Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Obstétrica e Ginecológica Portuguesa
versão impressa ISSN 1646-5830
Acta Obstet Ginecol Port vol.9 no.5 Coimbra dez. 2015
ORIGINAL STUDY/ESTUDO ORIGINAL
Amniocentesis in a tertiary referral centre: still the same old story?
Amniocentese num centro de referência terciário: alguma coisa de novo?
Sofia Monteiro*, Alexandra Matias**, Teresa Loureiro**, Manuela Cunha***, Ana Azevedo****, Nuno Montenegro*****
Centro Hospitalar de São João, Porto, Portugal
*MD, Serviço de Ginecologia e Obstetrícia, Centro Hospitalar de S. João
**PhD, Serviço de Ginecologia e Obstetrícia, Centro Hospitalar de S. João; Departamento de Ginecologia e Obstetrícia, Faculdade de Medicina da Universidade do Porto
***MD, Serviço de Ginecologia e Obstetrícia, Centro Hospitalar de S. João
****PhD, Departamento de Epidemiologia Clínica, Medicina Preditiva e Saúde Pública, Faculdade de Medicina da Universidade do Porto; Instituto de Saúde Pública da Universidade do Porto
*****PhD, Serviço de Ginecologia/Obstetrícia, Centro Hospitalar de S. João; Departamento de Ginecologia e Obstetrícia, Faculdade de Medicina da Universidade do Porto; Instituto de Saúde Pública da Universidade do Porto
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Overview and Aims: The main objective of this study was to describe the main clinical indications, diagnostic yield, complications and pregnancy outcomes regarding amniocentesis performed in a tertiary University Hospital, during an 8-year period.
Study Design: We developed an observational, retrospective study, of all amniocenteses performed between June 2003 and June 2011.
Population: All pregnant women consecutively submitted to amniocentesis in a tertiary University Hospital between June 2003 and June 2011. Only singleton gestations were included.
Methods: We searched the database of the Genetics Department for all products obtained by invasive procedures performed during pregnancy between June 2003 and June 2011, in order to identify the amniocenteses performed during that period. Maternal demographics, indication for amniocentesis, gestational age at the time of amniocentesis, procedure-related complications during pregnancy and pregnancy outcome data were extracted from patient’s physical and electronic medical records.
Results: A total of 1358 amniocenteses were included in the study. The proportion of amniocentesis performed due to maternal age decreased significantly and due to positive prenatal screening significantly increased over time (p<0,001).The indication with highest positive predictive value regarding abnormal fetal karyotype was parent carrier of chromosome abnormality (46.2%). Total pregnancy loss was 1.6%, post-procedural miscarriage rate was 0.74% and fetal loss risk within 2-weeks of procedure was 0.4%. There was no association between fetal loss and operator, number of needle insertions, transplacental puncture and bloody tap.
Conclusions: Counselling is complex and questions regarding procedure-related complications and fetal loss have been inconsistently reported. National and local institutional precise estimates are important to consider when advising women requesting amniocentesis.
Keywords: Abnormal karyotipe; Prenatal diagnosis; Amniocentesis.
Introduction
Amniocentesis is considered the first invasive diagnostic technique ever performed during pregnancy, initially as a method to detect severe fetal anemia. Only in the late 1960’s and early 1970’s it was used primarily for genetic diagnosis in high-risk patients1.
Since then and for more than 40 years, amniocentesis has played a leading role as an invasive diagnostic procedure for the detection of chromosomally abnormal fetuses.
For a long time, prenatal aneuploidy screening was based solely on maternal age and, therefore, that was the main indication for amniocentesis. The introduction of combined biochemical and ultrasound markers changed the paradigm of prenatal diagnosis: no longer should maternal age per se be considered the sole marker 2. Combined screening improved the sensitivity of screening and diminished false positive results, reducing the number of cases with indication for invasive procedures.
However, the definite diagnosis of chromosomal abnormalities in the antenatal period is still only possible through invasive techniques and, despite the shift towards earlier testing namely with chorionic villus sampling, amniocentesis is still a highly valued technique.
The main objective of this study was to characterize a population of pregnant women submitted to amniocentesis in a tertiary hospital, and to assess the diagnostic yield regarding fetal karyotype as well as pregnancy outcomes. This information is useful to counsel future pregnant women, as this represents a reference unit in Portugal and national data are scarce.
Methods
An observational retrospective study was performed in the Prenatal Diagnosis Unit of São João Hospital Center, with the approval of the Ethics Committee of the institution. The Prenatal Diagnosis Unit in São João Hospital Center, a tertiary and university hospital with differentiated perinatal care, performs invasive procedures for fetal karyotype studies not only in those cases screened in the Hospital, but also in cases referred from other hospitals, essentially from the North of Portugal, for diagnosis and counselling.
We searched the database of the Genetics Department for all products obtained by invasive procedures performed during pregnancy between June 2003 and June 2011, in order to identify the amniocenteses performed during that period. In this analysis only singleton gestations were included. From June 2003 to June 2011, a total of 1551 amniocentesis were performed, of which 1444 in singleton pregnancies.
Maternal demographics, indication for amniocentesis, gestational age at the time of amniocentesis, cytogenetic results, procedure-related complications during pregnancy and pregnancy outcome data were extracted from patient’s physical and electronic medical records. For patients delivering outside our hospital or whenever the information needed was not available, a questionnaire was sent by mail to be retrieved by one of the investigators (via regular post or email). Of the 1444 singleton pregnancies punctured, 86 (6%) were excluded due to missing information regarding operator, gestational age at procedure or outcome of pregnancy. Complete data were thus obtained for 1358 cases.
All procedures were performed under ultrasound guidance by senior staff obstetricians with special training in invasive procedures or supervised by them and performed by junior obstetricians. No procedures were performed by residents. Amniocentesis was performed with a 21-Gauge needle and, in the majority of cases, 15 mL of amniotic fluid was collected. From all amniotic fluid samples received in the laboratory, culture failed in 2 cases (0.14%).
The outcome was classified as termination of pregnancy (TOP), miscarriage if it concerned a spontaneous abortion before 24 weeks of gestation, intrauterine death or stillbirth after 24 weeks and delivery of a liveborn child. Postprocedural miscarriage rate was defined as spontaneous abortion or fetal demise (before 24 weeks gestation) after amniocentesis. Total pregnancy loss comprised all miscarriages, intrauterine deaths or stillbirths. Fetal loss and premature rupture of membranes within 2-weeks of procedure were also evaluated.
Statistical analysis was performed in Stata® version 11.1 for Windows (StataCorp LP, College Station, TX).
Results
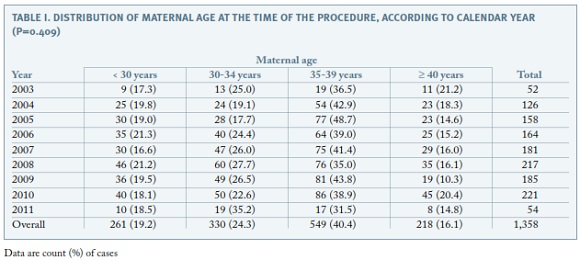
The distribution of maternal age at the time of the procedure is shown in Table I. Overall, 56.5% of the procedures were performed in women aged 35 years or more and the proportion of invasive tests performed in this age group remained the same during the study period (p=0.409).
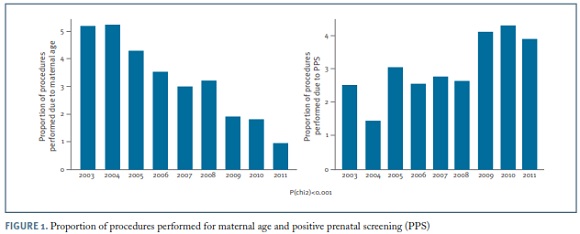
Regarding the clinical indications for amniocentesis, the most frequent was maternal age ≥ 35 years (32.2%), followed by positive prenatal screening (30.9%), either first trimester combined screening or second trimester triple screening or integrated screening ; ultrasound markers of aneuploidy (13.4%); ultrasonographically detected malformations (12.9%); seroconversion (maternal infections) (3.5%); previous child with chromosomal abnormality (3.3%); parent carrier of chromosome abnormality (1.9%); maternal anxiety (1.6%) and miscellaneous (0.4%). The proportion of amniocenteses performed due to maternal age decreased significantly along the study period, from over 50% to less than 10% (p<0.001). In contrast, the proportion of invasive procedures performed for positive prenatal screening increased significantly over time up to more than 40% (p<0.001) (Figure 1).The proportion of amniocentesis performed for the remaining indications did not have a meaningful variation along the 8-year period.
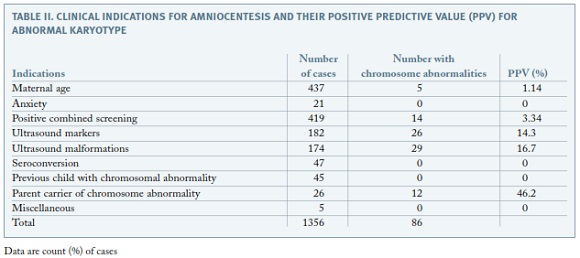
Out of the 1356 karyotypes obtained, 86 presented chromosomal abnormalities (6,3%). Of those, 38 (44%) had trisomy 21, 12 presented trisomy 18 (14%) and 9 had monosomy X (10%) The clinical indications with highest positive predictive values were parent carrier of chromosome abnormality (46.2%), ultrasonographically detected malformations (16.7%) and ultrasound markers of aneuploidy (14.3%) (Table II).
The amniocenteses were done between 12 and 39 weeks. The proportion of procedures performed before 16 weeks was 2.8% and it remained similar throughout the study period (p=0.16). Ninety-nine amniocenteses were performed at 24 weeks or more, representing 7.2% of the total of cases analysed.
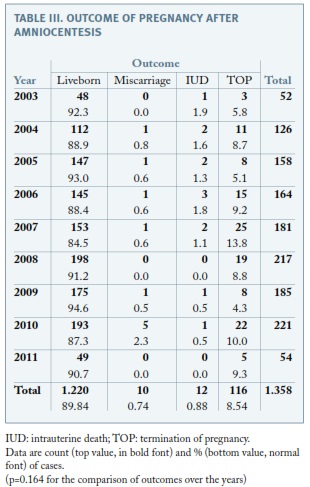
Regarding the outcome of pregnancy after amniocentesis, ten pregnancies ended in a miscarriage (< 24-week’gestation), representing a postprocedural miscarriage rate of 0.7%. Twenty-two pregnancies ended in a fetal death (ten before and twelve after 24 weeks’ gestation), representing a total pregnancy loss rate of 1.6%. There were 116 cases of TOP (8.5%) and the proportion did not change throughout the study, as none of the other outcomes (p=0.164) (Table III). Focusing on pregnancies presenting with a normal karyotype and that were not terminated, fetal loss was 1.56%, which is significantly different from fetal loss among those with abnormal karyotype (11%, p<0.001) but similar to total pregnancy loss rate.
Among pregnancies that were not interrupted, fetal loss risk within 2 weeks of the procedure was 0.4% (95%CI 0.1-0.9) and the incidence of premature rupture of membranes within 2 weeks was 0.37%. There was no association between the operator and fetal loss within 2 weeks of procedure (ranging from 0 to 2%, p=0.89). The proportion of amniocenteses with more than one needle insertion was 3%, brownish/hematic fluid occurred in 2.7% and transplacental puncture in 15%, with no association with fetal loss within 2 weeks of procedure (p= 0.12, p= 0.64 and p= 0.84, respectively).
Discussion
In this study, we present a review of our Prenatal Diagnosis Centre experience across 8 years. During the study period, of the 1358 samples studied, cytogenetic results were obtained in 99.9% of the cases. In fact our culture failure rate is very low probably because the vast majority of the amniocenteses were performed earlier in pregnancy when a greater concentration of fetal cells is found. This is in good agreement with the current literature and expectedly in our department prenatal cytogenetic diagnosis in amniotic fluid samples is a highly reliable method to obtain fetal karyotype3.
Regarding clinical indications for invasive testing, our results are similar to other published series 4, 5. Maternal age and positive combined prenatal screening are the most frequent indications for cytogenetic study, representing 63% of all clinical indications. However, during the study period, a few changes occurred in the prenatal diagnosis scenario. For many years, maternal age was the only marker associated to Down syndrome and represented the main clinical reason for referral for invasive diagnostic procedures. However, maternal age as selection criteria for invasive testing performed poorly. Nowadays, a much more individualized risk assessment is performed in most countries6. Biochemical markers, combined with nuchal translucency and further ultrasound markers, such as nasal bone, ductus venosus and tricuspid regurgitation, are progressively replacing maternal age as standard method for prenatal screening7. In our study, that is clearly revealed by the significant decrease of amniocentesis performed for maternal age, opposed to the increase in invasive testing performed because of positive prenatal screening. Nevertheless, the total number of amniocenteses performed did not decline as would be expected as a consequence of the more performant first trimester screening, because the total number of referrals kept steadily increasing. Only after 2011 there is an effective decline in the total number of amniocenteses because CVS was introduced routinely in the Department.
It is known that karyotype abnormalities range between 1% and 6.7% in amniocenteses results5. In our series, chromosome abnormalities were disclosed in 6.3% of the cases. Analyzing the frequency of chromosome abnormalities for each clinical indication, parental chromosomal rearrangements presented the highest PPV (46.2%). This was an expected finding, as progenitors with chromosomal rearrangements have a high probability of transmitting it to descendants. Excluding this indication, clinical indications with highest VPP values are ultrasound malformations and ultrasound markers of aneuploidy, with 16.7% and 14.3%, respectively. This is also in agreement with literature data, confirming ultrasound examination important role in prenatal diagnosis screening for chromosomal anomalies4,5. Low VPP value for positive prenatal screening , similarly to other studies5, might be explained by the fact that not only positive combined first-trimester screening were included, but also second trimester positive screenings and positive integrated screenings, which are less selective.
Despite recent advances that promoted chorionic villous sampling in invasive prenatal testing, amniocentesis still has a place in genetic diagnosis. Counseling should include information regarding procedure-associated complications. Fetal loss is considered the major risk of second-trimester amniocentesis, as maternal risks are small and extremely rare. In determining the total fetal loss rate, we have to acknowledge the spontaneous background loss rates in the study population and add it up to the procedure-related fetal loss. Spontaneous background losses have been difficult to assess. The only randomized trial ever performed, more than 25 years ago, reported a loss rate of 1.7% in the amniocentesis group and 0.7% in the control group8. However, in the present practice, performing such a trial would be impractical, for ethical concerns9. The traditional reported amniocentesis-related pregnancy loss, quoted by most of practitioners when counseling women, has been stated as 1%. However, recent studies suggest lower procedure-related fetal loss rates.
A retrospective cohort study compared the fetal loss rate in women who underwent a midtrimester amniocentesis with the fetal loss rate of those women that did not have any invasive procedure and reported a fetal loss rate attributable to the invasive procedure of only 0.13%10. Also, the fetal loss rate presented in the First and Second Trimester Evaluation of Risk (FASTER) trial was 0.06 %8. It may be concluded that the present numbers are consistent with a procedure-related miscarriage risk lower than 1%.
In Portugal, national data are limited and the referral profile of our centre further supports the importance of reporting information regarding procedure-associated fetal loss.
Definitions concerning what should really be considered fetal losses attributable to invasive procedure are far from being consensual. In fact, a systematic review aiming to compile complications related to amniocentesis, used various definitions for “procedure-related pregnancy losses” 11. As such, we chose to evaluate not only total pregnancy loss rate (all fetal deaths during pregnancy), but also post-procedural miscarriage rate (fetal death before 24 weeks) and fetal loss risk within 2-weeks of procedure. In our study, post-procedural miscarriage rate was of 0.74%, comparable to available literature numbers10,12. Total pregnancy loss rate was 1.62%, which is similar to others in literature 11,13. When excluding those pregnancies electively terminated, the risk of fetal loss within 2-weeks of procedure was of 0.4%, comparable to previously stated procedure-related fetal loss rates14.
As effective screening methods are being implemented, the number of invasive procedures is steadily declining, raising concerns regarding operator skills. As fetal loss after amniocentesis is fortunately low, other complications are studied in order to evaluate operator’s performance. Some of those complications were evaluated in previous studies as potential variables associated with fetal loss, such as multiple needle insertion and “bloody tap”.
Regarding multiple needle insertions, the proportion in previous studies is highly variable, ranging from 0.2% to 2.9 %11. In our study, the proportion of amniocentesis needing more than one needle insertion was 3%. It is unlikely that needle size has a significant importance in this matter, because series with similar needle size have lower multiple insertion rates. Our high rate might be due to the fact that, although the majority of amniocentesis was performed by highly skilled operators, 11.8% were performed by less experienced specialists, although supervised by senior operators. However, previous published studies show that there is no significant difference in fetal loss rate between only one needle insertion and multiple needle insertion10. That is supported in our study that showed no increase in fetal loss within 2-weeks of procedure in the group with multiple needle insertions.
About transplacental puncture, although there is no randomized approach to this question, a prospective case-control study showed no significant difference in fetal loss rate between non-transplacental and transplacental amniocentesis6. Our results sustain those found previously, since fetal loss rate within 2-weeks of procedure is similar with or without transplacental puncture.
Bloody tap, potentially a surrogate measure of operator performance, was found to be associated with fetal loss15. However, in our study, there was no significant difference in fetal loss rate within 2-weeks of procedure, regarding the presence of bloody tap.
Recent advances in prenatal diagnosis, such as the possibility of fetal DNA analysis in maternal blood and the increasing availability of CVS as a first trimester alternative for diagnosis, might potentially decrease the number of amniocentesis and, as such, evaluation of centre and operator’s performance assumes further importance. Recent studies in literature reinforce the need to analyse institutional data in order to provide benchmark data and quality control.
The present study aimed to analyse the clinical indications, outcomes and procedure related complications in our tertiary centre throughout an 8-year span. Along these years, despite a meaningful shift in aneuploidy screening, with advanced maternal age declining as referral for invasive procedures, amniocentesis remained a valuable, reliable method for cytogenetic analysis, with recognized clinical indications.
Although we collected a large quantity of data regarding amniocentesis, our study is limited by its retrospective design. However, to our knowledge, it represents the first available and complete portuguese data concerning this issue. Counselling is complex and important questions as procedure-related complications and associated fetal loss have been inconsistently reported, with significant variations from study to study. As such, availability of institutional precise estimates regarding these important questions would be useful when advising women requesting amniocentesis.
REFERENCES
1. Niermeijer MF, Sachs ES, Jahodova M, C Tichelaar-Klepper, W J Kleijer, H Galjaard. Prenatal diagnosis of genetic disorders. J Med Genet 1976 Jun;13(3):182-194. [ Links ]
2. Ogilvie CM. Prenatal diagnosis for chromosome abnormalities: past, present and future. Pathol Biol (Paris) 2003;51(3):156- -160. [ Links ]
3. Lam YH, Tang MH, Sin SY, Ghosh A. Clinical significance of amniotic-fluid-cell culture failure. Prenat Diagn 1998;18(4): 343-347. [ Links ]
4. Han SH, An JW, Jeong GY, Yoon HR, Lee A, Yang YH, Lee KP, Lee KR. Clinical and cytogenetic findings on 31,615 mid-trimester amniocenteses. Korean J Lab Med 2008; 28(5):378-385. [ Links ]
5. Mademont-Soler I, Morales C, Clusellas N, Soler A, Sánchez A. Group of Cytogenetics from Hospital Clínic de Barcelona. Prenatal cytogenetic diagnosis in Spain: analysis and evaluation of the results obtained from amniotic fluid samples during the last decade. Eur J Obstet Gynecol Reprod Biol 2011; 157(2):156-160. [ Links ]
6. Tabor A, Alfirevic Z. Update on procedure-related risks for prenatal diagnosis techniques. Fetal Diagn Ther 2010; 27(1):1-7. [ Links ]
7. Nicolaides KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol 2004;191(1):45-67. [ Links ]
8. Tabor A, Philip J, Madsen M, Bang J, Obel EB, Nørgaard- -Pedersen B. Randomised controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet 1986; 1(8493):1287-1293. [ Links ]
9. Eddleman KA, Malone FD, Sullivan L, Dukes K, Berkowitz RL, Kharbutli Y, Porter TF, Luthy DA, Comstock CH, Saade GR, Klugman S, Dugoff L, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, D’Alton ME. Pregnancy loss rates after midtrimester amniocentesis. Obstet Gynecol 2006; 108(5):1067-1072. [ Links ]
10. Odibo AO, Gray DL, Dicke JM, Stamilio DM, Macones GA, Crane JP. Revisiting the fetal loss rate after second-trimester genetic amniocentesis: a single center’s 16-year experience. Obstet Gynecol 2008; 111(3):589-595. [ Links ]
11. Mujezinovic F, Alfirevic Z. Procedure-related complications of amniocentesis and chorionic villous sampling: a systematic review. Obstet Gynecol 2007; 110(3):687-694. [ Links ]
12. Corrado F, Cannata ML, La Galia T, Magliarditi M, Imbruglia L, D’anna R, Carlo Stella N. Pregnancy outcome following mid-trimester amniocentesis. J Obstet Gynaecol 2012; 32(2):117-119. [ Links ]
13. Tabor A, Vestergaard CH, Lidegaard Ø. Fetal loss rate after chorionic villus sampling and amniocentesis: an 11-year national registry study. Ultrasound Obstet Gynecol 2009; 34(1):19-24. [ Links ]
14. Enzensberger C, Pulvermacher C, Degenhardt J, Kawacki A, Germer U, Gembruch U, Krapp M, Weichert J, Axt-Fliedner R. Fetal loss rate and associated risk factors after amniocentesis, chorionic villus sampling and fetal blood sampling.Ultraschall Med 2012; 33(7):E75-9. doi: 10.1055/s-0031-1299388. [ Links ]
15. Kong CW, Leung TN, Leung TY, Chan LW, Sahota DS, Fung TY, Lau TK. Risk factors for procedure-related fetal losses after mid-trimester genetic amniocentesis. Prenat Diagn 2006; 26(10):925-930. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Sofia Monteiro
Serviço de Ginecologia/Obstetrícia
Centro Hospitalar de S. João
Alameda Prof. Hernáni Monteiro
4200-319 Porto
E-mail: sofiambessa@gmail.com
Ackowledgements
The authors thank Prof. Filipa Carvalho, from the Department of Human Genetics, São João Hospital Center, for kindly giving us access to the Department’s information on invasive procedures.
Recebido em: 30-11-2014
Aceite para publicação: 22-02-2015