796-804. [ Links ]
12. Loibl S, Denkert C, von Minckwitz G. Neoadjuvant treatment of breast cancer - Clinical and research perspective. Breast. Elsevier Ltd; 2015;24:S73-7. [ Links ]
13. Hortobagyi GN, Connolly JL, D’Orsi CJ, Edge SB, Mittendorf EA, Rugo HS, et al. Breast. AJCC Cancer Staging Manual. 2017. p. 587-636. [ Links ]
14. Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Supplement 5):v8-30. [ Links ]
15. Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology / College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Clin Oncol. 2010;22314. [ Links ]
16. Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in Breast Cancer: Recommendations from the international Ki67 in breast cancer working Group. J Natl Cancer Inst. 2011;103(22):1656-1664. [ Links ]
17. Kuroi K, Toi M, Ohno S, Nakamura S, Iwata H, Masuda N, et al. Comparison of different definitions of pathologic complete response in operable breast cancer: a pooled analysis of three prospective neoadjuvant studies of JBCRG. Breast Cancer. Springer Japan; 2015;22(6):586-595. [ Links ]
18. McFarland DC, Naikan J, Rozenblit M, Mandeli J, Bleiweiss I, Tiersten A. Changes in pathological complete response rates after neoadjuvant chemotherapy for breast carcinoma over five years. J Oncol. Hindawi Publishing Corporation; 2016;2016. [ Links ]
19. Schlotter CM, Tietze L, Vogt U, Heinsen CV, Hahn A. Ki67 and lymphocytes in the pretherapeutic core biopsy of primary invasive breast cancer: Positive markers of therapy response prediction and superior survival. Horm Mol Biol Clin Investig. 2017;32(2):1-11. [ Links ]
20. Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Morisaki T. Prediction of treatment responses to neoadjuvant chemotherapy in triple - negative breast cancer by analysis of immune checkpoint protein expression. J Transl Med. BioMed Central; 2018;1-12. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Ana Raquel Neves
Centro Hospitalar e Universitario de Coimbra EPE
Coimbra, Portugal
E-mail: anaraquel.lneves@gmail.com
Recebido em: 26/11/2018
Aceite para publicação: 09/09/2019