Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Obstétrica e Ginecológica Portuguesa
Print version ISSN 1646-5830
Acta Obstet Ginecol Port vol.12 no.3 Coimbra Sept. 2018
ORIGINAL STUDY/ESTUDO ORIGINAL
Diagnostic accuracy of sonovaginography for deep infiltrating endometriosis
Sonovaginografia na avaliação da endometriose profunda
Joana Cruz*, Cátia Moreira**, Rita Cunha***, José Ferreira****, Margarida Martinho*****, Jorge Beires******
Centro Hospitalar de S. João
*Assistente Hospitalar de Ginecologia e Obstetrícia, Hospital Universitário de Genebra
**Assistente Hospitalar de Ginecologia e Obstetrícia, Centro Hospitalar de Trás-os-Montes de Alto Douro
***Interno de Formação Específica de Medicina Interna, Centro Hospitalar de Trás-os-Montes de Alto Douro
****Assistente Hospitalar Graduado de Ginecologia e Obstetrícia, Centro Hospitalar de São João
*****Assistente Hospitalar Graduado de Ginecologia e Obstetrícia, Centro Hospitalar de São João. Docente voluntária, Faculdade de Medicina da Universidade do Porto
******Diretor da Unidade Orgânica de Ginecologia e Medicina da Reprodução, Centro Hospitalar de São João
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Objective: The aim of this study was to assess the performance and accuracy of sonovaginography (SVG) for preoperative diagnosis and characterization of deep infiltrating endometriosis (DIE) lesions.
Study Design: A prospective study was conducted between January 2014 and January 2016, including all premenopausal women with clinical and/or imaging suspicion of DIE that underwent laparoscopic surgery. We performed consecutive evaluation with transvaginal ultrasound complemented by SVG of all women with clinical suspicion of DIE and assessed for suspected lesions in the anterior compartment (bladder and vesicouterine pouch), rectovaginal septum (RVS), pouch of Douglas (POD), uterosacral ligaments, vagina and rectosigmoid colon. Accuracy of SVG to identify lesions of DIE was assessed for the different sites of DIE, relative to laparoscopic and histological findings. The sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR-) were determined.
Results: Fifty nine women were evaluated by SVG and 19 (32%) underwent laparoscopic surgery. Median age was 35 years (range 26-42 years), nine women (47.4%) were nulliparous and five (26.3%) had infertility. Most common symptoms were dysmenorrhea (14/19) and deep dyspareunia (14/19). DIE was confirmed in all patients, 18/19 (94.7%) had involvement of the posterior and 3/19 (8.9%) of the anterior compartments. We found high sensitivity for the diagnosis of lesions involving the POD (92%) and RVS (90%). Our diagnostic sensitivity was lower for DIE lesions involving the anterior compartment (67%), rectosigmoid colon (50%), vagina (50%) and uterosacral ligaments (23%).
Conclusions: SVG is a simple, non-invasive method with good diagnostic sensitivity for DIE lesions involving particularly RVS and POD. This technique has a relatively short learning curve for experienced operators and provides an effective alternative to other more invasive and expensive imaging techniques.
Keywords: Ultrasound; Sonography; Deep infiltrating endometriosis; Endometriosis
Introduction
Endometriosis is a chronic gynecologic disorder defined as the presence of functional endometrial-like tissue outside the uterine cavity and myometrium1-4. It affects mainly women of reproductive age and its prevalence in the general female population is about 10-15%5-7. Deep Infiltrating Endometriosis (DIE) is defined as endometriosis which infiltrates the surface of the affected structures more than 5 mm1,2,5. This aggressive presentation affects 15-30% of women with endometriosis. The most common locations are the recto-vaginal septum (RVS), the pouch of Douglas (POD), the uterosacral ligaments (USLs), the vagina, the rectosigmoid (RS) colon and, less frequently, the bladder and the ureter8,9.
DIE is a progressive disorder causing severe pelvic pain (dysmenorrhea, deep dyspareunia, non-menstrual pain), heavy menstrual bleeding and other organ-specific symptoms (dysuria, hematuria, dyschezia, constipations, diarrhea). It strongly affects the quality of life of the patients and a radical surgical treatment is often required10.
Accurate site-specific diagnosis is essential to define the optimal therapeutic strategy for patients suffering from endometriosis. Imaging techniques emerge as non-invasive and accurate methods for diagnosing and precisely mapping lesions, as well as in evaluating the extension of DIE lesions to plan surgery and adequately counsel patients regarding the treatment8.
Transvaginal sonography (TVS) is considered the first-line imaging technique in the evaluation of women with suspected pelvic endometriosis, with great sensitivity (64-89%) and specificity (89-100%) for the diagnosis of endometriomas12. However, its accuracy for lesions involving the posterior compartment (RVS, POD, posterior vaginal fornix, USLs and RS) is much lower, less consistent and highly operator-dependent. Sonovaginography (SVG) is an adapted TVS technique that uses a saline solution to increase the acoustic window between the probe and surrounding structures, allowing a better evaluation of DIE lesions.
In this study, the authors assess the performance and accuracy of SVG for preoperative diagnosis and characterization of DIE lesions.
Material and methods
We conducted a prospective observational study at a tertiary-care university hospital, between January 2014 and January 2016. All pre-menopausal women with clinical and/or imaging suspicion of DIE, submitted to laparoscopic surgery were included. Patients gave oral informed consent. Institutional Ethics Committee consent was not request since SVG was performed as a complement to TVS, performed routinely to all our patients with gynecological complaints, and therapeutic strategy, medical versus surgical, was not based on SVG findings alone.
TVS and SVG
During the study period, all pre-menopausal women attending our Gynecology Department with clinical suspicion of endometriosis were evaluated by TVS complemented with SVG. The sonographic exams were performed by the same operator (J.F.), an Assistant in Gynecology with 16 years of experience in gynecologic ultrasound, using a VolusonTM 730 (General Electric, USA) transvaginal 5-9 MHz probe. The investigator was informed about the patient's clinical history, but blinded to the results of physical examination or previous imaging techniques. No bowel preparation was used before sonography.
The sonographic protocol consists of a routine evaluation of uterus and adnexa, including evaluation of uterus and ovaries mobility, sonographic signs of adenomyosis and adnexal masses. Endometriomas and other adnexal masses were described according to the International Ovarian Tumor Analysis (IOTA) terminology12. Obliteration of the POD was assessed by the degree of sliding of the posterior aspect of the cervix over the anterior rectum wall (sliding sign) by gently pressing the uterus with the probe.
SVG acoustic window was obtained by placing 50 ml of ultrasound-gel in the vagina. Endometriosis was defined as irregular hypoechoic lesions and characterized as: 1) nodules - well-circumscribed projected lesions; 2) thickening - infiltrative lesions; 3) indian head-like lesions - mixed lesions with both nodular and infiltrative components. Lesions were mapped by location: 1) anterior compartment (urinary bladder, vesicouterine pouch and distal ureter); 2) ureter; 3) vagina (including posterior vaginal fornix); 3) RVS; 4) POD; 5) USLs and 6) RS colon.
Surgical and Pathologic Findings
All women included in this study underwent laparoscopic surgery by clinical and imagological suspicion of DIE. Surgeries were performed by a Gynecologist Consultant with experience in advanced laparoscopic gynecologic surgery (M.M.). A Consultant in General Surgery with experience in advanced laparoscopic surgery performed interventions involving the excision of RS lesions. Location of all suspicious lesions was recorded and removed or biopsied for histological confirmation.
Diagnosis of DIE was made if at least one structure (bladder, ureter, vagina, RVS, USLs or RS colon) was involved. Histological confirmation included one of the following: 1) endometrial tissue in at least one resected/biopsied lesion13; 2) direct visualization of lesion suggestive of DIE and histological findings of fibrosis and/or smooth muscle cells hypertrophy14. Total obliteration of the POD that could not be surgically clarified (frozen pelvis)15 was also consider a surgical finding of DIE.
Statistical Analysis
We performed a descriptive statistical analysis using SPSS 23.0 (SPSS Inc., Chicago, USA). The accuracy of SVG to identify lesions of DIE was assessed for the different sites of DIE, relative to laparoscopic and histological findings. The sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR-) were determined with 95% confidence intervals (95% CI).
Results
During the 25-month study period, 59 women were evaluated by TVS complemented with SVG because of clinical suspicion of endometriosis. Of those, only 19 underwent laparoscopic surgery.
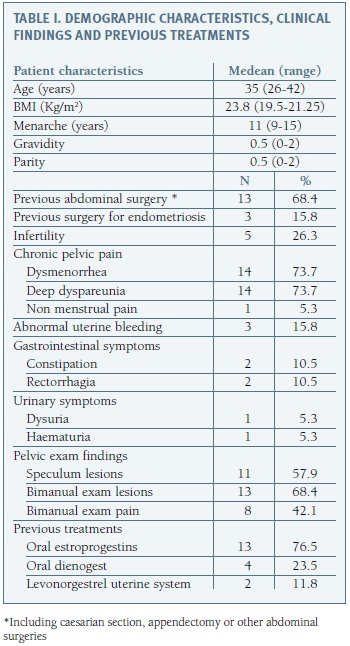
In Table I we report the demographic characteristics, medical history and clinical findings from the 19 women included in our final analysis. Patients median age was 35 years (range 26-42 years), nine women (47.4%) were nulliparous and five (26.3%) had criteria of infertility. Most common symptoms were dysmenorrhea (14 cases) and profound dyspareunia (14 cases). Physical examination revealed suspicious in 13 women, either nodules or tightness at bimanual touch (13 cases) or by direct visualization of macroscopic vaginal lesions (11 cases).
At the time of surgery 17 women (89.5%) were receiving hormonal treatment with oral estroprogestins (13/17 cases), dianogest (4/17 cases) or levonorgestrel intrauterine system (2/17 cases) and three women (13.6%) had already undergone surgical treatment for endometriosis.
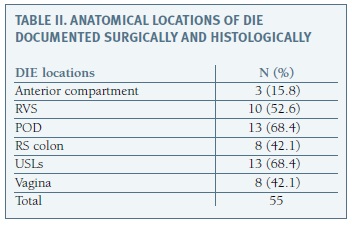
DIE was confirmed in all cases and specific lesion location is summarized in Table II. Posterior and anterior DIE was documented in 18/19 (94.7%) and 3/19 (8.9%) patients, respectively. Ovarian lesion (endometrioma) was found in two patients.
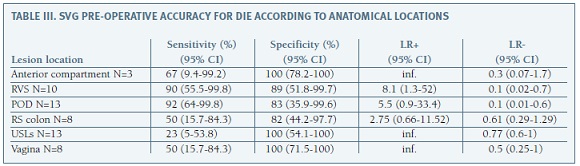
Table III shows pre-operative SVG accuracy for the diagnoses of DIE according to anatomical locations. Sensitivity was higher for the diagnosis of lesions involving POD (92%) and RVS (90%), with LR+ of 0.1 for both locations. Our diagnosis sensitivity was lower for DIE lesions involving the anterior compartment (67%), RS colon (50%), vagina (50%) and USLs (23%).
We found in five patients frozen pelvis in laparoscopy. The sensitivity and specificity of sliding sign was 40% (95% CI: 5.3 - 85.34) and 64% (95% CI: 35.1 - 87.2), respectively, with a LR+ of 1.12 (95% CI: 0.31 - 4.04) and a LR- of 0.93 (95% CI: 0.41 - 2.11).
Comment
Endometriosis is a chronic disease with a broad expression, regarding both the organs it affects as well as the severity of symptoms. Laparoscopic excision of endometriosis lesions is the gold standard treatment, but it is a difficult procedure and requires differentiated technical skills and sometimes long operating time. Accurate preoperative lesion mapping helps to predict the extent of the disease and to define the best therapeutic and surgical strategy. Different imaging techniques have been suggested for this purpose, including magnetic resonance imaging (MRI) and barium enema, but TVS is now considered the first-line imaging tool for the diagnosis of endometriosis, with the advantages of being inexpensive, easily accessible, patient acceptable and an innocuous technique16.
The use of SVG in the evaluation of DIE lesions is relatively new and only recently there has been consensus regarding nomenclature and standardization of the technique17. In 2014 we introduced SVG technique in our Department, initial protocol was slightly modified over the two years, as a result of updating the scientific knowledge and feed-back between our surgeon and sonographer, in order to optimize our diagnostic accuracy.
The main objective of this study was to understand if we could rely on TVU complemented with SVG whenever DIE is suspected to identify, describe and characterize DIE lesions for initial imaging evaluation and select patients that should be referred to MRI, avoiding unnecessary costs.
During these 25 months period a total of 59 TVS complemented with SVG were performed, but only 19 patients underwent surgery based on clinical and imaging findings. Despite our sample size, our preliminary results are quite appealing and point out an acceptable acuity and potential utility of extended TVU for posterior compartment's evaluation, especially for the diagnosis of lesions involving the RVS and POD. Regarding the diagnosis of lesions involving RVS, our accuracy was surprisingly high (sensitivity 90% and specificity 89%) and even superior from the one reported in a recent meta-analysis from Guerriero et al, where sensitivity and specificity for this location was from 49% and 98%, respectively18. Our detection rate for lesions involving the anterior compartment, vagina and RS was very similar to that described in this same meta-analysis.
Regarding lesions involving the USLs we achieved a lower sensitivity compared to data from published literature18 (23% versus 53%) and this finding is probably related to the examiner's experience and learning curve. Our ability to identify POD obliteration using the sliding signal was also significantly lower (sensitivity: 40%, specificity: 64%) than expected (sensitivity: 83%, specificity: 97%)19.
We are aware of our study limitations, particularly the small sample size. For this purpose, the inclusion of patients without DIE criteria, particularly those with endometrioma findings alone, could have been an advantage to increase our overall diagnostic sensitivity and specificity. An intestinal preparation would have been a plus in the diagnosis of intestinal endometriosis, however it was not used mainly due to the fact that the exam was performed preferably on the day of first appointment. All sonographic exams were performed by the same operator, which proved to be extremely useful and of advantageous for his learning curve and technique improvement, but limits the assessment of interobserver variability. Finally, we acknowledge our patient selection bias, since we only include women with DIE, probably overestimating our results.
In conclusion, our data suggest that SVG is a simple, non-invasive method with good diagnostic sensitive for DIE lesions particularly those involving posterior compartment (RVS and POD). This technique, used as a routine TVS complement, has a relatively short learning curve for experienced operators and may provide an effective technique for initial imaging evaluation and selection of patients that should be referred to other more invasive and expansive imaging techniques.
REFERENCES
1. Cimsit C, Yoldemir T, Guclu M, Akpinar IN. Susceptibility-weighted magnetic resonance imaging for the evaluation of deep infiltrating endometriosis: preliminary results. Acta Radiol. 2015;57(7):878-885. [ Links ]
2. De Venecia C, Ascher SM. Pelvic Endometriosis: Spectrum of Magnetic Resonance Imaging Findings. Semin Ultrasound CT MRI. 2015;36(4):385-393. [ Links ]
3. Di Paola V, Manfredi R, Castelli F, Negrelli R, Mehrabi S, Pozzi Mucelli R. Detection and localization of deep endometriosis by means of MRI and correlation with the ENZIAN score. Eur J Radiol. 2015:84(4):568-574. [ Links ]
4. Medeiros LR, Rosa MI, Silva BR, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet 2015;291:611-621. [ Links ]
5. Barcellos MB, Lasmar B, Lasmar R. Agreement between the preoperative findings and the operative diagnosis in patients with deep endometriosis. Arch Gynecol Obstet. 2016;293(4):845-850. [ Links ]
6. Bazot M, Stivalet A, Daraï E, et al. Comparison of 3D and 2D FSE T2-weighted MRI in the diagnosis of deep pelvic endometriosis: preliminary results. Clin Radiol. 2013;68:47-54. [ Links ]
7. Scardapane A, Lorusso F, Bettocchi S, et al. Deep pelvic endometriosis: accuracy of pelvic MRI completed by MR colonography. Radiol Med. 2013;118:323-338. [ Links ]
8. Saccardi C, Cosmi E, Borghero A, et al. Comparison between transvaginal sonography, saline contrast sonovaginography and magnetic resonance imaging in the diagnosis of posterior deep infiltrating endometriosis. Ultrasound Obstet Gynecol. 2012;40(4): 464-469. [ Links ]
9. Bazot M, Lafont C, Rouzier R, et al. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil Steril. 2009;92:1825-1833. [ Links ]
10. Ballweg ML. Impact of endometriosis on women's health: comparative historical data show that the earlier the onset, the more severe the disease. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):201-218. [ Links ]
11. Moore J, Copley S, Morris J, Lindsell D, Golding S, Kennedy S. A systematic review of the accuracy of ultrasound in the diagnosis of endometriosis. Ultrasound Obstet Gynecol. 2002;20(6): 630-634. [ Links ]
12. Timmerman D, Valentin L, Bourne TH, et al. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Grup. Ultrasound Obstet Gynecol. 2000;16:500-505 [ Links ]
13. Cornillie FJ, Oosterlynck D, Lauweryns JM, et al. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril. 1990;53:978-983. [ Links ]
14. Adamson GD, Nelson HP. Surgical treatment of endometriosis. Obstet Gynecol Clin North Am. 1997;24:375-409. [ Links ]
15. Reich H, McGlynn F, Salvat J. Laparoscopic treatment of cul-de-sac obliteration secondary to retrocervical deep fibrotic endometriosis. J Reprod Med. 1991;36:516-522. [ Links ]
16. Piketty M, Chopin N, Dousset B, et al. Preoperative work-up for patients with deeply infiltrating endometriosis: transvaginal ultrasonography must definitely be the first-line imaging examination. Hum Reprod. 2009; 24(3):602-607. [ Links ]
17. Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318-32. [ Links ]
18. Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46:534-545. [ Links ]
19. Reid S, Condous G. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2013;41(6):605-607. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Joana Cruz
E-Mail: joanadacruz@hotmail.com
Recebido em: 14/01/2018
Aceite para publicação: 12/03/2018