Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta Obstétrica e Ginecológica Portuguesa
Print version ISSN 1646-5830
Acta Obstet Ginecol Port vol.10 no.3 Coimbra Sept. 2016
CASE REPORT/CASO CLÍNICO
Benign metastasizing leiomyoma: pulmonary involvement from an uterine fibroid
Leiomioma benigno metastizante: envolvimento pulmonar a partir de um fibroma uterino
Emídio Vale-Fernandes*, Ana Sílvia Pires-Luís**, Vanda Patrício***, Cristina Ramalho***, Carlos Lopes****
Instituto Português de Oncologia do Porto Francisco Gentil, EPE
*Assistente Hospitalar de Ginecologia e Obstetrícia, Serviço de Ginecologia e Obstetrícia do Hospital de Braga
**Médica Interna de Anatomia Patológica, Serviço de Anatomia Patológica do Instituto Português de Oncologia do Porto Francisco Gentil, EPE. Laboratório de Histologia e Embriologia do Departamento de Microscopia do Instituto de Ciências Biomédicas de Abel Salazar da Universidade do Porto
***Assistente Graduada de Ginecologia e Obstetrícia, Serviço de Ginecologia do Instituto Português de Oncologia do Porto Francisco Gentil, EPE
****Assistente Graduado Sénior de Ginecologia e Obstetrícia, Serviço de Ginecologia do Instituto Português de Oncologia do Porto Francisco Gentil, EPE
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Benign Metastasizing Leiomyoma (BML) is a rare disorder in which uterine fibroids metastasize to distant sites. We present a case of a 57-year-old postmenopausal woman under hormone therapy with a previous total hysterectomy for uterine fibroids that performed chest radiography, for a history of weight loss, that showed multiple bilateral pulmonary nodules. The histological examination of these nodules revealed a morphological and immunohistochemical profile consistent with the diagnosis of pulmonary BML (supported by a history of primary uterine leiomyoma). The patient started treatment with tamoxifen, remaining asymptomatic and with stable pulmonary lesions.
Keywords: Fibroids, Uterine; Multiple Pulmonary Nodules; Benign Metastasizing Leiomyoma.
Introduction
The Benign Metastasizing Leiomyoma (BML) is a very rare entity described initially by Steiner in 1939 that featured the presence of nodules of well-differentiated smooth muscle tissue of the uterus in distant places1. Its clinical course is usually indolent, with the incidental finding of pulmonary nodules in chest radiography in women of childbearing age with prior or coincident history of uterine fibroids. Most patients with BML remain asymptomatic but there are cases with symptoms such as cough, chest pain, dyspnea and hemoptysis. The average length of time since hysterectomy until the appearance of nodules in the lung is about 15 years, but there are metastatic foci of leiomyoma up to 24 years after the histerectomy2-7. The younger BML case was reported on a 23-year-old woman8.
The designation of this entity reflects its morphological and biological features, namely a benign morphology, a smooth muscle phenotype and the ability to metastasize, mainly to the lung but also to lymph nodes, heart, breast, liver, esophagus, trachea, skeletal muscle, skin, scars and central nervous system9. Several hypotheses for the pathogenesis of BML were described10, such as a pulmonary hamartoma composed mainly by smooth muscle; a multifocal primary pulmonary smooth muscle neoplasm (leiomyoma, leiomyosarcoma); a vascular and/or lymphatic dissemination of a benign uterine leiomyoma, at least in some cases possibly secondary to surgical manipulation; a metastatic deposit of intravenous leiomyomatosis; and metastasis from a low grade uterine leiomyosarcoma (not recognized due to undersampling of the uterine tumour) or from a smooth muscle tumour that actually is a smooth muscle tumour of unknown malignant potential (STUMP) but fails to meet the morphological criteria to be classified as such11. Although a common pathological pathway had been suggested for BML, leiomyoma with vascular microinvasion and intravenous leiomyomatosis12, and excluding the cases of undersampled uterine smooth muscle tumours that subsequently metastasize, accumulating evidence suggests that BML is a clonal neoplasm originating from an uterine bland-appearing smooth muscle tumour, as it expresses estrogen receptors (ER) and progesterone receptors (PR)9,13,14 and has a similar telomere length pattern14, X-chromosome inactivation pattern15 and a non-random pattern of genetic alterations16 than the uterine tumour of the same patient. Besides this, BML shares some genetic alterations with a subset of leiomyoma, like 19q and 22q terminal deletion10, HMGA2 (12q15) and HMGA1 (6p21) rearrangements16 and loss of 7q16. BML also has a miRNA profile distinct from uterine leiomyosarcoma17, as well as a balanced karyotype instead of a complex and unbalanced karyotype like most leiomyosarcomas15. All these data taken together suggest that there is a subset of uterine smooth muscle tumours morphologically indistinguishable from leiomyomas that harbor genetic alterations which convey them the ability to metastasize10 mostly to the lung.
Case report
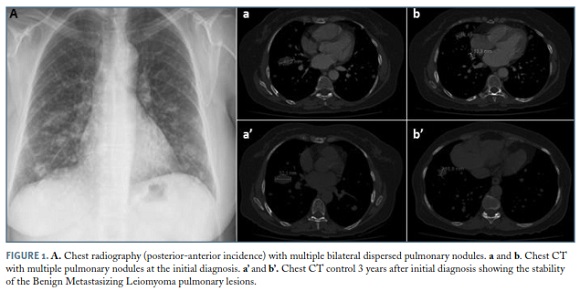
57-year-old postmenopausal woman under hormone therapy with previous total hysterectomy in 1995 for uterine fibroids that owing to a history of weight loss was admitted for further evaluation. The patient had lost about 20% of her body weight over a period of 10 months, showing no other relevant symptoms, including chest pain, cough, dyspnea and hemoptysis. Physical examination showed no pathological changes, particularly in terms of auscultation. The general analytical study had not any particular abnormality, namely anaemia or elevated sedimentation rate. The chest radiography revealed multiple bilateral pulmonary nodules (Figure 1A), which result was confirmed by computerized tomography (CT) (Figures 1a and 1b). The patient underwent further assessment with abdominopelvic CT scan that was negative. As there was only thoracic disease an excisional biopsy by thoracotomy was performed.
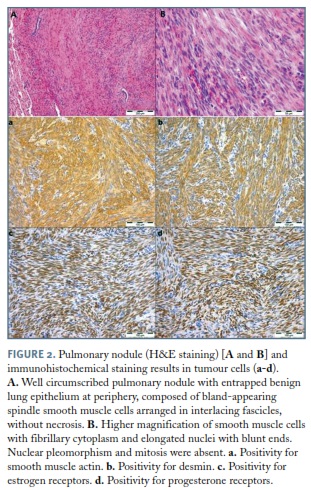
H&E staining showed well circumscribed but not encapsulated pulmonary nodules. These nodules were composed of spindle smooth muscle cells arranged in intersecting fascicles, sometimes with entrapment of benign lung epithelium consistent with an indolent progression with no atypia, mitosis or necrosis, excluding leiomyosarcoma (differential diagnosis based on morphology)18. Immunohistochemically, tumour cells were diffusely immunoreactive for Smooth Muscle Actin (SMA), desmin and caldesmon, supporting the smooth muscle phenotype9,13, as well as for ER and PR, which suggests a gynaecological origin and hormonal regulation for these pulmonary nodules (Figure 2)9,13. Epithelial Membrane Antigen (EMA), AE1/ /AE3, CD34 and CD31 were negative, excluding monophasic synovial sarcoma, intrapulmonary solitary fibrous tumour and sarcomatoid carcinoma18. Fluorescence in situ Hybridization (FISH) analysis in Formalin-Fixed Paraffin-Embedded (FFPE) slides did not detect 22q12 nor 19q13 deletion.
In cancer advisory group it was decided hormone therapy suspension and imaging reassessment in 2 months. There was no significant improvement in pulmonary lesions and then it was decided to start tamoxifen. The 3 years follow-up period reveals clinical and imaging stability of the lesions (Figures 1a’ and 1b’).
Discussion
A diagnosis of BML was rendered based on the characteristic morphological and immunohistochemical features, and in the clinical information of a previous hysterectomy for fibroids, even though 22q and 19q deletions were absent. At the present time, the absence of these deletions in an otherwise typical case did not preclude the diagnosis of BML since these deletions were described in only 5 cases10 and, although suggestive of BML, further studies are needed to assess their prevalence and pathological relevance in this entity.
Entities such as pulmonary lymphangioleiomyomatosis and leiomyosarcoma, that should be included in the differential diagnosis, have distinct morphological features. BML is characterized by solid nodules composed by a bland-appearing proliferation of smooth muscle cells, in a patient with a history of uterine leiomyomas, whilst in lymphangioleiomyomatosis cystic changes and clusters of round to spindle cells without significant nuclear pleomorphism or mitotic activity (LAM cells) are seen at the edges of the cysts, along pulmonary lymphatics, at the vessel walls and/or distal airways walls18. Immunohistochemical staining can be helpful in the differential diagnosis, as LAM cells are SMA positive and HMB45 positive whereas BML smooth muscle cells are SMA positive and HMB45 negative. Differential diagnosis of BML and leiomyosarcoma is based on morphology, as both entities are SMA and desmin positive, but in leiomyosarcoma intersecting fascicles of spindle cells with atypia, mitosis and necrosis are seen. In turn, immunohistochemical staining with CD34 and EMA might be helpful in the differential diagnosis of BML (CD34 and EMA negative) with monophasic synovial sarcoma (usually EMA and CD34 positive) and intrapulmonary solitary fibrous tumour (CD34 positive in 90-95% and EMA positive in 33%) and with sarcomatoid carcinoma, that can be focally immunoreactive for SMA but also is at least focally cytokeratin positive18.
Typical radiological findings of BML, on chest radiography, include diffuse and bilateral nodular opacities. CT usually reveal well-defined nodules, solitary or multiple, scattered in both lungs, ranging in size from a few millimeters to several centimeters. The miliary pattern, interstitial lung disease, cavitary nodules and multilocular cystic lesions are rarely observed. The absence of pleural and endobronchial involvement and absence of mediastinal lymphadenopathies are characteristic. Lung nodules may remain stable, decrease or increase in size with time2.
Pulmonary BML is rare but should be considered whenever there is presence of pulmonary nodules and concomitant or previous history of uterine fibroids, especially if there isn’t evidence of another cancer (diagnosis based on clinical, histopathologic and immunohistochemical features)2. As the standard treatment of BLM has not been established, an individual approach in specific clinical cases should be considered, which may involve the chemical or surgical castration, as well as an expectant attitude2. If feasible, the radical excision of BML lesions saving the surrounding parenchyma is recommended. However, as in most cases the lesions are multiple, surgical or chemical castration is the treatment of choice in BML, since these lesions are hormone-dependent tumours (presence of ER and PR). The pulmonary nodules tend to remain stable or even regress with treatment with GnRH (gonadotropin releasing hormone) agonists, selective estrogen receptor modulators (tamoxifen), progestins or aromatase inhibitors and with bilateral oophorectomy in premenopausal women. Some authors advocate an expectant and conservative approach because regression of BML lesions is described in situations occurring with natural decrease in estrogen levels (termination of pregnancy and menopause)2,4-6,19,20. In this clinical case it was decided to suspend the hormone therapy and start tamoxifen, keeping the patient in regular surveillance.
REFERENCES
1. Steiner PE. Metastazing fibroleiomyoma of the uterus. Report of a case and review of the literature. Am J Pathol. 1939;15:89-109. [ Links ]
2. Kołaczyk K, Chamier-Ciemińska K, Walecka A, Chosia M, Szydłowska I, Starczewski A, Grodzki T, Smereczyński A, Sawicki M. Pulmonary benign metastasizing leiomyoma from the uterine leiomyoma: a case report. Pol J Radiol. 2015;80:107-10.
3. Fasih N, Prasad Shanbhogue AK, Macdonald DB, Fraser-Hill MA, Papadatos D, Kielar AZ, Doherty GP, Walsh C, McInnes M, Atri M. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics. 2008;28(7):1931-48. [ Links ]
4. Abramson S, Gilkeson RC, Goldstein JD, Woodard PK, Eisenberg R, Abramson N. Benign metastasizing leiomyoma: clinical, imaging, and pathologic correlation. Am J Roentgenol. 2001;176(6):1409-13. [ Links ]
5. Ki EY, Hwang SJ, Lee KH, Park JS, Hur SY. Benign metastasizing leiomyoma of the lung. World J Surg Oncol. 2013;11:279. [ Links ]
6. Chen S, Zhang Y, Zhang J, Hu H, Cheng Y, Zhou J, Shen L, Chen H. Pulmonary benign metastasizing leiomyoma from uterine leiomyoma. World J Surg Oncol. 2013;11:163. [ Links ]
7. Jeon HW, Choi SH, Sung SW, Park JK. Pulmonary benign metastasizing leiomyoma: report of three cases. World J Surg Oncol. 2013;11:281. [ Links ]
8. Goyle KK, Moore DF Jr, Garrett C, Goyle V. Benign metastasizing leiomyomatosis: case report and review. Am J Clin Oncol. 2003;26(5):473-6. [ Links ]
9. Chen S, Liu RM, Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J Thorac Dis. 2014;6:E92-8. [ Links ]
10. Nucci MR, Drapkin R, Dal Cin P, Fletcher CD, Fletcher JA. Distinctive cytogenetic profile in benign metastasizing leiomyoma: pathogenetic implications. Am J Surg Pathol. 2007;31:737-43. [ Links ]
11. Esteban JM, Allen WM, Schaerf RH. Benign metastasizing leiomyoma of the uterus: histologic and immunohistochemical characterization of primary and metastatic lesions. Arch Pathol Lab Med. 1999;123:960-2. [ Links ]
12. Canzonieri V, D'Amore ES, Bartoloni G, Piazza M, Blandamura S, Carbone A. Leiomyomatosis with vascular invasion. A unified pathogenesis regarding leiomyoma with vascular microinvasion, benign metastasizing leiomyoma and intravenous leiomyomatosis. Virchows Arch. 1994;425:541-5. [ Links ]
13. Jautzke G, Muller-Ruchholtz E, Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas). A report on 5 cases. Pathol Res Pract. 1996;192:215-23. [ Links ]
14. Patton KT, Cheng L, Papavero V, Blum MG, Yeldandi AV, Adley BP, Luan C, Diaz LK, Hui P, Yang XJ. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod Pathol. 2006; 9:130-40. [ Links ]
15. Tietze L, Günther K, Hörbe A, Pawlik C, Klosterhalfen B, Handt S, Merkelbach-Bruse S. Benign metastasizing leiomyoma: a cytogenetically balanced but clonal disease. Hum Pathol. 2000;31(1):126-8. [ Links ]
16. Bowen JM, Cates JM, Kash S, Itani D, Gonzalez A, Huang D, Oliveira A, Bridge JA. Genomic imbalances in benign metastasizing leiomyoma: characterization by conventional karyotypic, fluorescence in situ hybridization, and whole genome SNP array analysis. Cancer Genet. 2012;205:249-54. [ Links ]
17. Nuovo GJ, Schmittgen TD. Benign metastasizing leiomyoma of the lung: clinicopathologic, immunohistochemical, and micro-RNA analyses. Diagn Mol Pathol. 2008;17(3):145-50. [ Links ]
18. Rao NM, Moran CA. Lymphangioleiomyomatosis. In: Pulmonary Pathology (1st Edition). Suster S (ed). Demos Medical Publishing; 2014:90-93.
19. Lopes ML, Carvalho L, Costa A. Benign metastasizing leiomyomas. Acta Med Port. 2003;16(6):455-8. [ Links ]
20. Teixeira BC, Mahfouz K, Escuissato DL, Costa AF, Noronha Ld. Solitary benign metastasizing leiomyoma: imaging features and pathological findings. J Bras Pneumol. 2014;40(2):193-5. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Emídio Vale-Fernandes
Hospital de Braga
E-mail: emidio.vale.fernandes@gmail.com
Conflicts of interest
The authors declare no conflict of interest in conducting this work.
Sources of funding
The authors declare that this work was not subject to any financing.
Recebido em: 9/10/2015
Aceite para publicação: 3/12/2015