Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Motricidade
versão impressa ISSN 1646-107X
Motri. vol.14 no.1 Ribeira de Pena maio 2018
ORIGINAL ARTICLE
Resistance exercise recovery morphology and AQP1 expression in denervated soleus muscle of Wistar rats
Keli Lovison1; Lizyana Vieira1; Regina Inês Kunz1; Suellen Ribeiro da Silva Scarton2; Juliana Sobral Antunes1; Jhenifer Karvat3; Ana Luiza Peretti1; Gladson Ricardo Flor Bertolini1; Rose Meire Costa Brancalhão1; Célia Cristina Leme Beu1; Lucinéia de Fátima Chasko Ribeiro1
1State University of Western Paraná, Cascavel, Brazil.
2State University of Londrina, Londrina, Brazil.
3Federal University of Santa Catarina, Florianópolis, Brazil.
ABSTRACT
It was our objective to analyse the effects of resistance exercise (climbing steps), which was started in the acute phase, on the histomorphometry and the expression of aquaporin 1 (AQP1) in the soleus muscle after sciatic nerve injury in Wistar rats. Twenty-four adult rats were randomly divided into the following four groups, containing six animals each: control (G1); exercise (G2); injury (G3); and exercise with injury (G4). Three days after the compression of the sciatic nerve, the animals in G2 and G4 were submitted to resistance exercise for 21 non-consecutives days. The exercise was conducted in two series of 10 consecutive ascents of the ladder, with an additional weight of 100g and with an interval of 60 seconds between sets for rest. After this period, the animals were sacrificed, and the soleus muscle was processed. The number of blood capillaries in G3 was 65.7% and 76.86% higher when compared with the G2 and G4, respectively. The morphological analysis revealed muscle damage in G3, hypertrophy in G2 and significant improvement in the muscle in G4. The AQP1 was immunolocalized in the endothelium of blood capillaries present in the muscle fibres, with different expressions in the groups. Resistance exercise initiated in the acute phase was an effective therapeutic modality in the recovery of morphological aspects in the soleus muscle after denervation.
Keywords: peripheral nerves, skeletal muscle, aquaporin, rehabilitation, injuries.
INTRODUCTION
Every year, in developed countries, the incidence of peripheral nerve damage is estimated to be between 13 to 23 cases per 100,000 inhabitants (Li et al. 2014). This mainly affects the economically active population and has a major impact on individuals, their families and society, as a whole (Sebben et al., 2011). Lesions to the sciatic nerve are characterized by pain along the nerve path or sciatica, low back pain, sensory disturbances and muscle weakness in the lower muscles (Kobayashi, Yoshizawa, & Yamada, 2011), such as the soleus muscle, which is extremely important in terms of standing and roam (Moore & Dalley, 2007). Discontinuation of neuromuscular communication leads to functional impairments because such discontinuation can result in a reduction in the cross-sectional area of muscle fibres (Junior et al. 2013), atrophy (Silva-Couto et al., 2012), an increase in capillary density (Wagatsuma, Tamaki, & Ogita, 2005) and an increase in intramuscular connective tissue (Caierão, Betini, Teodori, & Minamoto, 2008).
Although nerves have an intrinsic ability to recover after injury, the regeneration of the affected tissues remains an important clinical problem (Cheng et al., 2013). Rehabilitation programs usually include resistance-type physical exercises that consist of activities designed to focus on the restoration of muscle function by applying an overload (Cassilhas et al., 2013). Studies have shown the benefits of exercise on muscle regeneration such as preventing atrophy and restoring the contractile and metabolic properties of muscles (Marqueste, Alliez, Alluin, Jammes, & Decherchi, 2004; Tanaka, Tsubaki, & Tachino, 2005). However, the effect of exercise on the soleus muscle after compression injuries of the sciatic nerve is a much-discussed aspect, especially in relation to the type of exercise, its intensity, and the best time to start such exercise (Tanaka et al., 2005; Artifon, Silva, Ribeiro, Brancalhão, & Bertolini, 2013).
Aquaporins (AQPs) are membrane proteins that were initially identified as facilitative proteins of physiological processes in functional terms through the passive, bidirectional transport of water. Nowadays, it is known that this is only one of the functions, and AQPs also mediate signal transduction and they may have implications for a few physiological processes and pathophysiological conditions (Kitchen et al., 2015). Wakayama (2007) hypothesized that AQP1 might speed skeletal muscle regeneration because of its role in enhancing intramuscular endothelial function. Rivera, Martinez, Carrion, and Fahey (2011) showed an association between AQP1 C allele and adjusted body fluid loss in response to a 10 km races and suggested that athletes with the more active AQP1C allele might be able to train harder and recover faster. To our knowledge, only AQP3 was studied in laboratory animals (AQP3 knockout mice) exposed to exercise (Lim, Kim, Han, Kwak, & Bae, 2016) and the authors suggested that low exercise capacity in the mice was due to decreased glycerol efflux from the skeletal muscles. So, our study is the first to show the effects of exercise to AQP1 expression of the rat muscle.
Thus, the purposes of the present study were to analyse the effects of resistance exercise (climbing steps) on the histomorphometry and expression of AQP1 in the soleus muscle after compression of the sciatic nerve in Wistar rats.
METHOD
Participants
Twenty-Four male Wistar rats, with an approximate weight of 350-400grams, were kept in a light-dark photoperiod of 12 hours. The hygiene and temperature (23º-25ºC) were controlled and they were provided with food (Standard for rodents - Algomix® - Algomix Agroindustrial LTDA, Ouro Verde do Oeste, Brazil) and water ad libitum. The animals were randomly divided into the following four experimental groups, with six rats in each group: control (G1); exercise (G2); injury (G3); and injury with exercise (G4). This study was approved by the Ethics Committee Regarding the Use of Animals (CEUA) at the State University of Western Paraná (UNIOESTE) in 2012.
ProceduresFor the axonotmesis sciatic nerve experimental model, the animals in G3 and G4 were weighed and anesthetized with an intraperitoneal injection of ketamine (95 mg/Kg) and xylazine (12 mg/Kg). After checking the animal's state of consciousness (noted by the absence of motor response to the clamping of the tail and interdigital folds), it was positioned in the prone position, keeping the scapular and hindlimbs in abduction. Trichotomy was performed on the middle third of the right thigh and the area was disinfected with povidone-iodine (Povidine®). An incision was then performed parallel to the fibres of the biceps femoris muscle to expose the sciatic nerve, which was subsequently compressed using haemostatic forceps for 30 seconds. The pressure that was generated was standardized by closing the gripper on the second tooth of the rack and by the fact that this operation was always performed by the same researcher in order to minimize variations in the procedure (Câmara et al., 2012).
After clamping, the nerve was relocated and cutaneous suture was performed using simple stitches with monofilament nylon cord. Povidone-iodine (Povidine®) was applied over the incision and the animals were then housed in the same pre-surgical conditions.
The resistance exercise protocol was adapted from Hornberger and Farrar (2004). A vertical wooden ladder with 67 iron steps was used. The height of the ladder was 118 cm; it was 20.5 cm wide and was maintained at an inclination of 60°. A cage was attached at the top of the ladder that was 18.5 cm high and 15 cm wide, which served as shelter during the rest period between the sets of exercises.
Before the sciatic nerve compression surgery was performed all the animals (G1, G2, G3 and G4) were submitted to a protocol to familiarize them with the vertical ladder. Training was always performed in the afternoon by all animals.
On the third post-operative day (Gaffuri et al., 2011) the animals in G2 and G4 were submitted to resistance exercise (climbing steps) five days a week, with an interval of two days, totalling 21 days of treatment (Gorio, Carmignotto, Finesso, Polato, & Nunzi, 1983). The exercise was conducted in two series of 10 consecutive ascents of the ladder, with an additional weight of 100g and with an interval of 60 seconds between sets for rest.
Twenty-four hours after the treatment sessions, all the animals were weighed and anesthetized with ketamine (95 mg/Kg) and xylazine (12 mg/Kg). They were then euthanized using a guillotine and the right soleus muscle was dissected, placed on a flat surface to measure the muscle length with a digital calliper (Digimess®, São Paulo, Brazil), and longitudinally divided into two equal parts. The medial half was processed by routine histological analysis and the lateral half was used to count the number of sarcomeres (Coutinho et al., 2004)
The slides were prepared following routine laboratory protocol (Gafurri et al., 2011) and they were stained with haematoxylin and eosin (HE) for general analysis of the muscle tissue and Mallorys trichrome to analyse the connective tissue (Kiernan, 2015). The following measurements were performed: cross-sectional area; smaller diameter of the muscular fibre; density of the connective tissue; the number of fibres and blood capillaries. An evaluation of the morphology of the muscular fibres was also performed. The slides that were obtained were examined under a light microscope (Olympus®, BX60, Tokyo, Japan) and were measured in each image using Image-Pro Plus 6.0 software (Media Cybernetics®, Silver Spring, USA).
The GIMP (GNU Image Manipulation Program) 2.0 (GNU General Public License®, Berkeley, California) program was used to analyse the density of the connective tissue of the endomysium and perimysium (Bosi et al., 2008). The relative area of connective tissue (area density) was calculated by dividing the total number of pixels in the photomicrograph by the total of pixels marking connective tissue.
The muscle fibres and the blood capillaries present in the photomicrographs were morphologically identified, marked and analysed by image programs (Camilo; Rocha; Choppard, 2004).
The analysis of the number of sarcomeres was performed using a light microscope (Olympus®, BX60, Tokyo, Japan) at 40x magnification. Using this magnification, the images of the longitudinal muscle fibres were taken for further analysis utilizing the Image-Pro Plus 6.0 (Media Cybernetics®, Silver Spring, USA) program (Silva et al., 2013).
For the immunolocalization of the aquaporin 1 (AQP1), 5 μm histological sections were subjected to antigen retrieval and the slides were then incubated overnight in a humidified chamber with anti-AQP1 primary antibodies (1:80, rabbit, Anti-aquaporin1, Millipoire Inc, Temecula, CA, USA) and were then washed in PBS. Incubation was performed with secondary antibody (1:100, goat anti-rabbit IgG peroxidase, Sigma, St. Louis, MO, USA) for one hour, followed by washing in PBS for subsequent development with diaminobenzidine (DAB) (approximately 40 seconds). The sections were counterstained with haematoxylin and the images were captured using a light microscope (Olympus®, BX60, Tokyo, Japan) and digitized for analysis using the Image Pro-Plus 6.0 program (Media Cybernetics®, Silver Spring, USA).
Statistical AnalysisThe data regarding the effects of the resistance exercise (climbing steps) on the peripheral nerve injury in the soleus muscle were analysed using the GraphPad Prism 6.0 program and were presented as mean and standard deviation. After verification of the normality of the data by the Shapiro-Wilk test, the statistical analysis was performed with Two-way ANOVA (analysis of variance) with Tukeys post-test and statistical significance of p <0.05 was considered for all the analyses.
RESULTS
Histomorphometric analysis of the soleus muscle
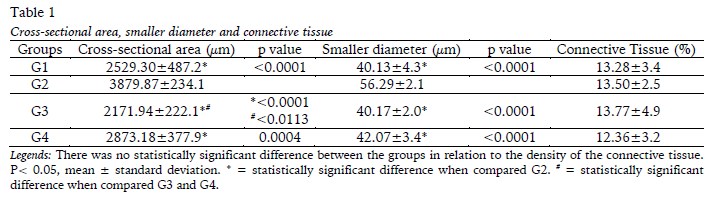
The histomorphometric analyses of the soleus muscle in relation to the cross-sectional area, the smaller diameter, and the density of the connective tissue of the endomysium and the perimysium are presented in Table 1. Exercise (G2) promoted the increase of the cross-sectional area of the fibre in relation to G1, G3 and G4, [F(1,20)=52.14; p<0.0001], as well as increase the smaller diameter of the fibre in relation to the other groups [F(1,20)=49.80; p<0.0001]. Furthermore, muscle fibre area was higher in G4 compared to G3 [F(1,20)=5.190 ;p=0.0338]. There were no changes in connective tissue density, with F(1,20)=0.1615; p=0.6920.
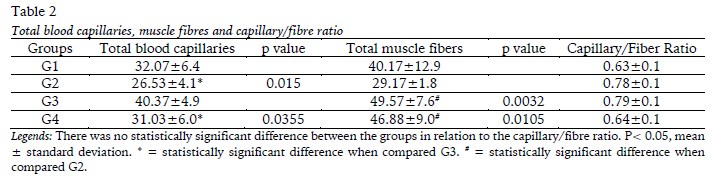
The totals for blood capillaries, muscle fibres and capillary/fibre ratio for the soleus muscle are shown in Table 2. The injury (G3) was responsible for the increase of total capillaries in relation G2 and G4 [F(1,20)=8.263; p=0.0094]. There was an increase in total muscle fibers per field of analysis in G3 and G4 compared to G2, with F(1,20)=14.55; p=0.0011. The capillary/fibber ratio showed no changes between the groups [F(1,20)=0.001881; p=0.9658].
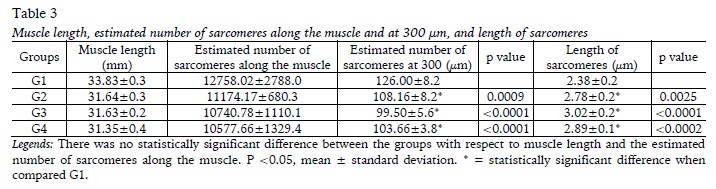
The analysis of the soleus muscle related to muscle length, estimated number of sarcomeres along the muscle (and in 300 µm), as well as the length of the sarcomeres are shown in Table 3. There were no changes in soleus muscle length [F(1,20)=0.9356; p=0.3450] and in the estimated number of sarcomeres along the muscle [F(1,20)=1.630;p=0.2164] between the groups. There was an increase in sarcomere length in G2, G3, G4 when compared to G1[F(1,20)=14.94;p=0.0010]. At the same time, the estimated of sarcomeres at 300 μm was less in these groups when compared to G1 [F(1,20)=16,14; p=0,0007].
Morphological and immuno-histochemical analysis of the soleus muscle
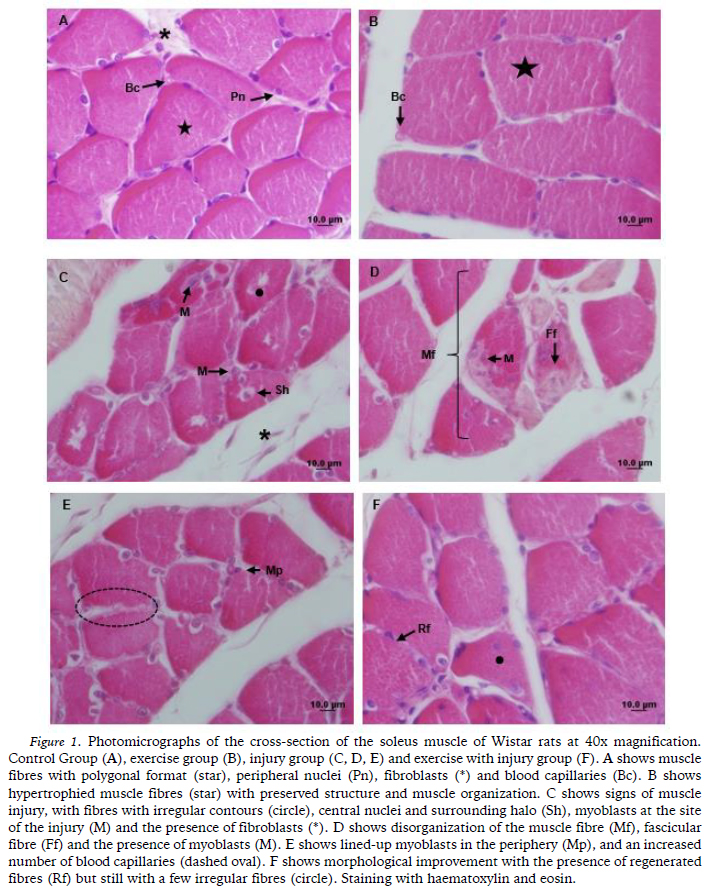
In G1, the soleus muscle showed normal morphology, with polygonal muscle fibres with rounded angles and regular contours, myonuclei on the periphery, in the subsarcolemmal position with fascicular arrangement and with loose connective tissue in the endomysium and perimysium (Figure 1A). In G2, most of the fibres were hypertrophied compared to the other groups but they maintained a polygonal shape and fascicular pattern with the nuclei located at the periphery, thereby preserving the muscle structure and organization (Figure 1B).
In G3, the soleus muscle showed signs of damage resulting from denervation. The fibres were disorganized with irregular contours and centralized nuclei, many of which showed a circumferential halo and myoblasts in the area of the injury (Figure 1C). Signs of degeneration were also observed in the cytoplasm due to the lack of organization of the myofibrils (Figure 1D) and lined-up myoblasts were visualized at the cell periphery (Figure 1E). An increase was observed in the amount of blood capillaries in the animals in G3, with intense neovascularization (Figure 1E), which was confirmed by the morphometric findings.
In G4, improvement was observed in the morphology, with the regeneration of the majority of the muscle fibres, whose characteristics were similar to G1 (Figure 1F). However, some fibres with central nuclei and irregular contours were also observed.
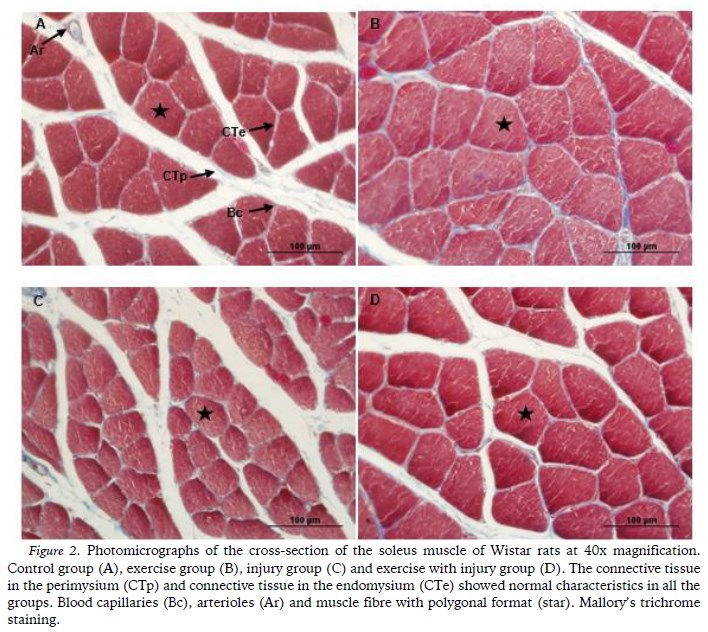
In relation to the density and the structural organization of the connective tissue in the endomysium and the perimysium, there were no morphological differences between the groups, all of which were arranged in typical arrangements with alignment of the fibres and without alterations (Figure 2).
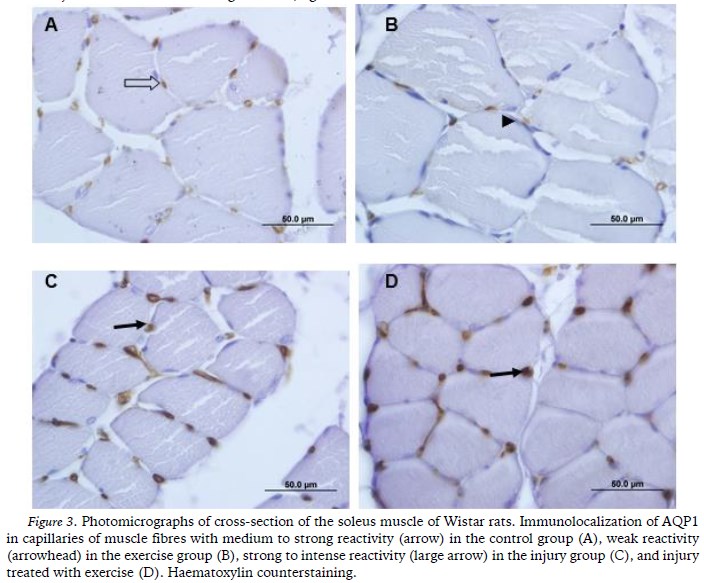
AQP1 was immunolocalized in the endothelium of the blood capillaries that were present in the muscle fibres of all the groups. The reactivity was medium to strong in G1 (Figure 3A) and weak in G2 (Figure 3B). However, in G3 (Figure 3C) and G4 (Figure 3D) strong to intense reactivity were observed.
DISCUSSION
The sciatic nerve compression model used in this study reproduced axonotmesis-type injuries (Silva-Couto et al., 2012), which results in disruption of neuromuscular communication. The absence of stimulus changed the morphology of the soleus muscle of the animals in G3, revealing features that were typical of muscle damage caused by denervation. Muscle fibres with irregular contours and centralized nuclei were also observed by Polônio Mazzer, Barbieri, and Matielo-Sverzut (2010) in the tibialis anterior muscle of rats subjected to a complete section of the sciatic nerve. Salvini Durigan, Peviani, and Russo (2012) reported that such structural changes in muscle fibres compromised organ function.
Blood supply plays an essential role in the morphological and functional recovery of muscles after nerve injury (Hudlicka, 2011; Wagatsuma et al, 2005). The neovascularization that was observed in G3 enables the supply of nutrients and oxygen that are necessary for tissue repair. Another study (Camillo, Rocha, & Chopard, 2004), also reported an increased amount of blood capillaries in the soleus muscle after sectioning of the sciatic nerve and this characteristic seems to be a response to the proliferation of endothelial cells that are stimulated by vascular endothelial growth factor (VEGF) (Zhao, Huang, Wu, & Huang 2016).
The sciatic nerve injury in the present study also promoted changes in the expression of AQP1 in the soleus muscle. AQP1 has been located in red blood cells, the endothelial cells of capillaries, kidneys, bladder, liver, hippocampus, choroid plexus and other structures. Although its expression in skeletal muscle is still controversial, there is agreement about the positive expression of this AQP in the endothelial cells of muscle capillaries (Wakayama, 2007). Thus, the results regarding the immunolocalization of AQP1 obtained in the present study were consistent with the literature because AQP1 was immunolocalized in the endothelium of the soleus muscle of the animals in all of the groups.
The strong to intense reactivity of AQP1 in the endothelium of the animals from G3 and G4 was a striking result in the present study and it was evident in an unprecedented manner in the soleus muscle. Wakayama (2007) argued that the regeneration of skeletal muscle could be increased by the over-expression of AQP1. That hypothesis is consistent with our results and it also may be based on other functions of AQPs, such as facilitating cell migration. Thus, it is believed that the strong reactivity of AQP1 that was observed in the present study was due to the role of AQP in assisting in the remodelling of the soleus muscle.
Despite the numerous deleterious morphological characteristics that were observed in the soleus muscle of animals in G3, 21 days after the nerve injury it was possible to observe some signs of muscle regeneration, such as the presence of centralized nuclei in the fibres, which was indicative of protein synthesis (Karalaki, Fili, Philippou, & Koutsilieris, 2009). Tanaka, Tsubaki, and Tachino (2005) also observed that the soleus muscle recovered spontaneously six weeks after tibial nerve injury.
The plasticity of muscle tissue in response to injury depends, among other factors, on the functional role of satellite cells (Karalaki et al., 2009; Wakayama, 2007). These cells are in a quiescent state in healthy muscles, however, in the case of an injury they proliferate in the basal lamina, and with the aid of growth factors that act in chemotaxis, proliferation, and differentiation, the satellite cells form myoblasts. When they cross the basal lamina the myoblasts release enzymes (trypsin and pronase), which are capable of dissolving the membrane and reaching the site of the injury (Carlson, 2014). Evidence suggests that these cells are able to fuse with muscle fibres in order to repair the injured segment, but they may also merge to form myotubes, differentiating themselves and originating a new muscle fibre (Wakayama, 2007).
Even though atrophy is frequently observed after muscle denervation (Russo et al., 2010; Karalaki et al., 2009) this was not observed in the present study, i.e., there was no decrease in the cross-sectional area and the diameter of the muscle fibres 21 days after the injury was produced. The myogenic differentiation factor (myoD) is directly involved in this process and it increases after denervation because the myoD is capable of inducing proliferation and differentiation of the satellite cells, thus preventing the appearance of muscular atrophy (Russo et al., 2010).
Different forms of muscle stress can cause changes in the number of sarcomeres (Ceylan et al., 2014). The exercise performed in G2 and the nerve damage that was observed in G3 was responsible for increasing the length of the sarcomeres and concomitantly decreasing their number at 300 µm. This adjustment occurs so that there is perfect overlap of the actin and myosin filaments, which allows the optimal development of tension during contractions (Lima et al., 2007) thereby showing the adaptability of sarcomeres in the soleus muscle in relation to compressive sciatic nerve injury.
In terms of the animals submitted to resistance exercise (climbing steps), there were no degenerative morphological changes, although an increase in the cross-sectional area and the smaller diameter of the muscle fibres was observed. This hypertrophy was a result of increased muscle protein synthesis. Without pre-existing injuries, the action of resistance exercise (climbing steps) has been shown to be effective in inducing muscle hypertrophy in the forelimbs and the hindlimbs (Marqueste et al., 2004).
The potential effect of resistance exercise on muscle physiology was a determining factor in the morphological improvement of the soleus muscle of the animals that were subjected to injuries and subsequently exercised. Aspects of regeneration in the majority of the muscle fibres were observed in these animals in comparison with animals in G3. Thus, the resistance exercise (climbing steps), which was started in the acute post-lesion phase, had a protective effect on the morphology of the soleus muscle. Salvini et al. (2012) also reported that muscular contraction induced by electrical stimulation has a protective effect on denervated muscles.
The beneficial effects of exercise in denervation models started at an early stage of reinnervation were also reported by Marqueste Alliez, Alluin, Jammes, and Decherchi (2004) who observed that treatment using a treadmill was able to increase resistance to fatigue, as well as restoring the contractile properties and mechanosensitivity of the tibialis anterior muscle. A study by Tanaka et al. (2005) found that the use of a treadmill prevented atrophy of the soleus muscle after injury to the tibial nerve. These results were attributed to an increase in protein synthesis as a result of the mechanical stimulation induced by exercise, and an increase in motor units due to the regeneration of nerve fibres (Betz, Caldwell, & Ribchester, 1980).
Considering that, to our knowledge, this was one of the first researches that used as a therapeutic measure the resistance exercise of stair climbing in the nerve injury, in which the load used was based on Ilha et al. (2008). We would suggest that further studies should be performed considering the weight of the animal, the best load, as well as the intensification of this overload throughout the treatment, so as to have the maximum possible expression of AQP1 and that this may determine the acceleration of recovery.
CONCLUSION
Compression of the sciatic nerve was capable of promoting the effects of muscle injury and AQP1 was immunolocalized in the endothelium of blood capillaries, and 21 days after axonotmesis of the soleus muscle the intrinsic potential for recovery was evident. It is important to start resistance exercise in the acute phase after denervation because the resistance exercise (climbing steps) acted as a protective factor in regeneration.
REFERENCES
Artifon, E. L., Silva, L. I., Ribeiro, L. F. C., Brancalhão, R. M. C., & Bertolini, G. R. F. (2013). Treinamento aeróbico prévio à compressão nervosa: análise da morfometria muscular de ratos. Revista Brasileira de Medicina do Esporte, 19(1), 66-69. doi: 0.1590/S1517-86922013000100014 [ Links ]
Betz, W. J., Caldwell, J. H., & Ribchester, R. R. (1980). The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. Journal of Physiology, 303, 265-279. [ Links ]
Bosi, P. L., Delfino, G. B., Durigan, J. L. Q., Cancelliero, K. M., Polacow, M. L. O, & Silva, C. A. (2008). Metformina minimiza as alterações morfométricas no músculo sóleo de ratos submetidos à imobilização articular. Revista Brasileira de Medicina do Esporte, 14(5), 436-439. doi: 10.1590/S1517-86922008000500007 [ Links ]
Caierão, Q. M., Betini, J., Teodori, R. M., & Minamoto, V. B. (2008). O efeito do intervalo da estimulação elétrica no músculo desnervado de rato. Revista Brasileira de Fisioterapia, 12(2), 143-148. doi:10.1590/S1413-35552008000200011 [ Links ]
Câmera, C. N. S., Brito, M. V. H., Silveira, E. L., Silva, D. S. G., Simões, V. R. F, & Pontes, R. W. F. (2011). Histological analysis of low-intensity laser therapy effects in peripheral nerve regeneration in Wistar rats. Acta Cirurgica Brasileira, 26(1), 12-18. doi: 10.1590/S0102-86502011000100004 [ Links ]
Camillo, A. C., Rocha, R. C., & Chopard, R. P. (2004). Structural and microvascular study of soleous muscle of wistar rats after section of the sciatic nerve. Arquivos de Neuro-Psiquiatria, 62(3), 835-838. doi: 10.1590/S0004-282X2004000500018 [ Links ]
Cassilhas, R. C., Reis, I. T., Venâncio, D., Fernandes, J., Tufik, S., & Mello, M. T. (2013). Animal model for progressive resistance exercise: a detailed description of model and its implications for basic research in exercise. Revista Motriz, 19(1), 178-184. doi: 10.1590/S1980-65742013000100018 [ Links ]
Carlson, B. M. (2014). The biology of long-term denervated skeletal muscle. European Journal Translational Myology – BAM, 24(1), 5-11. doi: 10.4081/ejtm.2014.3293 [ Links ]
Ceylan, O., Seyfettinoglu, F., Dulgeroglu, A. M., Avci, A., Bayram, B., & Bora, O. (2014). A. Histomorphological comparison of immobilization and desnervation atrophies. Acta Orthopaedica et Traumatologica Túrcica, 48(3), 320-325. doi: 10.3944/AOTT.2014.2993 [ Links ]
Cheng, F. C., Sheu, M. L., Su, H. L., Chen, Y. J., Chen, C. J., Chiu, W.T, Sheehan. J., & Pan, H. C. (2013). The effect of exercise on mobilization of hematopoietic progenitor cells involved in the repair of sciatic nerve crush injury. Journal of Neurosurgery, 118(3), 594-605. doi: 10.3171/2012.8.JNS111580 [ Links ]
Coutinho, E. L., Gomes, A. R. S., França, C. N., Oishi, J., & Salvini, T. F. (2004). Effect of passive stretching on the immobilized soleus muscle fiber morphology. Brazilian Journal of Medicine and Biology and Research, 37(12), 1853-1861. doi: 10.1590/S0100-879X2004001200011 [ Links ]
Fernandes, T., Roque, F. R., Magalhães, F. C., Carmo, E. C., & Oliveira, E. M. (2012). O treinamento físico aeróbio corrige a rarefação capilar e as alterações nas proporções dos tipos de fibra muscular esquelética em ratos espontaneamente hipertensos. Revista Brasileira de Medicina do Esporte, 18(4), 267-272. doi: 10.1590/S1517-86922012000400010 [ Links ]
Frasson, N. F. V., Taciro, C., & Parizotto, N. A. (2009). Análise nanoestrutural da ação do ultra-som terapêutico sobre o processo de regeneração do tendão de ratos. Revista Fisioterapia e Pesquisa, 16(3), 198-204. doi: 10.1590/S1809-29502009000300002 [ Links ]
Gaffuri, J., Meireles, A., Rocha, B. P., Rosa, C. T., Artifon, E.L., Silva, L. I., Moreira, N. B., & Bertolini, G. R. F. (2011). Avaliação do exercício físico como fator de analgesia em um modelo experimental de ciatalgia. Revista Brasileira de Medicina do Esporte, 17(2), 115-118. doi: 10.1590/S1517-86922011000200009 [ Links ]
Gorio, A., Carmignotto, G., Finesso, M., Polato, P., & Nunzi, M. G. (1983). Muscle reinnervation – II. Sprouting, synapse formation and repression. Neuroscience, 8(3), 403-416. doi: 10.1016/0306-4522(83)90188-4 [ Links ]
Hornberger, T. A. J., & Farrar, R. P. (2004). Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Canadian Journal of applied physiology, 29(1), 16-31. doi: 10.1139/h04-002 [ Links ]
Hudlicka, O. (2011). Microcirculation in skeletal muscle. Muscle, Ligaments and Tendons Journal, 1(1), 3-11. [ Links ]
Ilha, J., Araujo, R. T., Malysz, T., Hermel, E. E., Rigon, P., Xavier, L. L., & Achaval, M. (2008). Endurance and resistance exercise training programs elicit specific effects on sciatic nerve regeneration after experimental traumatic lesion in rats. Neurorehabilitation and Neural Repair, 22(4), 355-366. doi: 10.1177/1545968307313502 [ Links ]
Kiernan, J. (2015). Histological and Histochemical Methods: Theory and Practice (5th Ed.). Banbury: Scion Publishing Limited. [ Links ]
Junior, G. M. R., Bueno, C. R. S., Heubel, A., Bortoluci, C. H. F., Simionato, L. H., Daré, L. R., Silva, M. P., Dias, D. V. (2013). Efeito da corrente alternada simétrica sinusoidal na musculatura estriada esquelética desnervada experimentalmente. Salusvita, 32(3), 211-225. [ Links ]
Karalaki, M., Fili, S., Philippou, A., & Koutsilieris, M. (2009). Muscle Regeneration: Cellular and Molecular Events. In vivo, 23(5), 779-796. [ Links ]
Kitchen, P., Day, R. E., Salman, M. M., Conner, M.T., Bill, R. M., & Conner, A. C. (2015). Beyond water homeostasis: diverse functional roles of mammalian aquaporins. Biochimica et Biophysica Acta, 1850(12), 2410-2421. doi:10.1016/j.bbagen.2015.08.023 [ Links ]
Kobayashi, S., Yoshizawa, H., & Yamada, S. (2004). Pathology of lumbar nerve root compression. Part 2: morphological and immunohistochemical changes of dorsal root ganglion. Journal Orthopaedic Research, 22(1), 180-188. doi: 10.1016/S0736-0266(03)00132-3 [ Links ]
Li, R., Liu, Z., Pan, Y., Chen, L., Zhang, Z., & Lu, L. (2014). Peripheral nerve injuries treatment: a systematic review. Cell Biochemistry and Biophysics. 68(3). 449-454. doi: 10.1007/s12013-013-9742-1 [ Links ]
Lima, S. C., Caierão, Q. M., Durigan, J. L. Q., Schwarzenbeck, A., Silva, C. A, Minamoto, V. B, & Guirro, R. R. J. (2007). Curto período de imobilização provoca alterações morfométricas e mecânicas no músculo de rato. Revista Brasileira de Fisioterapia, 11(4), 297-302. [ Links ]
Lim, J. H., Kim, D. H., Han, D. W., Kwak, J. Y., Bae, H. R. (2016). The effect of AQP3 deficiency on fuel selection during bout of exhausting exercise. Pflugers Arch. – European. Journal of Physiology, 468, 1283-1293. doi: 10.1007/s00424-016-1827-4. [ Links ]
Marqueste, T., Alliez, J. R., Alluin, O., Jammes, Y., & Decherchi, P. (2004). Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. Journal of Applied Physiology, 96(5), 1988-1995. doi: 10.1152/japplphysiol.00775.2003 [ Links ]
Moore, K. L., & Dalley, A. F. (2007). Anatomia Orientada para a Clínica. Rio de Janeiro: Guanabara Koogan. [ Links ]
Polônio, J. T., Mazzer, N., Barbieri, C. H., & Matielo-Sverzut, A. C. (2010). Eletroestimulação seletiva mantem estrutura e função do tibial desnervado de ratos. Acta Ortopédica Brasileira, 18(2), 85-89. doi: 10.1590/S1413-78522010000200005 [ Links ]
Rivera, M. A., Martinez, J. L, Carrion, A., & Fahey T. D. (2011). AQP-1 association with body fluid loss in 10-km runners. International Journal Sports of Medicine, 32, 229-233. doi: 10.1055/s-0030-1268489 [ Links ]
Russo, T. L., Peviani, S. M., Durigan, J. L.Q, Gigo-Benato, D., Delfino, G. B., & Salvini, T. F. (2010). Stretching and electrical stimulation reduce the accumulation of MyoD, myostatin and atrogin-1 in denervated rat skeletal muscle. Journal of Muscle Research and cell motility, 31(1), 45-57. doi: 10.1007/s10974-010-9203-z [ Links ]
Salvini, T. F., Durigan, J. L. Q., Peviani, S. M., & Russo, T. L. (2012). Efeitos da eletroestimulação e do alongamento muscular sobre a adaptação do músculo desnervado – implicações para a fisioterapia. Revista Brasileira de Fisioterapia, 16(3), 175-183. doi: 10.1590/S1413-35552012005000027 [ Links ]
Sebben, A. D., Cocolichio, F., Schmitt, A. P. V., Curra, M. D., Silva, P. V., Tres, G. L., & Silva, J. B. (2011). Efeitos de fatores neurotróficos sobre o reparo de nervo periférico. Scientia Médica, 21(2), 81-89. [ Links ]
Silva-Couto, M. A., Gigo-Benato, D., Tim, C. R., Parizotto, N. A., Salvini T. F., & Russo, T. L. (2012). Effects of low-level laser therapy after nerve reconstruction in rat denervated soleus muscle adaptation. Revista Brasileira de Fisioterapia, 16(4), 320-327. [ Links ]
Silva, L. I., Meirelhes, A., Nascimento, C. M., Rocha, B. P., Rosa, C. T., Ribeiro, L. F. C., Brancalhão, R. M. C., & Bertolini, G. R. F. (2013). Avaliação de parâmetros histomorfométricos em sóleos de ratos submetidos à remobilização por salto em meio aquático. Revista Brasileira de Medicina do Esporte, 19(3), 219-222. [ Links ]
Tanaka, S., Tsubaki, A., & Tachino, K. (2005). Effect of exercise training after partial denervation in rat soleus muscles. Journal of Physical Therapy Science, 17(2), 97-101. doi: 10.1589/jpts.17.97 [ Links ]
Wagatsuma, A., Tamaki, H., & Ogita, F. (2005). Capillary supply and gene expression of angiogenesis-related factors in murine skeletal muscle following denervation. Experimental Physiology, 90(3), 403-409. doi: 10.1113/exoohysiol.2004.029769 [ Links ]
Wakayama, Y. (2007). Skeletal muscle regeneration may be enhanced by overexpression of aquaporin 1 in intramuscular capillary endothelial cells. Medical Hypotheses, 68, 856-859. doi: 10.1016/j.mehy.2006.07.056 [ Links ]
Zhao, H., Huang, H., Wu, J., & Huang, P. (2016). Specialization of mitochondrial and vascular oxidant modulated VEGFR in the denervated skeletal muscle. Cell Signalling, 25(11), 2106-2114. doi: 10.1016/j.cellsig.2013.06.014. [ Links ]
Acknowledgments:
Nothing to declare
Conflict of interests:
Nothing to declare.
Funding:
Nothing to declare.
Manuscript received at March 29th 2017; Accepted at September 5th 2017
Correspondence to: Rua Universitária, 2069, 85819-110 Cascavel, PR, Brazil. E-mail: keli.lovison@hotmail.com