Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Portuguesa de Pneumologia
versão impressa ISSN 0873-2159
Rev Port Pneumol v.16 n.2 Lisboa abr. 2010
Bronchopulmonary dysplasia: Clinical practices in five Portuguese neonatal intensive care units
H Guimarães1, G Rocha1, G Vasconcellos1, E Proença2, ML Carreira3, MR Sossai4, B Morais4, I Martins5, T Rodrigues6, M Severo6
1 NICU, Hospital de S. João, Porto
2 Maternidade Júlio Dinis, Porto
3 Hospital de Santo António, Porto
4 Hospital Fernando da Fonseca, Amadora/Sintra
5 Hospital Pedro Hispano, Matosinhos
6 Departamento de Epidemiologia, Faculdade de Medicina da Universidade do Porto, Portugal/Department of Epidemiology, Faculty of Medicine of Porto University, Portugal
Abstract
With the advent of surfactant, prenatal corticosteroids (PNC) and advances in technology, the survival rate of extremely low birth weight (ELBW) infants has improved dramatically. Rates of bronchopulmonary dysplasia (BPD) vary widely among neonatal intensive care units (NICUs) and many studies using multiple interventions have shown some improvement in BPD rates. Implementing potentially better practices to reduce BPD has been an effort made over the last few decades.
Aim: To compare five Portuguese NICUs in terms of clinical practices in very low birth weight (VLBW) infants, in order to developbetter practices to prevent BPD.
Patients and methods: 256 preterm neonates, gestational age (GA) < 30 weeks and/or birthweight (BW) < 1250g admitted to five Portuguese NICUs (centers 1 to 5) between 1st January 2004 and 31st December 2006, were studied. VLBW infants with major malformations, grade IV intraventricular haemorrhage in the first week of life and metabolic or neuromuscular disease were excluded. BPD was defined as oxygen dependency at 36 weeks of postconceptional age. We considered a practice to be improved as clinically significant whenever a decrease greater than 10% in the prevalence of BPD adjusted for the practice, GA and BW was achieved compared to BPD prevalence adjusted only for GA and BW.
Results: The overall prevalence of BPD was 12.9%. Our results revealed that PNC use should be improved in centers 2, 4 and 5; fluid policy in center 5; oxygen therapy in centers 1 and 3 and sepsis prevention in centers 1 and 2. Patent ductus arteriosus (PDA) treatment should be improved in center 2.
Conclusion: The implementation of potentially better practices to reduce lung injury in neonates in Portuguese NICUs, according to each NICU, must be addressed to increase the prescription of PNC, to use a lower FiO2, to be careful with fluid administration in the first weeks of life and to prevent PDA and sepsis. It is necessary to follow guidelines, recommendations or protocols to improve quality in the prevention of BPD.
Key-words: Bronchopulmonary dysplasia, neonatal intensive care, preterm infants, better practices, mechanical ventilation, oxygen therapy, prenatal corticosteroids, sepsis, patent ductus arteriosus.
Displasia broncopulmonar: Práticas clínicas em cinco unidades de cuidados intensivos neonatais
Resumo
Com o advento do surfactante, dos corticosteróides prénatais e dos avanços na tecnologia, a sobrevida dos recém-nascidos de extremo baixo peso tem melhorado dramaticamente. As taxas de displasia broncopulmonar (DBP) variam amplamente entre unidades, e vários estudos, avaliando resultados de múltiplas intervenções, têm mostrado alguma melhoria na prevalência da DBP. A implementação de potenciais boas práticas na DBP tem sido adoptada por muitos serviços nas últimas décadas.
Objectivo: Comparar cinco unidades portuguesas de cuidados intensivos neonatais no que se refere as práticas clínicas no tratamento dos recém-nascidos de muito baixo peso, para desenvolver e melhorar as boas práticas na prevenção da DBP.
População e métodos: Foram estudados 256 recém-nascidos com a idade gestacional inferior a 30 semanas e/ou peso ao nascer inferior a 1250 g, admitidos nas cinco unidades portuguesas (centros 1 a 5) entre 1 de Janeiro de 2004 e 31 de Dezembro de 2006. Foram excluídos os recém-nascidos com malformações major, hemorragia intraventricular grau IV na primeira semana de vida e com doença metabólica ou neuromuscular. Definimos DBP como a dependência do oxigénio às 36 semanas de idade pós-concepcional. A ecessidade de melhorar determinada prática foi considerada significativa sempre que se verificava uma melhoria superior a 10% na prevalência da DBP ajustada para a prática, idade gestacional e peso ao nascer, comparada com a prevalência ajustada só para a idade gestacional e peso ao nascer.
Resultados: A prevalência global da DBP foi de 12,9%. Os resultados mostram que o uso de corticosteróides pré-natais deve ser melhorado nos centros 2, 4 e 5; a política de fluidos deve ser melhorada no centro 5; o uso de oxigénio deve ser melhorado nos centros 1 e 3; a prevenção da sépsis deve ser melhorada nos centros 1 e 2. O tratamento do canal arterial patente deve ser melhorado no centro 2.
Conclusão: Neste estudo, a implementação de boas práticas para reduzir a lesão pulmonar nos recém-nascidos, de acordo com cada unidade, deve ser dirigida ao aumento da prescrição de corticosteróides pré-natais, ao uso de menor FiO2, ao uso criterioso de líquidos na primeiras semanas de vida, à prevenção do canal arterial patente e da sépsis. Guidelines, recomendações ou protocolos são necessários na melhoria da qualidade na prevenção da DBP.
Palavras-chave: Displasia broncopulmonar, cuidados intensivos neonatais, recém-nascidos de pré-termo, boas práticas, ventilação mecânica, oxigénio, corticosteróides pré-natais, sépsis, canal arterial patente.
Introduction
Bronchopulmonary dysplasia (BPD) definition has changed since the first description of the disease by Northway et al. in 19671-5.
The pathogenesis of BPD is clearly multifactorial and well-known specific pathogenic risk factors include prematurity, respiratory distress syndrome (RDS), oxygen toxicity, barotrauma and volutrauma of mechanical ventilation (MV), inflammation and infection, excessive fluids and patent ductus arteriosus (PDA)6.
With the advent of surfactant, prenatal corticosteroids (PNC) and advances in technology, the survival rate of extremely low birth weight (ELBW) infants has improved dramatically. Despite these improvements the incidence of BPD in ELBW infants has remained stable over the last decades. Rates of BPD vary widely among NICUs and according to gestational age. In a recent study, where BPD was defined as the need for oxygen supplementation at 36 weeks postconceptional age (PCA), the incidence was 52% in infants with birth weight (BW) of 501-750g, 34% in infants with BW 751-1000g, 15% in infants with BW 1001-1200g and 7% in infants with BW 1201-1500g5.
Several studies using multiple interventions have shown some improvement in BPD rates. However experience suggests that some of these interventions have not been effective when translated into clinical practice in neonatal intensive care units (NICUs).
A large chasm between what we know and what we do has been reported in many áreas of medicine, and seems to be true in the prevention of BPD as well7. Implementing potentially better practices to reduce BPD has been an effort made over the last few decades8-11.
The aim of our study was to evaluate and compare clinical practices in the management of these very low birth weight (VLBW) newborns in five Portuguese NICUs in order to develop better practices in the prevention of BPD.
Patients and methods
VLBW infants with gestational age (GA) less than 30 weeks and/or BW less than 1250 grams admitted to five Portuguese NICUs between 1st January 2004 and 31st December 2006 and alive at 36 weeks of PCA were included. VLBW infants with major malformations, grade IV intraventricular haemorrhage (IVH) in the first week of life and metabolic or neuromuscular disease were excluded. A protocol was developed based on clinical information registered in the hospital charts: maternal history, prenatal corticosteroids, newborn demographical and clinical data, surfactant administration, ventilatory support, oxygen supplementation and fluid administration until 36 weeks of PCA. Neonatal sepsis, patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), (IVH) and periventricular leukomalacia (PVL) were also registered.
BPD was defined as oxygen dependency at 36 weeks of PCA and had characteristic chest radiographs12. Gestational age (in this study we considered the completed weeks) was assessed by menstrual age (women with regular menstrual cycles), ultrasound exa mination (when a discrepancy of two or more weeks existed between the age derived by menstrual dating and the age derived sonographically, or in the absence of a menstrual date)13 or the New Ballard Score (in the absence of obstetrical indexes)14. RDS (hyaline membrane disease (HMD)) was defined according to the Rudolf AJ et al. criteria15. Proven neonatal sepsis was defined as any systemic bacterial or fungal infection documented by a positive blood culture. Hemodynamically significant PDA was diagnosed on the basis of the echocardiograph findings. The Bell criteria was used for the diagnosis and staging of NEC16. Staging of ROP was performed according to the International Classification17,18.IVH was classified according to Papile LA19. PVL was classified according to de Vries L and Rennie JM20.
Chi-square (or Fisher´s Exact Test) and ANOVA were used, respectively, to compare qualitative and quantitative variables among centers.
We considered a practice to be improved as clinically significant whether there was a decrease greater than 10% in the prevalence of BPD adjusted for the practice, compared to BPD prevalence adjusted only for GA and BW. The adjusted prevalence was estimated considering the mean for GA and BW and the practice in all centers.
The NICU with less prevalence of BPD was considered the reference center for establishing a comparison among the five centers. Odds ratios (OR) estimated by logistical regression and the respective 95% confidence intervals (95% CI) were used to measure the magnitude of the association between the centers and BPD. Statistical analysis was performed using the statistical package SPSS 17.0.
Results
A sample of 256 newborns met the inclusioncriteria. The global prevalence of BPD was 12.9% (33/256). The demographical characteristics of the study population stratified by center are shown in Table I. We found statistically significant differences among the five centers in BW, GA and HMD but not in sex.
Table I – Demographical and clinical characteristics of the study population stratifi ed by center
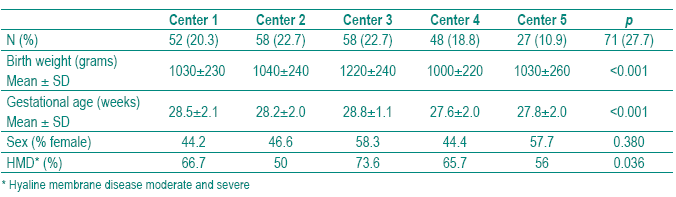
The clinical and therapeutical aspects of the study population stratified by center are shown in Table II. We did not find significant differences among centers in surfactant ministration. Center 5 was considered the reference center for establishing the comparison among the five centers as it was the NICU with less BPD prevalence (2.7%), after adjusting for GA and BW (Table III). All centers used surfactant mainly as a rescue therapy rather than as a prophylactic treatment. In Center 5, seven (9.9%) preterm infants received prophylactic surfactant within 15 minutes of birth (Tables II and III). Curosurf (poractant alpha) was used in Center 4 and 5 in all newborns; in Centers 1, 2 and 3 in 48%, 65% and 33%, respectively. Center 3 used Survanta (beractant) in 67% of cases (p<0.001). The five centers revealed different practices concerning PNC, MV, oxygen therapy and fluids. The incidence of sepsis and PDA was also significantly different. The adjusted BPD by center, calculated assuming the mean of GA, BW of all sample and the mean prevalence of each clinical and therapeutical characteristic, is given in Table III.
Table II – Clinical and therapeutical aspects stratifi ed by center
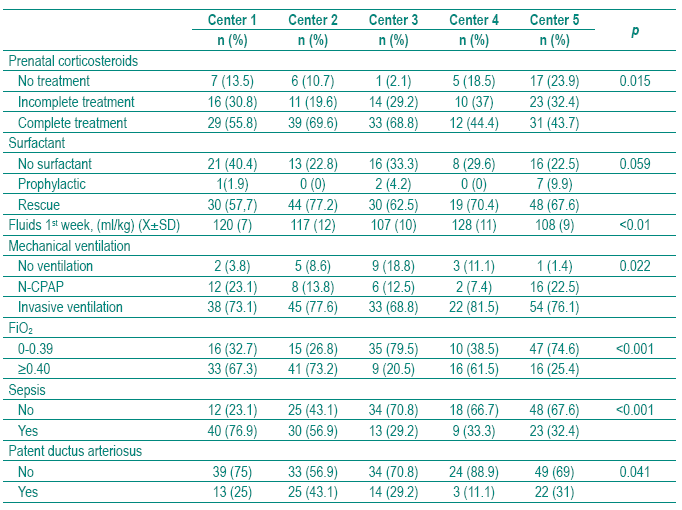
Table III – Adjusted prevalences of bronchopulmonary dysplasia by center calculated assuming the mean of gestational age, birth weight of all sample and the mean prevalence of each clinical characteristic
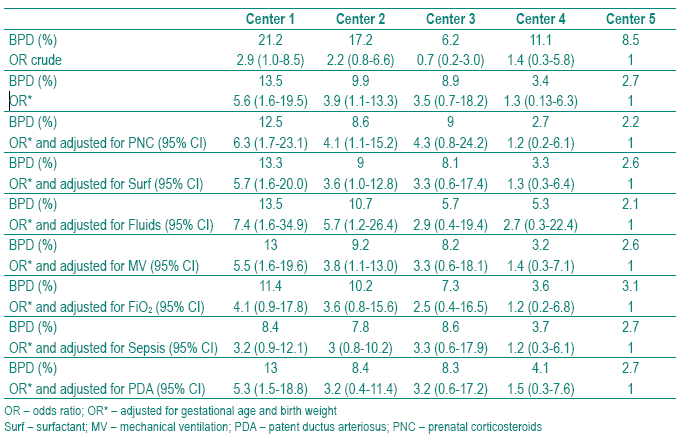
The prevalence of BPD adjusted for GA and BW varied from 13.5 % in Center 1 to 2.7 % in Center 5. Comparing the practices among NICUs, our results showed that PNC treatment can be improved in Centers 2, 4 and 5; fluid administration policy in Centers 3 and 5 and oxygen therapy in Centers 1 and 3; Centers 1 and 2 can improve their policy of sepsis prevention, and the Center 2 PDA prevention also (Table IV).
Table IV – Practices to be improved by center (+)

We did not find significant differences among NICUs in surfactant treatment and MV practices (Tables II and III).
The major pathology (NEC, ROP, IVH, PVL) observed in our patients was significantly different among the centers, ranging from 0% in Center 4 to 21.1% in Center 5.
Centers 1, 2 and 3 registered 15.4, 7 and 8.3% respectively of the cases (p=0.019).
Mortality rates by center in this group of preterm newborns were 18% in Center 1, 24% in Center 2, 19% in Center 3, 10% in Center 4 and 19% in Center 5.
Discussion
Despite increased knowledge and improving technology, BPD rates remain high. Its incidence varies among institutions, ranging from 15 to 50% of all VLBW infants21.
These differences could be due in part to the definition of BPD and to the decision to administer oxygen that is not uniform, as there is no consensus in the literature and neonatologists have widely divergent practices regarding oxygen saturations targets. In this study we used the BPD definition of oxygen dependency at 36 weeks of PCA and the prevalence of BPD ranged from 13.5% in Center 1 to 2.7% in Center 5. To decrease the significant differences, efforts must be made to identify infants treated with oxygen who are able to maintain saturations exceeding 90% in room air4,22. Another aspect which may explain these differences in the prevalence of BPD is the management of these preterm infants. After four decades since the original description by Northway, its clinical presentation, evidence as to its pathogenesis and epidemiology has changed, and the understanding of this process has provided new possibilities for BPD prevention. As BPD is a multifactorial disease, a multifaceted approach to the management of preterm infants is necessary to prevent it.
Knowing that BPD rates differ greatly among centers we analysed and compared practices in five centers to develop and implement potentially better practices to reduce BPD, as has been done over the last few decades in many other centers.
Prenatal corticosteroids – RDS is a serious complication of preterm birth and the primary cause of early neonatal mortality and disability that has been reduced by administering corticosteroids to the mother before anticipated preterm birth23,24.
In spite of the beneficial effect PNC has on foetal pulmonary maturity, we found significant differences among the five NICUs of the study. As shown in Table II, in Center 5, 23.9% of the mothers did not receive PNC and in Center 3, 68.8% received a full course. Centers 2, 4 and 5 decreased the BPD rate when adjusted to BW, GA and the mean of this practice of all centers. This means that they must improve PNC treatment in order to reduce their BPD rate (Tables III and IV).
The evidence supports the continued use of a single vs. multiple course of PNC to accelerate foetal lung maturation in women at risk of preterm birth, with a single course considered routine for preterm delivery23-26.
Repeated doses of PNC reduce the occurrence and severity of neonatal lung disease and the risk of serious health problems in the first few weeks of life. These short-term benefits for babies support the use of repeat doses of PNC for women at risk of preterm birth. However, these benefits are associated with a reduction in some measures of weight and head circumference at birth, and there is still insufficient evidence on the longer-term benefits and risks. National Institutes of Health recently recommended that no more than one course of PNC be used routinely outside clinical trials25. In this study all mothers treated with PNC received only a single course (complete or incomplete) of PNC.
Surfactant – Exogenous surfactant therapy to prevent or to treat HMD in premature infants clearly reduced neonatal mortality and survival without BPD27. Although some studies show that treatment with exogenous surfactant may decrease the incidence or severity of BPD, the fact is that surfactant use has not clearly reduced the incidence of BPD28. This may be due, in part, to its effect on improving survival of extremely immature infants who would have died without surfactant therapy.
We also know that oxidant injury and lung inflammation in extremely premature infants are associated with the development of BPD. Surfactant dysfunction resulting from these events may also contribute to the pathogenesis of BPD, justifying exogenous surfactant treatment to decrease inflammation and improve RDS29.
Nowadays exogenous surfactant is used worldwide in neonatal intensive care27-34.
Early surfactant replacement therapy with extubation to nasal continuous positive airway pressure (N-CPAP) compared with later selective surfactant replacement and continued mechanical ventilation with extubation from low ventilator support has been shown to be associated with less need for MV, lower incidence of BPD and fewer air leak syndromes30.
Evidence has shown a benefit to multiple versus single doses of exogenous surfactant in the prevention or treatment of neonatal RDS31.
In Portugal, about 50% of VLBW infants have received surfactant for RDS since 1996, and a schedule of multiple doses has been adopted33.
In this study we did not find any difference among centers in surfactant administration.
All centers used surfactant mainly as a rescue rather than a prophylactic therapy. We know that early rescue (<30 minutes of age) surfactant therapy is an effective method to minimize overtreatment of some preterm infants who may not develop RDS32. Nevertheless, in Center 5, seven (9.9%) preterm infants received prophylactic surfactant (Tables II and III).
Recently, the results of eight prospective, randomized controlled trials and two retrospective studies involving the natural surfactant preparations, treatment with poractant alpha revealed a significant decreased in mortality, decreased need for additional doses, faster weaning from oxygen and reduced hospital costs than treatment with beractant or calfactant. These differences in outcome may be due to differences in phospholipid and SP-B content and the amount of antioxidant phospholipids, plasmalogens, anti-inflammatory properties and viscosity among these three surfactants34.
The result of this large trial may explain the choice of Curosurf (poractant alpha) as the most used surfactant in four centers and only Center 3 used Survanta (beractant) in a higher percentage (67% of cases). Another reason for this choice is the small volume of Curosurf needed to treat these extremely preterm infants.
Fluids – Excessive fluid intake and/or delayed weight loss and prolonged PDA are well-known major pathogenic mechanisms for BPD. Infants with BPD have increased lung water and are susceptible to gravityinduced collapse and alveolar flooding in the dependent lung with focal tissue damage being distributed heterogenously.
High fluid volumes in the first days of life may increase neonatal morbidity and is associated to increased risk of PDA. Therefore fluid restriction, with the goal of reducing BPD risk, is standard treatment in the care of the premature infant10,35, 36.
Comparing fluid administration among the five centers showed significant differences. This study showed that this practice can be improved in Center 5, as we can see when adjusted to the mean of this practice in all centers (Tables 3 and 4). Attention must be paid to decrease fluids in the first weeks of life, allowing physiological weight loss in these very immature preterm infants.
However, evidence supporting fluid restriction is inconclusive and we must remember that restricting fluids may also restrict calorie intake37.
Mechanical ventilation – Invasive ventilation via the endotracheal tube is one of the most common therapeutic interventions performed in preterm infants with respiratory failure. MV ventilation using conventional or high-frequency ventilation and surfactant therapy has become the standard of care in management of preterm infants with RDS.
However, BPD remains a major morbidity with adverse pulmonary and nonpulmonary outcomes in preterm infants despite these interventions. Ventilator-associated lung injury appears to be related to the duration of invasive ventilation via the endotracheal tube rather than the mode of ventilation. Randomized controlled trials comparing conventional mechanical ventilation and high-frequency ventilation, using optimal ventilatory strategies, have shown no significant difference in rates of BPD. Use of noninvasive ventilation, such as N-CPAP, has shown a significant decrease in postextubation failure as well as reduced incidence of BPD10,38-40.
In this study we found no significant difference in mechanical ventilation among centers (Table III). In Center 3, 18.8% of preterm infants were not ventilated, which could be due to the fact that they were heavier than in the other four centers. N-CPAP was used in Center 1 and 5, respectively in 23.1% and 22.5%.
Invasive ventilation (SIPPV=synchronized intermittent positive pressure ventilation; SIMV=synchronized intermittent mandatory ventilation; HFOV=high frequency oscillatory ventilation) was used in 81.5% of cases in Center 4, and in other Centers ranged from 68.8% in Center 3 and 77.6% in Center 2.
N-CPAP or early surfactant therapy with early extubation to N-CPAP rather than continued mechanical ventilation has been adopted by many centres, particularly in Scandinavia, as part of the treatment of newborns with RDS. It has been suggested that BPD is less of a problem in centres adopting such a policy. Results from randomized trials suggest prophylactic or early N-CPAP may reduce BPD, but further studies are required to determine the relative contributions of an early lung recruitment policy, early surfactant administration and N-CPAP in reducing BPD. In addition, the optimum method of generating and delivering N-CPAP needs to be determined. The efficacy of N-CPAP in improving long-term respiratory outcomes needs to be compared with the newer ventilator techniques and the optimum timing of delivery of surfactant administration41.
In Portugal efforts have been made to begin an optimal ventilatory strategy in the delivery room in preterm infants with RDS with application of sustained inflation to establish functional residual capacity (NCPAP), followed by early surfactant therapy if needed and extubation as soon as possible to noninvasive ventilation. After the Portuguese Consensus on newborn management in the delivery room published in 2004, most NICUs standardised their practices.
This is probably the reason why we did not find significant differences among centers in respiratory support strategies.
Oxygen – Oxygen is the most commonly used therapy in NICUs as an integral part of respiratory support. The objective of oxygen therapy is to achieve adequate delivery of oxygen to the tissues without creating oxygen toxicity. However current evidence for optimal oxygen saturation for extremely premature infants is scarce. We still know very little about how much oxygen these babies actually need, or how much oxygen it is safe to give, especially in the first few weeks of life41-44.
Avoiding hyperoxemia is an important goal during respiratory support and neonatal exposure to 100% oxygen is almost never necessary. Much lower fraction of inspired oxygen (FiO2) during the neonatal period can also lead to oxygen toxicity if oxygen is used when it is not necessary. Even brief neonatal exposure to pure oxygen must be avoided45.
In the STOP-ROP trial (supplemental therapeutic oxygen for prethreshold retinopathy), babies in the supplemental oxygen arm (target saturations of 96-99%) had evidence of adverse pulmonary outcome compared to those in the conventional oxygen arm (target saturations of 89-94%)46.
The recent studies of Saugstad and coworkers showed that oxygen saturations levels in ELBW infants should be kept between 85 and 93% or possibly between 88 and 95%, but should definitely not exceed 95% and fluctuations should be avoided44.
Recent data show that a lower FiO2, less than 0.45, confers greater advantage in reducing the incidences of air leak syndromes and BPD compared to a higher FIO2 (more than 0.45), in the treatment of RDS30.
In our study, Centers 2, 4 and 5 appear to have more accurate FiO2 administration practices and oxygen therapy should be improved in Centers 1 and 3 (Tables III and IV). Although evidence for BPD protection by reducing oxygen exposure is not well demonstrated, we know the toxicity of oxygen and its free radicals, and better practices in oxygen supplementation must be implemented in our NICUs11,47.
Sepsis – Inflammation (and infection), either antenatal or postnatal, is likely to be a major trigger for the lung inflammation that plays a role in the pathogenesis of BPD6,48, 49.
Although there is recent evidence that premature infants born to mothers with chorioamnionitis are at increased risk of developing BPD48,50, other studies were unable to confirm this association51. In this study the association between histological chorioamnionitis and BPD was not analysed because placental histological data were missing.
The presence of nosocomial infections during the first month of life increases the risk of BPD in preterm infants requiring prolonged mechanical ventilation, another risk factor for the disease52,53.
In our study, early and late or nosocomial infections were included in the group of sepsis, because of the small numbers of cases in each center. Neonatal sepsis was observed in all centers with a significant prevalence that ranged from 29.2 to 76.9% (Table II). Centers 1 and 2 registered higher rates of sepsis, which were higher than the national average of 35%33. Rates of early–and late-onset septicaemia of 5 and 29.4% respectively in VLBW infants have recently been published54.
As we can see in these two centers (Centers 1 and 2) must improve their policy of sepsis prevention to decrease the rate of BPD (Tables III and IV).
It is crucial to reduce neonatal sepsis in our preterm infants in all NICUs, as even in centers with a low rate of sepsis we can lower the BPD rate still further.
Patent ductus arteriosus – The most common congenital heart disease in the newborn population, PDA, accounts for significant morbidity in preterm newborns. With the increasing survival of extremely premature infants, a large number of them are developing chronic lung disease, but the severity of the lung damage is considerably less than that observed in the classic form of BPD. Because many of these infants have only a mild initial respiratory distress and therefore do not receive aggressive ventilation, it is clear that factors other than oxygen toxicity and mechanical ventilation are involved in the pathogenesis of this new milder type of BPD6,52.
In this study an 11.1%,rate of PDA was observed in Center 4, and a rate of 43.1% in Center 2. These percentages differ from the rate of PDA in VLBW infants in Portugal that was 20% in a five-year study (1996-2000)33.These differences may be due to the method used for diagnosis that is mostly clinical but should be confirmed by Doppler ultrasound.
Center 2 can improve practices on prevention of PDA, as when we adjusted the BPD rate to the mean of practices of all centers the BPD rate decreased from 9.9 to 8.4%, more than the 10% defined as significant (Tables III and IV).
Clinical and epidemiological data strongly suggest that the presence of PDA plays a major role in the development of BPD in these infants. For this reason, efforts to prevent BPD in ELBW infants should include an aggressive approach to an early closure of the hemodynamically significant PDA55-58.
However it has also been assessed that in randomized controlled trials, neither a significant reduction, nor even a trend towards a reduction on BPD was observed47.
Major pathology – In major pathology we included NEC (> grade IIA), ROP (> grade 3), IVH (grades III-IV) and PVL. The major pathology observed was significantly different among the centers. Centers 1, 2, 3, 4 and 5 registered 15.4, 7, 8.3, 0 and 21.1% of major pathology respectively (p=0.019).
In Center 5 we observed a major pathology prevalence of 21.1% and in Center 4 no cases of major pathology were registered.
Nonetheless, Center 4 contributed with the most immature babies. This fact can be due in part to the transfer to other NICUs of preterms with major complications that needed special treatment, namely neurological, ophthalmological or digestive surgery.
Center 1 and Center 5 are NICUs with surgery facilities, which can, in part, explain the higher prevalence of major pathology of 15.4 and 21.1% respectively of the cases.
In a previous study in Portugal including VLBW infants, we found IVH in 27%, NEC in 10%, ROP in 9%, PVL in 6%, of preterm infants less than 1500g33.
In a recent Spanish study, intraventricular haemorrhage grades III to IV (8.1%) and cystic leukomalacia (2.6%) were the most relevant brain ultrasound findings and NEC was observed in 6.9% of VLBW infants54.
In a ten-year period investigating trends in mortality and morbidity in very preterm infants there were no changes in the rates of IVH (grades III-IV), ROP (grades > 3), seizures or NEC. The increasing rate of sepsis was present in infants <28 gestational weeks, whereas the increase in BPD was demons trated in the whole study population <32 gestational weeks59.
The mortality rates observed in this group of preterm newborns in the five NICUs of the study varied from 10% in Center 4 to 24% in Center 2. The differences can be explained, in part, by the same fact that also explains the low prevalence of major pathology, the transfer of the preterm infants that needed surgery. However it was observed that Center 4 is the center that showed less practices to be improved (Table IV).
Conclusion
Bronchopulmonary dysplasia is one of the most common long-term complications in very premature infants. Its incidence has been increasing over the past two decades in parallel with an improvement in the survival of this population.
BPD results from the interaction of multiple factors that can injure the immature lung. For this reason prevention must be based on the elimination of all the factors implicated in its pathogenesis.
The significant differences in BPD prevalence observed among centers, reflecting different practices in the management of ELBW infants, suggest that efforts must be put into developing and adopting better practices in BPD prevention.
The implementation of potentially better practices to reduce lung injury in neonates in Portuguese NICUs according to each NICU, must be addressed to increase the prescription of PNC, to use a lower FiO2, to be careful with fluid administration in the first weeks of life and to prevent PDA and sepsis.
Guidelines, recommendations or protocols must be followed to improve quality in the prevention of BPD.
Bibliography
1. Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 1967; 276(7):357-368.
2. Bancalari E, Abdenour GE, Feller R, Gannon J. Bronchopulmonary dysplasia: clinical presentation. J Pediatr 1979; 95(5 Pt 2):819-823.
3. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988; 82(4):527-532.
4. Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, Everette R, Peters N, Miller N, Muran G, Auten K, Newman N, Rowan G, Grisby C, Arnell K, Miller L, Ball B, McDavid G; National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004; 114(5):1305-1311.
5. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K; National Institutes of Child Health and Human Development Neonatal Research Network Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005; 116(6):1353-1360.
6. Sosenko IRS, Bancalari E. New developments in the presentation, pathogenesis, epidemiology and prevention of bronchopulmonary dysplasia. In: The Newborn Lung Eduardo Bancalari. Consulting Ed: Richard A Polin. Saunders Elsevier 2008: 187-207.
7. Crossing the Quality Chasm: A new health system for the 21st century. Washington DC: National Academy 2001.
8. Payne NR, LaCorte M, Karna P, Chen S, Finkelstein M, Goldsmith JP, Carpenter JH. Breathsavers Group, Vermont Oxford Network Neonatal Intensive Care Quality Improvement Collaborative. Reduction of bronchopulmonary dysplasia after participation in the Breathsavers Group of the Vermont Oxford Network Neonatal Intensive Care Quality Improvement Collaborative. Pediatrics 2006; 118(Suppl 2):S73-S77.
9. Payne NR, LaCorte M, Sun S, Karna P, Lewis-Hunstiger M, Goldsmith JP. Breathsavers Group. Evaluation and development of potentially better practices to reduce bronchopulmonary dysplasia in very low birth weight infants. Pediatrics 2006; 118 Suppl 2:S65-S72.
10. Burch K, Rhine W, Baker R, Litman F, Kaempf JW, Schwarz E, Sun S, Payne NR, Sharek PJ. Implementing potentially better practices to reduce lung injury in neonates. Pediatrics 2003; 111(4 Pt 2):e432-e436.
11. Sharek PJ, Baker R, Litman F, Kaempf J, Burch K, Schwarz E, Sun S, Payne NR. Evaluation and development of potentially better practices to prevent chronic lung disease and reduce lung injury in neonates. Pediatrics 2003; 111(4 Pt 2):e426-e431.
12. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respi Crit Care Med 2001; 163:1723-1729.
13. MacDonald H. American Academy of Pediatrics. Committee on Fetus and Newborn. Perinatal Care at the Threshold of Viability. Pediatrics 2002; 110:1024-1027.
14. Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New ballard score, expanded to include extremely premature infants. J Pediatr 1991; 119:417-423.
15. Rudolph AJ, Smith CA. Idiopathic respiratory distress syndrome of the newborn. J Pediatr 1960; 57:905-921.
16. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Ped Clin N Am 1986; 33:179-201.
17. An International classification of retinopathy of prematurity.Pediatrics 1984; 74:127-133.
18. The international classification of retinopathy of prematurity revisited. International Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol 2005; 123:991-999.
19. Papile LA, Burstein J, Burstein R. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birthweights less than 1500g. J Pediatr 1978; 92:529-534.
20. de Vries L, Rennie JM. Preterm brain injury. In: Rennie JM, Roberton NRC. Textbook of neonatology, 3rd edition. Churchill Livingstone. London 1999: 1252-1270.
21. Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 2003; 8(1):63-71.
22. Walsh M, Engle W, Laptook A, Kazzi SN, Buchter S, Rasmussen M, Yao Q. National Institute of Child Health and Human Development Neonatal Research Network. Oxygen delivery through nasal cannulae to preterm infants: can practice be improved? Pediatrics 2005; 116(4):857-861.
23. Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev 2007; 18(3):CD000065.
24. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006; 19(3):CD004454
25. National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: repeat courses – National Institutes of Health Consensus Development Conference Statement, August 17-18, 2000. Obstet Gynecol 2001; 98(1):144-150.
26. Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database Syst Rev 2007; 18;(3):CD003935.
27. Collaborative European Multicenter Study Group. Surfactant replacement therapy for severe neonatal respiratory distress syndrome: an international randomized clinical trial. Pediatrics 1988; 82(5):683-691.
28. Chotigeat U, Promwong N, Kanjanapattanakul W, Khorana M, Sangtawesin V, Horpaopan S. Comparison outcomes of surfactant therapy in respiratory distress syndrome in two periods. J Med Assoc Thai 2008; 91(Suppl 3):S109-S114.
29. Halliday HL, O´Neil CP. What is the evidence for drug therapy in the prevention and management of bronchopulmonary dysplasia? In: The newborn lung. Neonatal questions and controversies. Eduardo Bancalari. Ed Richard A Polin. Saunders Elsevier 2008:208-232.
30. Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs.selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev 2007; 17(4):CD003063.
31. Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev 2009;21(1):CD000141.
32. Ramanathan RJ. Surfactant therapy in preterm infants with respiratory distress syndrome and in nearterm or term newborns with acute RDS. Perinatol 2006; 26(Suppl 1):S51-S56; discussion S63-S64.
33. VLBW infants in Portugal. National Multicenter Study 1996-2000. Portuguese Neonatal Network. Bial Award of Clinical Medicine 2002. ISBN 972-99224-0-3.
34. Ramanathan R. Choosing a right surfactant for respiratory distress syndrome treatment. Neonatology 2009; 95(1):1-5.
35. Oh W, Poindexter BB, Perritt R, Lemons JA, Bauer CR, Ehrenkranz RA, Stoll BJ, Poole K, Wright LL; Neonatal Research Network. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr 2005;147(6):786-790.
36. Stephens BE, Gargus RA, Walden RV, Mance M, Nye J, McKinley L, Tucker R, Vohr BR. Fluid regimens in the first week of life may increase risk of patent ductus arteriosus in extremely low birth weight infants. J Perinatol 2008; 28(2):123-128.
37. Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in pre-term infants. Cochrane Database Syst Rev 2008; (1):CD000503.
38. Ramanathan R. Optimal ventilatory strategies and surfactant to protect the preterm lungs. Neonatology 2008; 93(4):302-308.
39. Bohlin K, Jonsson B, Gustafsson AS, Blennow M. Continuous positive airway pressure and surfactant. Neonatology 2008; 93(4):309-315.
40. Ramanathan R, Sardesai S. Lung protective ventilatory strategies in very low birth weight infants. J Perinatol2008;28(Suppl 1):S41-S46.
41. Patel D, Greenough A. Does nasal CPAP reduce bronchopulmonary dysplasia (BPD)? Acta Paediatr 2008; 97(10):1314-1317.
42. Saugstad OD. Take a breath – but do not add oxygen (if not needed). Acta Paediatr 2007; 96(6):798-800.
43. Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology 2008; 94(3):176-182.
44. Tin W, Gupta S. Optimum oxygen therapy in preterm babies. Arch Dis Child Fetal Neonatal. 2007; 92(2):F143-F147.
45. Sola A. Oxygen in neonatal anesthesia: friend or foe? Curr Opin Anaesthesiol 2008; 21(3):332-339.
46. STOP-ROP Multicenter Study Group. Supplemental therapeutic oxygen for pretreshold retinopathy of prematurity (STOP-ROP), a randomized controlled trial. I: Primary outcomes. Pediatrics 2000; 105:295-310.
47. Tin W, Wiswell TE. Adjunctive therapies in chronic lung disease: examining the evidence. Semin Fetal Neonatal Med 2008; 13(1):44-52.
48. Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med 2006; 11(5):354-362.
49. Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal 2008; 93(6):F455-F461.
50. Groneck P, Schmale J, Soditt V, Stützer H, Götze-Speer B, Speer CP. Bronchoalveolar inflammation following airway infection in preterm infants with chronic lung disease. Pediatr Pulmonol 2001; 31(5):331-338.
51. Rocha G, Proença E, Quintas C, Rodrigues T, Guimarães H. Chorioamnionitis and lung damage in the extremely low birth weight infant. Rev Port Pneumol 2007; 3(5):745-754. [ Links ]
52. Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr 1996;128(4):470-478.
53. Liljedahl M, Bodin L, Schollin J. Coagulase-negative staphylococcal sepsis as a predictor of bronchopulmonary dysplasia. Acta Paediatr 2004; 93(2):211-215.
54. Moro M, Pérez-Rodriguez J, Figueras-Aloy J, Fernández C, Doménech E, Jiménez R, Pérez-Sheriff V, Quero J, Roques V. Predischarge morbidities in extremely and very low-birth-weight infants in spanish neonatal units. Am J Perinatol 2008; 17.
55. Golombek SG, Sola A, Baquero H, Borbonet D, Cabañas F, Fajardo C, Goldsmit G, Lemus L, Miura E, Pellicer A, Pérez JM, Rogido M, Zambosco G, van Overmeire B; Primer Grupo de Consenso Clínico SIBEN (Golombek SG, Sola A, Clyman R, van Overmeire B, Goldsmit G, Natta D, Zambosco G, Miura E, Péerez JM, Weissheimer C, Baquero H, García Harker J, Oviedo Barrantes AN, Morgues M, Tapia JL, Domínguez F, Majano M, Cabañas F, Pellicer A, Cruz H, Fajardo C, Rogido M, Lemus L, Origel AV, Lacarruba JM, Lee M, Tresierra J, Guimarães H, Bustos R, Borbonet D, Perales JL. First SIBEN clinical consensus: diagnostic and therapeutic approach to patent ductus arteriosus in premature newborns. An Pediatr 2008; 69(5):454-481.
56. Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2008; 23;(1):CD003481.
57. Mosalli R, Alfaleh K. Prophylactic surgical ligation of patent ductus arteriosus for prevention of mortality and morbidity in extremely low birth weight infants. Cochrane Database Syst Rev 2008; 23;(1):CD006181.
58. McCurnin D, Seidner S, Chang LY, Waleh N, Ikegami M, Petershack J, Yoder B, Giavedoni L, Albertine KH, Dahl MJ, Wang ZM, Clyman RI. Ibuprofen-induced patent ductus arteriosus closure: physiologic, histologic, and biochemical effects on the premature lung. Pediatrics 2008; 121(5):945-956.
59. Lundqvist P, Källén K, Hallström I, Westas LH.Trends in outcomes for very preterm infants in the southern region of Sweden over a 10-year period. Acta Paediatr 2009; 98(4):648-653.
Correspondência/Correspondence to:
Hercilia Guimarães
Serviço de Neonatologia/Departamento de Pediatria – Hospital de São João
Alameda Professor Hernâni Monteiro
4202-451 Porto
Telephone: 00351 225095816
Fax: 225505919
E-mail: herciliaguimaraes@gmail.com
Recebido para publicação/received for publication: 09.06.29
Aceite para publicação/accepted for publication: 09.09.08













