Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Psicologia
versão impressa ISSN 0874-2049
Psicologia vol.24 no.2 Lisboa jul. 2010
The evolving empathy: hardwired bases of human and non-human primate empathy
A empatia em evolução: As bases estruturais da empatia em primatas humanos e não-humanos
Rita de Castro1; Augusta Gaspar2,*; Luís Vicente3
1Departamento de Biologia Animal, Faculdade de Ciências, Universidade de Lisboa
2Instituto Universitário de Lisboa (ISCTE-IUL), Centro de Investigação e Intervenção Social (Cis-IUL), Lisboa, Portugal
3Centro de Biologia Ambiental, Departamento de Biologia Animal, Faculdade de Ciências, Universidade de Lisboa, Campo Grande, 1749-016 Lisboa, Portugal.
ABSTRACT
Empathy has always been hard to operationalize. A communication gap between psychologists and neurobiologists delayed the study of empathic processes for long, but in recent years, with the discovery of mirror neurons, with the finally found neurological substrate of the much discussed “embodiment of observed behaviours”, envisaged by psychophysiologists, a revolution is in the way we understand emotion. Neuroscientists are coming ever closer to social psychologists in finding the substrate for the proposed relations between gender, mimicry, emotional contagion and empathy. Furthermore, they are stumping on evidence of empathy in non-human animals. In this paper we describe different types and components of empathy, with a particular emphasis on thePerception-Action Model (Preston and de Waal, 2002), and overview the discovery of the mirror neurons and its implication to empathy and its biological evolution.
Keywords: evolution of empathy, altruism, mirror neurons, perception-action model, non-human primates, emotion
RESUMO
A empatia foi desde sempre um conceito de difícil operacionalização. Durante muito tempo existiu um fosso na comunicação entre psicólogos e neurobiólogos que retardou o estudo dos processos empáticos. Nos últimos anos, com a descoberta do sistema de neurónios-espelho, passou finalmente a conhecer-se o substrato neurológico já conhecido em psicofisiologia - o ‘embodiment'dos comportamentos observados. Deu-se assim uma revolução no modo como a empatia é compreendida e os neurocientistas e psicólogos sociais encontram-se agora numa rota de aproximação, em busca da compreensão das relações longamente propostas entre género, imitação, contágio emocional e empatia. Ao mesmo tempo, é impossível não tropeçar na evidência de existência de empatia em outros animais. Neste artigo revêem-se definições de empatia, incluindo tipos e graus de empatia, dando-se particular ênfase ao modelo inclusivo de Preston e de Waal (2002), o Modelo da Percepção-Acção,e recapitula-se a descoberta dos neurónios-espelho e as suas implicações para a definição de empatia, bem como para o suporte da evolução biológica da mesma.
Palavras-chave: evolução da empatia, altruísmo, neurónios-espelho, modelo da percepção-acção, primatas não humanos, emoção
On the definition of empathy and its human-centred preconceptions
The word “empathy” derives from the Greek εμπΑθειΑ (empatheia), meaning passion or affection - εv (en), "in, at" + πεθιΑ (pathos), "feeling” (Liddell & Scott, 1940). The word was then adapted into Einfühlung (“feeling into”), a German word used to describe the human ability to symbolize inanimate objects of nature and art (Gallese, 2007; Preston & de Waal, 2002). Theodore Lipps extended the concept of Einfühlung to the domain of inter-subjectivity, which he characterized in terms of inner inhibited imitation of the perceived movement of others, without any intervening labelling, associative or cognitive perspective-taking processes (Gallese, 2007; Preston & de Waal 2002;). Empathy is not easy to define, as it involves the domains of emotion and cognition, and is conditioned by the inherent variability inside species, sex and age groups, as well as environmental context (de Waal, 2008). Its overall meaning is accepted as the sharing of the emotional experience of another individual, so it is primarily an affective state but most often it involves cognitive abilities such as perspective taking, knowledge of emotion and even another deeply interconnected emotional reaction - sympathy - often referred to as a synonym of empathy, that it is not (Eisenberg & Mussen, 1989). However, the details of what it comprises and how its components relate are not consensual (see Blair, 2003; Eisenberg, 2000) and throughout the years many authors published their own distinct definitions of empathy, some of which are presented in Table 1.
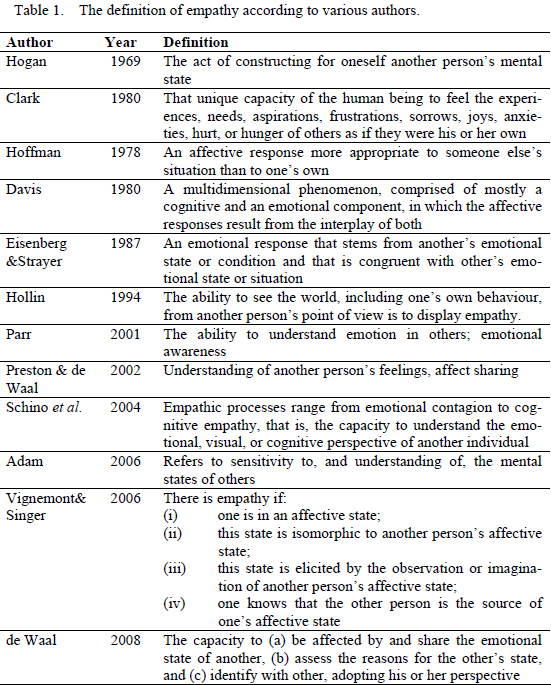
Until recently most papers on Empathy addressed it as the ability to understand the emotional and mental states of others, a concept deeply connected with self-awareness and with having a Theory of Mind (ToM) - a view that, despite its popularity, restricted the empathic process to cognition (Bekoff, Allen & Burghardt, 2002; Gallup, 1977; Preston & de Waal, 2002). Assessment of these processes is a difficult task, since researchers have to infer from observable behaviours, emotions and mental states that are not directly observable (de Waal, 2008). It is much easier to elaborate paradigms to assess those processes in humans than in non-humans. Thus, not surprisingly, the most widely accepted view of empathy outside the human sphere has been in tune with Clark’s (1980) view of empathy as uniquely human, albeit biologically rooted in evolutionary newer areas of the cortex.
In this paper we review evidence showing that this view has been overtaken. First and foremost, there is ample and ever-growing evidence that understanding the emotions of others involves (although it is not limited to) automatic and unconscious embodying mechanisms that occur with no effortful processing or cognitive perspective-taking (e.g. de Vignemont & Singer, 2006; Dimberg & Thunberg, 1998; Preston & de Waal, 2002; Rizzolatti, Gallese & Keysers, 2004). Activation of brain areas such as the insula, amygdala and premotor cortex in response to emotional stimuli, and resulting emotion congruent visceral-motor responses reveal that the brain is equipped to deal with the understanding of emotions in oneself and in others (de Vignemont & Singer, 2006; Rizzolatti et al., 2004). The absence of empathy has for long been pinpointed as a defining feature of anti-social personalities (Cleckley, 1941; Miller & Eisenberg, 1988), but now a new step has been given as we are witnessing across the social sciences a recognition of the relevance of empathy as a socially facilitating tool, be it in the school grounds, the family or the workplace (e.g., Goleman & Boyatzis, 2008).
This socially-instrumental empathy might have had important advantages throughout the evolution of pre-human societies and even more ancient primate societies. Empathy is no longer seen as an obstacle to the biological urges of power and personal satisfaction, as Clark (1980) saw it, but as a vital component in the coordination of ones social life, incidentally benefiting the entire community. Emotions are often the contextual element that signs the intent of an action, which animals are required to understand in order to lead a successful social life (Rizzolatti, Fogassi, & Gallese, 2006). Although non-human animals may not be able to express or understand such a vast array of emotions as humans do, it is widely accepted that basic emotions, those that organize behaviour crucial to survival - anger, joy, sadness, fear and disgust, as well as pain- are common in the animal world, sharing neurophysiological systems and behaviour outcomes in all mammals (see Panksepp, 2004). These basic emotions are thought to be easier to empathize with than secondary emotions such as jealousy (de Vignemont & Singer, 2006; Grandjean, Sander, & Scherer, 2008; Jabbi & Keysers, 2008). Emotions are known to be social facilitators and allow the formation of social bonds, since being in an emotional state serves as a form of communication, as it reflects the position of the individual within a specific social context (Gallese, 2007).
Empathy has been accepted as an “umbrella term” for affective phenomena such as emotional contagion, sympathy and empathic perspective taking, which can co-occur in a species or manifest separately in different animals (Davis, 1980; de Waal 2008; Preston and de Waal, 2002). We begin by defining each of these assumed affective components.
Emotional contagion: Also known as the neighbour effect, emotional transference, self-centred vicarious arousal or affective resonance (e.g. Eisenberg, 2000; Parr 2001; Videan, Fritz, Schwandt, & Howell, 2005), it is the process by which the observer (the subject) is affected by another individual’s emotional or arousal state (the object) and does not perceive it to affect or influence his own (de Waal 2008; Videan et al., 2005). Behaviour synchronization can occur in social animals, as it works as a survival facilitating tool (Bekoff, 2002; de Waal, 2008). Emotional contagion has been considered the most basic form of affective communication, and one of the primary ways of learning and experiencing the emotional states of others (Bekoff et al., 2002; de Waal, 2008). Emotional contagion has been proposed as the lowest common denominator of all empathic processes, presenting continuity between humans and other animals (de Waal, 2008). Even Hoffman (1978, 1990), who authored a developmental theory of empathy in which the empathic reaction depends heavily on one’s understanding of oneself as distinct from others (self awareness), recognizes that children experience empathic distress by emotional contagion even before they acquire self awareness. The principle of emotional contagion, by comparison with empathic mechanisms of higher complexity, which according to Hoffman are linked to the development of cognitive abilities, has been proposed to be quite simply the activation of emotional expressions and vocalizations leading to physiological changes which in turn initiate a matching emotional state in the subject and observer, thus allowing a shared emotional experience, non-dependent of conscious processing (de Waal, 2008). In support of this idea, voluntary facial activity replicating prototypical emotional facial expressions was reported to generate emotion specific patterns of autonomic activity (Jabbi & Keysers, 2008). But emotional contagion may occur even if such expressive feedback does not. Observable examples of emotional contagion have long been reported and include the reflex-like spread of fear and distress, such as the take-off of a flock of birds because one is startled, or the contagion of cry in a room full of newborns when one starts crying (Bekoff et al., 2002; de Vignemont & Singer, 2006; de Waal, 2008). Emotional contagion can also result from intentional communication, such as the loud temper tantrums of infants, whose goal is recruiting the mother into changing her behaviour and providing more attention to the juvenile - and the mother becomes distressed as well. This mother-infant bond facilitation is observed in human and non-human animals (de Waal, 2008; Panksepp, 2004; Preston & de Waal, 2002): Guinea pigs’ mothers diencephalon activity mirrors the activity of distress and pain in the brains of their distressed vocalizing young (Panksepp, 2004) and humans also mirror the brain activity of the experience of pain when watching others undergoing a painful experience they have already experienced (e.g, Hutchinson, Davis, Lozano, Tasker, & Dos-trovsky, 1999; Morrison, Llyoid, Pellegrino, & Roberts, 2002). The areas that are most active during these affective experiences largely overlap across species (e.g., the anterior and dorsal cingulate cortex and the insula). Researchers tend to make sense of the “contagiousness” of negative experiences in terms of the advantages of preparedness to respond to aversive, potentially dangerous stimuli or situations, for instance by automatically activating avoidance behaviours (e.g., Jackson, Meltzoff, & Decety, 2005; Morrison et al., 2002; Panksepp, 2004).
Sympathy or empathic concern: An evolutionary landmark of empathy is believed to have taken place with the association of appraisal of the affective state of others and their emotional context to emotional contagion (de Vignemont & Singer 2006; de Waal 2008; Gallese 2007; Gruen & Mendelsohn 1986). Data on rats and macaque monkeys show that individuals will inhibit their behaviour if they perceive it to be responsible for a con-specific’s distress (for reviews of experiments involving observing and reacting to a conspecific’s pain and distress see de Waal, 2006, 2008). Sympathy is not to be confused with personal distress, where one seeks to alleviate his/her own suffering, regardless of the suffering of the other: Sympathetic individuals do not exhibit any reproduction of a perceived emotion in another, but will rather react with concern or sorrow to the sight of a distressed individual (Boesch & Boesch-Acherman, 2000; Eisenberg & Mussen, 1989; Gruen & Mendelsohn, 1986). This occurs both in humans and other animals. For example, some experiments revealed that not only children will react with concern for a family member, but pets such as cats and dogs also exhibit signs of concern (Clutton-Brock, 1999; de Waal, 2008). Rhesus monkeys embrace, mount or even pile on top of a screaming conspecific in an attempt to reduce their own negative arousal (de Waal, 2008).
A large portion of prosocial behaviour is believed to be grounded on Sympathy (e.g. Eisenberg, 2000). Consolation has been pointed as the most significant behaviour outcome of sympathetic concern (de Waal, 2008): An individual initiates contact with a distressed peer, even without being solicited, with reassuring behaviour (Preston & de Waal, 2002; Koski, Koops & Sterck, 2007). It has been observed in captive apes, wild chimpanzees, large-brained birds and human children (Preston & de Waal, 2002; Schino, Aureli, Geminiani, & Rosati, 2004; de Waal 2008). Despite intensive observations, it has never been observed in monkeys (Boesch & Boesch-Acherman, 2000; Schino et al., 2004; de Waal 2008). Macaque mothers fail to console their offspring after a fight, ignoring the bodily cues of distress presented by the infants (de Waal 2008; Plotnik, de Waal & Reiss, 2006; Schino et al., 2004). Monkeys, when compared with chimpanzees, seem to lack significantly important mental skills. Even regarding mirror self-recognition, while chimpanzees appear to be aware of the reflection as an image of them, monkeys do not behave in such way, independently of how many hours they are exposed to the mirror (Gallup, 1977). In a neuroanatomical comparative perspective, it has been found that an area deeply connected to the anterior insula and involved in processing emotional states and overall interoceptive awareness, the frontal operculum, has no homologue in the monkey’s insula (Jabbi & Keysers, 2008). A milestone of emotional awareness was probably made possible by this phylogenetically new region, yet another evidence of an evolutionary gap between monkeys and the Hominoidea and of the similarity of the emotional and social experiences of the later (Schino et al., 2004; de Waal, 2008; Jabbi & Keysers, 2008).
Empathy Perspective-taking: From a psychological point of view, empathy undertakes emotional perspective-taking, as the subject is required to understand and adopt the object’s point of view and emotions, engaging in imagination and mental state attribution (for reviews see Eisenberg, 2000; Miller & Eisenberg, 1988). Such requirements constitute the main reason why empathy in non-humans has been very hard to accept. Some authors still argue to this day that our closest cousins do not possess the ability to understand mental or emotional states in others (Povinelli & Giambrone, 2001). Accumulating data contradict this opinion. Apes show some level of perspective-taking both in their spontaneous social behaviour and under experimental conditions (Boesch & Boesch-Acherman, 2000; de Waal, 2006). Also, de Waal (2008) referred targeted helping as a proof of empathy; in his words, for an individual to move beyond being sensitive to others toward an explicit other-orientation requires a shift in perspective, becoming sensitive to the specific needs of others and behaving accordingly (de Waal, 2008; Plotnik et al., 2006;). Mere perspective-taking is not enough for the display of empathy. Emotional engagement needs to occur as well, and de Waal (2008) provides examples. Tree bridging in an orangutan female is one such example of empathic perspective taking, in which the mother anticipated the offspring need to move from one tree to another and provided a bridge with her body. Boesch and Boesch-Acherman (2000) reported chimpanzees hiding from a dominant male (in an aggressive display) if they perceived themselves to be the target of aggression, and moving accordingly as not to be seen; the dominant male showed some levels of awareness for such escapist behaviours, as he would go look for the hidden individuals, engaging in perspective-taking as well.
Chimpanzees are also known to cater to the needs of an injured individual, assessing the adequate care required by the wound, adjusting their behaviour accordingly and displaying different emotions depending on the nature or severity of it, and this behaviour is not limited to kin, but was reported to extend to other group members (Boesch & Boesch-Acherman 2000; Goodall, 1986). Other examples of empathic behaviours in chimpanzees include the removal of a foreign body from a companion’s eye (Miles, 1963), helping an elder chimpanzee climb a tree (de Waal, 2007; Goodall, 1986), consoling a defeated male after a power struggle (Aureli, Fraser, & Stahl, 2008; de Waal & van Roosmalen 1979), and helping a trapped bird escape (de Waal, 2006). Elephants reassure distressed companions and help injured ones by supporting or lifting them if they’re too weak to stand; dolphins support sick companions near the surface to keep them from drowning, stay close to females in labour and help release companions trapped in fishing nets (de Waal, 2008).
Empathic perspective-taking assumes the understanding that the new emotional state derives from an external source (the object’s emotional state), an awareness absent in emotional contagion (Gallese 2007; Hoffman, 1990). Such concept comprises the notion of self, as self knowledge is used in the reproduction of the observed emotion and on the inferring of other’s mental states and intentions (Hoffman, 1990; Oberman & Ramachandran, 2007; Preston & de Waal, 2002). Gallup (1977) proposed that in both development and phylogeny, mirror self-recognition appears together with the ability to sympathize, empathize and attribute intent and emotions to others -the Co-emergence Hypothesis - which seems to be supported by the substantially different results between monkeys and apes when it comes to mirror self-recognition, consolation and targeted help (Gallup, 1977; 1998; Plotnik etal., 2006; de Waal, 2008). Monkeys do not exhibit any behaviour that suggests mirror self-recognition, independently of how many hours they are exposed to the mirror (Gallup, 1977). Besides the great apes, only elephants and dolphins exhibit mirror self-recognition (Bekoff et al., 2002; de Waal 2008; Plotnik et al., 2006; Reiss & Marino, 2001). And indeed it is in regard to these two animal species that we find the most compelling reports on consolation and targeted helping (de Waal, 2008). Both possess a high level of encephalization and an impressive cognitive and behavioural complexity (e.g., Plotnik et al., 2006; Reiss & Marino 2001). Plotnik et al. (2006) also suggested that the elephant and dolphin’s mirror self-recognition could be related with a convergent evolution most likely related to complex sociality and cooperation. Reiss and Marino (2001) suggest different neurological substrates of mirror self-recognition for dolphins and primates, as both lines have diverged about 65-70 million years ago.
Empathy, albeit automatic by nature, can be modulated by several factors - empathic responses are thought to be heightened by individual traits, similarity, familiarity, social closeness, and positive experience with the object (Davis, 1980; Eisenberg, 2000; Hoffman, 1990; Preston & de Waal, 2002). Morphology and biomechanics seem to play an important role in the triggering of empathy; monkeys do not display empathic reactions to the sight of albino rats being shocked, nor to the sight of a puppet monkey in distress (Preston & de Waal, 2002). However, monkeys will starve themselves if getting food implies shocking a conspecific, and that response is heightened if the individual has been shocked before and is familiar with the other individual (Masserman, Wechkin, & Terris, 1964). Humans respond very differently depending on the perceived nature of a relationship or situation; for example an individual can empathize with another individual’s pleasure or distress if he/she perceives their relationship as cooperative, and show an antagonic response (distress to perceived pleasure or pleasure to perceived distress) if the relationship is perceived as competitive (reviewed by de Waal, 2008). With functional imagining resonance (fMRI), it is possible to register activation of the anterior cingulated cortex (ACC) and the anterior insula (AI) at the sight of a likeable character in pain, and no activation if the character is reported to be unlikeable; also, activation of pain related areas in the brain are smaller if the pain observed is perceived as necessary (e.g.: to cure the observed individual) (de Vignemont & Singer, 2006). It appears that empathy can be biased into activation with positive relationships and suppressed with negative relationships (de Waal, 2008). Like humans, chimpanzees engage in aggressive behaviours and “warfare” towards individuals of rival groups (de Waal, 2005; 2008; Wrangham, 1986). Intragroup tensions tend to be less serious and are usually resolved with reconciliatory approaches such as kissing and embracing (de Waal 2005; 2008).
Some authors prefer to acknowledge empathy as a concept with two separable, complementary systems: cognitive empathy (mental perspective taking) and emotional empathy (the vicarious sharing of emotion) (Smith, 2006). Cognitive empathy relates to the understanding and predicting of behaviour in others, particularly in terms of attributed mental states (Smith, 2006). It can be used in manipulating others to our benefit (Smith, 2006), or helping with simple task-solving problems (Call, 2001). Empathic processes do not require conscious awareness, but can be augmented by cognitive capacities in evolution and development, so that empathy is possible even in the absence of the object of distress, recurring to imitation or effortful processing (Preston & de Waal, 2002). Other authors add to these dimensions a motor Empathy, which is very much in line with the unconscious mirror-activity described by Gallese, Rizollati, Iacoboni and other collaborators, who found the MNS.
The empathic machinery of human and non-human primates
The Mirror Neuron System
A turning point in the enlightening of empathy in non human primates (at least in the group that encompasses both humans and macaques - Cercopithecinae or Old World Monkeys) was the discovery of the homologous brain circuitry of emotion response and simulation known as the Mirror Neuron System or MNS (Rizzolatti et al., 1996). Empathy switched from being interpreted as a psychological phenomenon, where one was required to consciously deal with abstract ideas and to create and function in terms of abstract realities (Clark, 1980) to being recognized as a bodily phenomenon. The core argument is that, through its own neural and bodily representations, it is possible for the observer to experience the subjective state of another individual, given that the neural representations of that particular state are activated in the observer (de Vignemont & Singer, 2006; Rizzolatti et al., 2004; 2006; van der Gaag et al., 2007). This mechanism removes conceptual reasoning from the equation, allowing the individual to understand the observed action through direct simulation using a brain mirror mechanism. The similarity between individuals allows the enhancement of the observer’s matching motor and autonomic responses (de Waal, 2008). The discovery of mirror neurons took place in the early 1990s, when Rizzolatti, Gallese, Fadiga, and Fogassi were investigating neuron controlled responses of hand movements in macaques and incidentally observed that some neurons in the macaques’ brain not only fired at the performance of certain motor actions (e.g., grasping), but also fired at the perception of the same actions being performed by someone else, in the case, a human (Keysers & Gazzola, 2006; Keysers & Perrett, 2004; Rizzolatti et al., 2006).
Three areas in the monkey’s brain become active at the performance of motor actions by other individuals: The superior temporal sulcus (STS), the anterior inferior parietal lobule (area 7b or PF) and the ventral premotor cortex (area F5) (Keysers & Perrett, 2004; Keysers & Gazzola, 2006; Rizzolatti et al., 2006). The superior temporal sulcus connects reciprocally with area PF, which also connects reciprocally with area F5; there are no direct connections between the superior temporal sulcus and area PF (Keysers & Perrett, 2004; Keysers & Gazzola, 2006). The singularity of area F5 lies in the fact that virtually all neurons that fire when the monkey observes an action being performed by someone else, also fire when the action is performed by the monkey, whether it can see its own action or not (Keysers & Perrett, 2004; Keysers & Gazzola, 2006; Rizzolatti et al., 2006). These neurons were hence named mirror neurons (Keysers & Perrett, 2004; Keysers & Gazzola, 2006; Rizzolatti et al., 2006). Studies using functional imaging resonance (fMRI), positron emission tomography (PET) and magneto-encephalography (MEG) have located three areas in the human brain which become active with observation of actions: The caudal inferior and frontal gyrus and adjacent premotor cortex (Broadman areas (BAs) 44 and 6) corresponding to the monkey’s area F5, the rostral inferior parietal lobule (IPL) corresponding to the monkey’s area PF, and caudal sectors of the temporal lobe, in particular the posterior superior temporal sulcus (pSTS) and adjacent MTG corresponding to the monkey’s STS (Keysers & Perrett 2004; Keysers & Gazzola 2006; Rizzolatti et al., 2006). The IPL and BA44/6 have important roles in the observation and execution of motor actions and, like in the monkey’s PF and F5, some neurons behave in a mirroring fashion (Keysers & Gazzola 2006). These mirror neurons fire during the performance of simple goal-directed actions (e.g, grasping), during the sight of the performed action or an audio stimulus of a perceived action, with no need for external reward (de Waal 2008; Iacoboni, Moinar-Szakacs, Gallese, Buccino, Mazziota, & Rizzolatti, 2005; Rizzolatti et al., 2004; 2006). Mirror neurons are thought to encode templates for specific actions, thus eliminating the need for explicit reasoning about basic observed actions and facilitating the learning process by imitation (Rizzolatti et al., 2006). After establishing that the brain uses the same hardwired connections to code observed and performed actions, investigators tried to understand if the mirror neuron system was capable of coding the intention of an observed action. Iacoboni and colleagues found that, in humans, actions embedded in context yielded a greater signal response in the parietal-cortical circuit for grasping than activities with absence of context. In monkeys, experiences with placing food in a container or in the mouth fired different sets of neurons, making evident that the final goal of the action matters and is understood by the brain (Iacoboni etal., 2005; Rizzolatti et al., 2004; 2006). Rizzolatti established that the patterns of neuron activity associated with observed actions were true representations in the brain of the act itself, regardless of who was performing it, working as an “offline simulation” of an observed situation through the embodiment of the observed individual’s behaviours and emotions (Oberman & Ramachandran, 2007; Rizzolatti et al., 2006). After the discovery of mirror neurons, it became obvious that the brain -of a rhesus macaque or a human -is organized in circuits that overlap observed and performed information, and which have been proposed as the mechanism for the unconscious to understand actions (Keysers & Gazzola, 2006), sensations and emotions in others (de Vignemont & Singer 2006; Gallese 2007; Keysers & Gazzola 2006; Rizzolatti et al., 2003; 2004; 2006). Pain also seems to be interpreted through such a shared circuit (Decety et al., 2006; Keysers & Gazzola 2006; Moriguchi et al., 2007). The experiencing of pain or the perception of pain in someone else activates a common circuitry which includes the anterior cingulated cortex (ACC) and the anterior insula (Keysers & Gazzola 2006).
This shared circuit cross-talks with shared-circuits of actions, as the observation of pain influences motor responses in the subject (Keysers & Gazzola, 2006). The insula appears to be linked with most of the brain, specifically with regions associated with gustation (basal ganglia, amygdala, ACC, orbito-cortical cortex), somato-sensation (SI, SII and posterior insula), high level vision (STS), memory and semantics (temporal pole and hippocampus, the pre-motor cortex and the cingulated motor cortex (Gallese 2007; Jabbi etal., 2008; Rizzolatti etal., 2006;). Electrical stimulation of the monkey’s insula produces movement in several body parts (Rizzolatti et al., 2004). In humans, electrical stimulation of the anterior insula produces unpleasant sensations in the throat and mouth, as well as the sensation of nausea (Rizzolatti et al., 2003; Keysers & Gazzola, 2006). Although the human insula is larger than the monkey’s, they are, according to Keysers and Gazzola (2006) strikingly similar. A mirror system for disgust has since been found, involving the insula and the adjacent frontal operculum (IFO) (Keysers & Gazzola, 2006; Rizzolatti et al., 2003; 2006), for the production and observation of the same emotion facial expression (Gallese 2007; Singer et al., 2004).
Such knowledge provided empathy with a physiological dimension that did not exist before - and by steering the concept away from a purely psychological definition, it allowed empathy to be established as a phenomenon that occurs in children, who may not possess the tools necessary for an “online-simulation” of the emotions of others (de Vignemont & Singer, 2006; Hallenbeck, 1981), as well as in animals equipped with a nervous system developed enough to allow these complex processes (de Waal, 2008). Chimpanzees subjected to emotionally charged images react with changes in the brain and peripheral skin temperature in a strikingly familiar fashion to humans (Parr 2001; de Waal 2008). Humans and chimpanzees share a very similar structure of emotional communication and recognition (e.g. Bard & Gardner, 1996; Gaspar, 2006; Hirata 2009; Leavens, Hopkins, & Bard, 2005), and chimpanzees seem to be able to differentiate emotional expressions very similar to human ones. Chimpanzees also exhibit anatomical asymmetries in areas of the brain that are thought to be homologous with language related areas in humans (Parr & Hopkins, 2000). As mentioned before, the insula receives information from olfactory and gustatory receptors, as well as from the anterior sectors of the ventral bank of the superior temporal sulcus, the same area that shows activation in the monkey regarding the sight of faces (Gallese 2007; Rizzolatti et al., 2003; 2004).
Effects of brain damage on human empathy
The recognition of affect expressive behaviour is at the base of empathic responses. Cross-species similarities have been reported in the effects of damage to brain areas involved in emotion recognition. For example, bilateral damage to the human amygdala causes impaired recognition of facial cues of fear and anger in others (Adolphs, Tranel, Damasio, & Damasio, 1994; Calder, Young, Rowland, Perret, Hodges, & Etcoff, 1996). Macaques also have amygdala neurons that respond selectively to emotional facial expressions of conspecifics and lesions to these neuron populations impair their recognition as well (for a review see Adolphs, Tranel, Damasio, & Damasio, 1995).
Gazzola, Azzizzadeh and Keysers (2006) found that people who are more empathic (based on a self-report questionnaire) have stronger activation both in the MNS for hand actions and the MNS for emotions, providing more direct support to the idea that the MNS is linked to empathy via the reactivation of emotion circuits in the brain that are active during self experience of those emotions (e.g., anterior insula, amygdala, secondary sensory cortices) (Keysers & Gazzola, 2006; Keysers et al., 2006; Jabbi & Keysers, 2008; Jabbi et al., 2008;). Damage to the frontal brain also supports the connection between neural processes and empathy. Adjacent frontal operculum (IFO) lesions cripple the recognition of disgust (Rizzolatti et al., 2003; Jabbi et al., 2008). The IFO area is related to the recognition of disgust, pleasure and pain, and is also activated during autobiographical recall and the attribution of taste to images of food. Most likely, IFO is related with interoceptive awareness (Rizzolatti et al., 2003; Jabbi et al., 2008). Individuals with early onset damage to the prefrontal cortex exhibit standard behaviours of sociopathy, impaired empathy, autism, Asperger’s syndrome and schizophrenia (Preston & de Waal, 2002; Shamay-Tsoory et al., 2005). However, because autistic individuals have difficulty in the production, imitation and recognition of expressions - an overall defective embodied simulation - this disorder is likely to be characterized by an early impairment in the perception-action pathway (Preston & de Waal, 2002) or altered regulation of the Mirror Neuron System (de Vignemont & Frith 2007; Gallese 2007). The deficit in imitation and empathic responses by autistic children agrees with de Waal’s Russian doll model (Preston & de Waal, 2002; de Waal, 2008). Autistic children also show delayed or absent responses in the mirror self-recognition test (Gallup, 1998).
Why has empathy evolved?
Is empathy useful? Does it have a function that translates into one’s survival or kin survival that explains its favouring by natural selection? Or at least its neutral selection, since phenomena such as empathic concern at one end or altruistic acts at the other, does not seem to have been selected against? These are questions that may cross our minds especially if we think of empathy as a culturally constructed luxury that only humans can afford.
The ability to recognize emotional states in conspecifics and act on them, is one of the most important skills in social life, as it gives rise to prosocial behavior and ultimately to altruistic acts, and its absence to antisocial conduct (Iacoboni etal., 2005; Maibom, 2008).
The lives of social animals are built upon within group cooperation. Empathy, not only allows the individual to learn from others and their experience, but it also allows the active learning of the surrounding environment through behavioural cues “leaked” by others. Different types of empathy seem to encompass their own specific advantages: According to Smith (2006) cognitive empathy might have been selected because of the enormous complexity of human social environments, providing important prosocial insight, whereas emotional empathy might have been selected because it promotes inclusive fitness, inhibiting violence and fostering group cohesion through intrinsic prosocial motivational rewards (de Vignemont & Singer 2006; de Waal 2008; Smith 2006). Emotional empathy is a basic fitness tool to attend to an offspring, especially to emotional vocalizations of an out of sight young (Panksepp, 2004; 2006). Smith further suggests that the combination of the two brings balance to social interactions, as cognitive empathy buffers the urge to help triggered by emotional empathy, and selects what kind of help is most appropriate in a given situation, while emotional empathy buffers violent and manipulative impulses, as well as the possible Machiavellian uses of cognitive empathy (Smith, 2006).
Primates in particular are known to learn much about their surroundings by observing conspecifics (Scherer 1984; Quiatt & Reynolds, 1993; Cunningham & Janson 2007; van der Gaag et al., 2007). Animals capable of predicting the behaviour of their peers, especially in novel situations, possess clear advantage over those who do not possess this ability and, consequently, higher fitness (Quiatt & Reynolds 1993; Call 2001). This emotional awareness serves in the formation of long-lasting relationships, and facilitates the pursuit of shared interests and coordination of group activity (Parr, 2001). This interpretation contradicts the old school of thought, where empathy was considered a liability to the animalistic impulses of personal satisfaction and search for power (Clark, 1980). Although the behavioural evidence of non-human empathy is still scant, mostly because until recently no one was looking for it outside the human species, there is evidence on homology of emotional systems and the MNS, as seen in the previous section, that at least some non-human primates possess the neural basis that allows for the display of empathic behaviour (Brothers 1990; Rizzolatti et al., 1996) and a steady accumulation of reports of empathic behaviour is taking place, as seen in the examples of the various components of empathy provided in the first section (for more examples of emotional contagion, helping behaviour and seemingly altruistic behaviour in primates and other mammals, see Bekoff, 2007, and de Waal 1996, 2006).
Preston & de Waal (2002) proposed the Perception-Action Mechanism model (PAM) to explain why some animals are so inclined to help others. According to this model, the observer (or subject), through his own neural and bodily representations, accesses the emotional state of the individual experiencing the situation (the object), because perception and action share a common code of representation in the brain; the familiarity or proximity between the two individuals would result in a much more detailed representation of the situation by the subject, which will in turn translate into a richer and more accurate pattern of response (Preston & de Waal, 2002). The PAM mechanism supports behaviours such as mother-infant responsiveness, alarm, social facilitation, amongst others, as it underpins sympathetic concern and perspective-taking and motivates behavioural outcomes (Preston & de Waal 2002; de Waal 2008). In the words of Preston and de Waal (2002, p. 6), «having a nervous system that responds automatically with empathy to situations where they must respond creates the appearance of reciprocity and maximizes inclusive fitness» In order to relate the different affective phenomena, de Preston & de Waal (2002) proposed the Russian Doll model, which also links imitation to the empathic process. Imitation is, in Heyes’ (1998) definition, the spontaneous reproduction of novel acts yielding disparate sensory inputs when observed and executed. Examples of imitation in primates (“aping”) include contagious yawning in chimpanzees, eating at the sight of others eating, contagious scratching in monkeys, as well as neo-natal imitation of facial expressive behaviour, similarly to human children (Anderson et al., 2004; Bard 2007; Nakayama 2004;). Several monkey species, although exhibiting social facilitative behaviours, show no evidence of imitation (Bekoff et al., 2002). It appears that the tendency to imitate is as spontaneous as the empathic response but tends to decrease with age, whilst prosocial behaviours, such as helping, increase (Preston & de Waal 2002; de Waal 2008). Imitation is an important socially facilitating tool - the chameleon effect of imitation is found to create affiliation and fondness (de Vignemont & Singer 2006; Trivers 2006). The relation between observation and imitation is supported by neurophysiological data regarding emotional facial behaviour: Both activate the same group of brain structures, including the ventral premotor cortex, the insula and the amygdala (Gallese 2007). And de Waal (2008) argues that empathy and imitation share the same motivational structure, which includes shared representations, identification through physical similarity, automaticity and spontaneity.
Through empathic perspective-taking, an individual has a faster access route to another individual’s emotions, and the reproduction of the observed emotion through its own neural system allows the individual to predict future behaviours from the arousal of its own motivational and action systems (de Vignemont & Singer 2006; Keysers & Gazzola 2006). The activation of shared-circuits allows an (unconscious) connectedness of all individuals within a social group through a neural basis, which according to Gallese (2007), finds its phylogenetic and ontogenetic roots in the social sharing of affect.
As noted before, the ability to understand behaviours and to predict them can prove to be a huge fitness advantage (Call, 2001). Empathy can serve as a great tool to acquire information about the surrounding environment - for example, seeing a conspecific eating food and looking disgusted will allow the individual to infer that the food is bad and should not be eaten; watching an individual get burnt and observing his pain will trigger the mirror neurons related with coding pain in the observer, generating a state of “intentional atunement” (Gallese 2007; Rizzolatti et al., 2003). This pain the observer feels is a result of his own stored memories and the emotion he observes in the other individual that is reproduced by his own system through Hebbian associations, allowing him to make the connection between fire and pain, and preserving him from the actual experience to understand how it feels like (de Vignemont & Singer, 2006; Gallese, 2007; Keysers & Gazzola, 2006). Singer, Seymour, O'Doherty, Kaube, Dolan, and Frith (2004) proposed that the re-representations form the basis for our ability to form subjective representation of feelings that allow us to predict the effects of emotional stimuli with respect to the self; they serve as a neural basis for our ability to understand the emotional importance of a particular stimulus for another person and to predict its likely associated consequences. From a functional perspective, fully detailed representations of stimulus are only useful when concerning one’s own body, where information such as intensity and location play important aspects when dealing with a possible unpleasant stimulus (Singer et al., 2004). When assessing another individual’s reaction to pain, the relevance is not as much of a sensory-discriminative kind, but rather the balance between the relevance of the stimulus reproduced and its observable unpleasantness (Singer et al., 2004).
The mirror neuron system could have evolved to simplify the comprehension of others’ behaviour - in a mechanic and direct way without recurring to a complex cognitive machinery (Rizzolatti et al., 1996; 2006). Such system would be a stepping stone in efficiency regarding time and energy consumption, since coding templates for simple behaviours would remove the explicit reflective mediation and allow a more complex cognitive process to develop through the simple coded ones (Rizzolatti et al., 2004). An individual develops a generalization from his own behaviour, and observing the same behaviour in others triggers the stored memory (Rizzolatti et al., 1996; Holton & Langton 1998). The observer understands the observed action because he knows the expected outcome from his previous experiences, since the visual cues allow the access of the experiential motor knowledge (Keysers & Gazzola 2006; Rizzolatti et al., 2006). Translating the actions, feelings and emotions of others into our own neural language, allows for a connection through primary representations of the states of others and, in such reality, when someone is asked to elaborate on someone else’s actions, feelings, and emotions, it’s not that different from elaborating about oneself (Keysers & Gazzola, 2006).
Empathy may lead to cooperative and altruistic behaviour, although not always, but it certainly seems to set the motivation to do so even if the beneficiary of the altruistic behaviour is not a close kin or a potential reciprocator of altruism. Research is still needed to explore whether an absence of control of these non-conscious emotional reactions increases the likelihood of acting upon them with manifestations of sympathy, such as altruistic acts or consolation. Based on the reviewed evidence, there is no doubt that human empathy related behaviour has been primed by evolution and that it largely depends on both emotional and cognitive processes. And, although we see reflections of empathy in other animals, some could have evolved independently of our own empathy. Do we know how far back do our sympathy, consolation and altruistic behaviours go back in time? The species Homo sapiens sapiens is ca. 200.000 years old and descends from a long lineage of 7-5 million years of Hominines (Kumar et al., 2005; Sibly & Ahlquist 1984). Our five MA ancestors were physically and genetically very much like chimpanzee’s ancestors, and we know from paleoanthropological evidence that our estimated 300.000 year old relatives, the Neanderthals, took care of their elderly and ill fellows, who crippled by serious bone disease such as bone cancer, osteoarthritis or damaged and even absent teeth (Stringer & Gamble, 1993; Tappen, 2005), and couldn’t have survived without extensive assistance from other members of their clans. The Mirror Neuron System similarities and the empathic behaviour in other primates suggest it has its roots in a far more distant past. From its advantages in both human and non-human primates we can certainly envisage its advantages in the lives and survival of other social mammals, of whom we have only heard anecdotal reports of empathic behaviour (Bekoff, 2007; de Waal 1996; 2006) and so far no knowledge of a MNS.
Looks like in its new interdisciplinary path, Psychology will no longer attend to prejudice about our biological selves and remain isolated from Physiology or Neuroscience in its quest to understand brain and behaviour, human or non-human, for these are inextricable realities.
References
Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. (1995). Fear and the human amygdala. The Journal of Neuroscience, 15(9), 5879-5891. [ Links ]
Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. (1996). Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature, 372, 669-72. [ Links ]
Anderson, J. R., Myowa-Yamakoshi, M., & Matsuzawa, T. (2004). Contagious yawning in chimpanzees. Proceedings of the Royal Society of London B.,271, S468-S470. [ Links ]
Aureli, F., Fraser, O. N., & Stahl, D. (2008). Stress reduction through consolation in chimpanzees. Proceedings of the National Academy of Sciences(105), 25. [ Links ]
Bard, K. (2007). Neonatal imitation in chimpanzees (Pan troglodytes)tested with two paradigms. Animal Cognition, 10, 233-242. [ Links ]
Bard, K. A., & Gardner, K. H. (1996). Influences on development in infant chimpanzees: Enculturation, temperament, and cognition. Reaching into thought: The minds of the great apes (pp. 235-256). New York, Cambridge University Press [ Links ]
Bekoff, M. (2007). The emotional lives of animals. Novato, CA: New World Library.
Bekoff, M., Allen, C., & Burghardt, G. M. (2002). When traditional methodologies fail: Cognitive studies of great apes. In M. Bekoff, C. Allen & G. M. Burghardt (Eds.), The cognitive animal: Empirical and theoretical perspectives on animal cognition (pp. 335-343). Cambridge: The MIT Press. [ Links ]
Blair, R. J. R. (2003). Neurobiological basis of psychopathy. British Journal of Psychiatry, 182, 5-7. [ Links ]
Boesch, C., & Boesch-Acherman, H. (2000). The Chimpanzees of the Tai Forest. Oxford, UK, Oxford University Press. [ Links ]
Brothers, L. (1990). The neural basis of primate communication. Motivation and Emotion, 14(2), 81-90. [ Links ]
Calder, A. J., Young, A. W., Rowland, D., Perrett, D. I., Hodges, J. R., & Etcoff, N. L. (1996). Facial emotion recognition after bilateral amygdala damage: Differentially severe impairment of fear. Cognitive Neuropsychology, 13,699-745. [ Links ]
Call, J. (2001). Chimpanzee social cognition. TRENDS in Cognitive Sciences, 5(9), 388-393. [ Links ]
Clark, K. B. (1980). Empathy: A neglected topic in psychological research. American Psychologist, 35(2), 187-190. [ Links ]
Cleckley, H. (1941). The mask of sanity. St. Louis, MO: C. V. Mosby.
Clutton-Brock, J. (1999). A natural history of domesticated mammals.Cambridge: Press Syndicate of the University of Cambridge. [ Links ]
Cunningham, E., & Janson, C. (2007). A socioecological perspective on primate cognition, past and present. Animal Cognition, 10, 273-281. [ Links ]
Davis, M. H. (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 10,85. [ Links ]
de Vignemont, F., & Frith, U. (2007). Autism, morality and empathy. In W. Sinnott-Armstrong (Ed.), Moral psychology, volume 3: The neuroscience of morality (pp. 273-280). Cambridge, Mass: MIT Press.
de Vignemont, F., & Singer, T. (2006). The empathic brain: How, when and why?. TRENDS in Cognitive Sciences, 10(10), 435-441. [ Links ]
de Waal, F. B. M. (1996). Good natured: The origins of right and wrong in humans and other animals. Cambridge, MA: Harvard University Press.
de Waal, F. B. M. (2005). A century of getting to know the chimpanzee. Nature, 437,56-59. [ Links ]
de Waal, F. B. M. (2006). Primates and philosophers: How morality evolved. Princeton, NJ: Princeton University Press.
de Waal, F. B. M. (2007). With a little help from a friend. PLoS Biology, 5, 1406-1408. [ Links ]
de Waal, F. B. M. (2008). Putting the altruism back into altruism: The evolution of empathy. Annual Review of Psychology, 59, 279-300. [ Links ]
de Waal, F. B. M., & van Roosmalen, A. (1979). Reconciliation and consolation among chimpanzees. Behavioral Ecology and Sociobiology 5,55-66. [ Links ]
Decety, J., Jackson, P. L., Brunet, E., & Meltzoff, A. N. (2006). Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel. Neuropsychologia, 44, 752-761. [ Links ]
Dimberg, U., & Thunberg, M. (1998). Rapid facial reactions to emotional facial expressions. Scandinavian Journal of Psychology, 39,39-45. [ Links ]
Eisenberg, N. (2000). Empathy and sympathy. In M. Lewis and J. M. Haviland-Jones (Eds.), Handbook of Emotions (2nd Ed.) (pp. 677-691). New-York: The Guilford Press. [ Links ]
Eisenberg, N., & Mussen, P. H (1989). The roots of prosocial behavior in children. Cambridge, UK: Cambridge University Press [ Links ]
Eisenberg, N., & Strayer, J. (1987). Empathy and its development. Cambridge: Cambridge University Press. [ Links ]
Gallese, V. (2007). Commentary on toward a neuroscience of empathy: Integrating affective and cognitive perspectives. Neuro-Psychoanalysis, 9, 146-151. [ Links ]
Gallup, G. G. (1977). Self-recognition in primates: A comparative approach to the bidirectional properties of consciousness. American Psychologist, 32,329-338. [ Links ]
Gallup, J., G. (1998). Can animals empathize?. Scientific American, 9, 66-71. [ Links ]
Gaspar, A. (2006). Universals and individuality in facial behaviour: Past and future of an evolutionary perspective. Acta Ethologica, 9,1-14. [ Links ]
Goleman, D., & Boyatzis, R. (2008) Social intelligence and the biology of leadership. Harvard Business Review, 36(2),74-81. [ Links ]
Goodall, J. (1986). The chimpanzees of Gombe. Patterns of behavior.Chicago University Press. [ Links ]
Grandjean, D., Sander, D., & Scherer, K. R. (2008). Conscious emotional experience emerges as a function of multilevel, appraisal-driven response synchronization. Consciousness and Cognition, 17, 484-495. [ Links ]
Gruen, R. J., & Mendelsohn, G. (1986). Emotional responses to affective displays in others: The distinction between empathy and sympathy. Journal of Personality and Social Psychology, 51(3), 609-614. [ Links ]
Hallenbeck, P. N. (1981). A reply to Clark's empathy. American Psychologist,February: 225. [ Links ]
Heyes, C. M. (1998). Theory of mind in nonhuman primates. The Behavioral and Brain Sciences, 21(1), 101-134. [ Links ]
Hirata, S. (2009). Chimpanzee social intelligence: Selfishness, altruism, and the mother-infant bond. Primates, 50, 3-11. [ Links ]
Hoffman, M. L. (1978). Empathy: Its development and prosocial implications. Nebraska symposium on motivation: Social cognitive development. University of Nebraska Press, J. H. E. H. C. B. Keasey. [ Links ]
Hoffman, M. L. (1990). Empathy and justice motivation. Motivation and Emotion, 4,151-172. [ Links ]
Hogan, R. (1969). Development of an empathy scale. Journal of Consulting and Clinical Psychology, 33, 307-316. [ Links ]
Hollin, C. (1994). Forensic (criminological) psychology. In A. Colman (Ed.), Companion encyclopedia of psychology (pp. 1231-1253). London: Routledge. [ Links ]
Holton, R., & Langton, R. (1998). Empathy and animal ethics. In D. Jamieson (Ed), Singer and his critics (pp. 209-232). Oxford: Basil Blackwell. [ Links ]
Hutchinson, W. D., Davis, K. D., Lozano, A. M., Tasker, R. R., & Dostrovsk, J. O. (1999). Pain-related neurons in the human cingulated cortex. Nature Neuroscience, 2, 403-405. [ Links ]
Iacoboni, M., Moinar-Szakacs, I., Gallese, V., Buccino, G., Mazziota, J. C., & Rizzolatti, G. (2005). Grasping the intentions of others with one's own mirror neuron system. PLoS Biology, 3(3), 0001-0007. [ Links ]
Jabbi, M., Bastiaansen, J., & Keysers, C. (2008). A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS one, 3(8), 239. [ Links ]
Jabbi, M., & Keysers, C. (2008). Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion, 8(6),775-780. [ Links ]
Jackson, P. L, Meltzoff, A. N. & Decety, J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage, 24,771-779 [ Links ]
Keysers, C., & Gazzola, V. (2006). Towards a unifying neural theory of social cognition. In Anders, Ende, Junghofer, Kissler & Wildgruber (Eds.), Progress in brain research Vol. 156 (pp. 379-401). Amsterdam: Elsevier. [ Links ]
Keysers, C., Gazzola, V., & Aziz-Zadeh, L. (2006). Empathy and the somatotopic auditory mirror system in humans. Current Biology, 16, 1824-1829. [ Links ]
Keysers, C., & Perrett, D. I. (2004). Demystifying social cognition: A Hebbian perspective. TRENDS in Cognitive Sciences, 8(11), 501-507. [ Links ]
Koski, S. E., Koops, K., & Sterck, E. H. M. (2007). Reconciliation, relationship quality, and postconflict anxiety: Testing the integrated hypothesis in captive chimpanzees. American Journal of Primatology, 69,158-172. [ Links ]
Kumar, S., Filipski, A., Swarna, V., Walker, A., & Hedges, S. B. (2005). Placing confidence limits on the molecular age of the human-chimpanzee divergence. Proceedings of the National Academy of Sciences, 102(52), 18842-18847. [ Links ]
Leavens, D. A., Hopkins, W. D., & Bard, K. A. (2005). Understanding the point of Chimpanzee pointing: Epigenesis and ecological validity. Current Directions in Psychological Science, 14 (4), 185-189. [ Links ]
Maibom, H. L. (2008). The mad, the bad, and the psychopath. Neuroethics, 1,167-184. [ Links ]
Masserman, J. H., Wechkin, S., & Terris, W. (1964). Altruistic behaviour in rhesus monkeys. The American Journal of Psychiatry, 121,584-585. [ Links ]
Miller, P. A., & Eisenberg, N. (1988). The relation of empathy to aggressive and externalizing/antisocial behavior. Psychological Bulletin, 103, 324-344. [ Links ]
Miles, R. W. (1963). Chimpanzee behaviour: Removal of foreign body from companion's eye. Proceedings of the National Academy of Sciences of the United States of America, 49(6), 840-843. [ Links ]
Moriguchi, Y., Decety, J., Ohnishi, T., Maeda, M., Mori, T., Nemoto, K., Matsuda, H., & Komaki, G. (2007). Empathy and judging other’s pain: An fMRI study of alexithymia. Cerebral Cortex, 17, 2223-2234. [ Links ]
Morrison, I., Llyoid, D., Pellegrino, G., & Roberts, N. (2002). Vicarious responses to pain in anterior cingulated cortex: Is empathy a multisensory issue?. Cognitive, Affective & Behavioral Neuroscience 4(2), 270-278. [ Links ]
Nakayama, K. (2004). Observing conspecifics scratching induces a contagion of scratching in Japanese monkeys (Macaca fuscata). Journal of Comparative Psychology, 118(1), 20-24. [ Links ]
Oberman, L. M., & Ramachandran, V. S. (2007). The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological Bulletin133(2), 310-327. [ Links ]
Panksepp, J. (2004). Affective neuroscience. The foundations of human and animal emotions. Oxford: Oxford University Press. [ Links ]
Panksepp, J. (2005). Affective consciousness: Core emotional feelings in animals and humans. Consciousness and Cognition, 14, 30-80. [ Links ]
Parr, L. A. (2001). Cognitive and physiological markers of emotional awareness in chimpanzees (Pan troglodytes). Animal Cognition, 4,223-229. [ Links ]
Parr, L. A., & Hopkins, W. D. (2000). Brain temperature asymmetries and emotional perception in chimpanzees, Pan troglodytes. Physiology & Behavior, 71, 363-371. [ Links ]
Plotnik, J. M., de Waal, F. B. M., & Reiss, D. (2006). Self-recognition in an Asian elephant. Proceedings of the National Academy of Sciences,103(45), 17053-17057. [ Links ]
Povinelli, D. J., & Bering, J. M. (2002). The mentality of Apes revisited. Current Directions in Psychological Science, 11, 115-119. [ Links ]
Povinelli, D. J., & Giambrone, S. (2001). Reasoning about beliefs: A human specialization?. Child Development, 72(3), 691-695. [ Links ]
Preston, S. D., & de Waal, F. B. M. (2002). Empathy: Its ultimate and proximate bases. The Behavioral and Brain Sciences, 25(1),1-71. [ Links ]
Quiatt, D., & Reynolds, V. (1993). Primate behavior: Information, social knowledge and the evolution of culture. Cambridge: Cambridge University Press. [ Links ]
Reiss, D., & Marino, L. (2001). Mirror self-recognition in the bottlenose dolphin: A case of cognitive convergence. Proceedings of the National Academy of Sciences,98(10), 5937-5942. [ Links ]
Rizzolatti, G., Fogassi, L., & Gallese, V. (2006). Mirrors in the mind. Scientific American, November, 54-61. [ Links ]
Rizzolatti, G., Gallese, V., Fadiga, L., & Fogassi, L. (1996). Action recognition in the premotor cortex. Brain, 119, 593-609. [ Links ]
Rizzolatti, G., Gallese, V., & Keysers, C. (2004). A unifying view of the basis of social cognition. Trends in Cognitive Sciences, 8(9), 396-403. [ Links ]
Rizzolatti, G., Wicker, B., Keysers, B., Plailly, J., Royet, J.-P., & Gallese, V. (2003). Both of us disgusted in my insula: The common neural basis of seeing and feeling disgust. Neuron, 40, 655-664. [ Links ]
Scherer, K. R. (1984). On the nature and function of emotion: A component process approach. In K. R. Scherer & P. Ekman (Eds.), Approaches to emotion (pp. 293-317). Hillsdale: NJ: Erlbaum.
Schino, G., Aureli, F., Geminiani, S., & Rosati, L. (2004). Behavioral and emotional response of Japanese Macaque (Macaca fuscata) mothers after their offspring receive an aggression. Journal of Comparative Psychology, 118(3), 340-346. [ Links ]
Scott, R., Liddell, H. G., Mackenzie, R., & Jones, H. S. (1996/1940). A Greek-English lexicon. Clarendon: Oxford University Press. [ Links ]
Shamay-Tsoory, S. G., Lester, H., Chisin, R., Israel, O., Bar-Shalom, R., Peretz, A., Tomer, R., Tsitrinbaum, Z., & Aharon-Peretz, J. (2005). The neural correlates of understanding the other’s distress: A positron emission tomography investigation of accurate empathy. Neuroimage, 27,468-472. [ Links ]
Sibly, C. G., & Ahlquist, J. E. (1984). The phylogeny of hominid primates as indicated by DNA-DNA hybridization. Journal of Molecular Evolution, 20,2-15. [ Links ]
Singer, T. B., Seymour, J., O’Doherty, H., Kaube, R. J., Dolan & Frith, C. D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157-1162. [ Links ]
Smith, A. (2006). Cognitive empathy and emotional empathy in human behavior and evolution. The Psychological Record, 56, 3-21. [ Links ]
Stringer, C., & Gamble, C. (1993). In search of the Neanderthals. New York: Thames & Hudson Inc. [ Links ]
Tappen, N. C. (2005). The dentition of the old man of La Chapelle-aux-Saints and inferences concerning Neanderthal behavior. American Journal of Physical Anthropology,67(1), 43-50. [ Links ]
Trivers, R. (2006). Reciprocal altruism: 30 years later. In P. M. Kappeler & C. P. van Schaik (Eds.), Cooperation in primates and humans: Mechanisms and evolution (pp. 67-83). Berlin: Springer. [ Links ]
van der Gaag, C., Minderaa, R. B., & Keysers, C. (2007). Facial expressions: What the mirror neuron system can and cannot tell us. Social Neuroscience,2(3-4), 179-222. [ Links ]
Videan, E. N., Fritz, J., Schwandt, M., & Howell, S. (2005). Neighbor effect: Evidence of affiliative and agonistic social contagion in captive Chimpanzees (Pan troglodytes). American Journal of Primatology, 66, 131-144. [ Links ]
Wrangham, R. W. (1986). Ecology and social evolution in two species of chimpanzees. In D. I. Rubenstein & R. W. Wrangham (Eds.), Ecology and social evolution: Birds and mammals. Princeton: Princeton University Press. [ Links ]
Augusta Gaspar, Instituto Universitário de Lisboa (ISCTE-IUL), Centro de Investigação e Intervenção Social (Cis-IUL), Av. das Forças Armadas, 1649-026 Lisboa, Portugal
E-mail:augusta.gaspar@iscte.pt













