Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Portuguesa de Pneumologia
Print version ISSN 0873-2159
Rev Port Pneumol vol.15 no.6 Lisboa Nov. 2009
Reclassifying bronchial-pulmonary carcinoma: Differentiating histological type in biopsies by immunohistochemistry
Lina Carvalho 1
Abstract
The current state of molecular knowledge on lung cancer demands a histological classification which goes beyond small-cell and non-small-cell carcinoma to provide support for tailored therapy in aiding in understanding of the drugs currently available.
As diagnosis and follow-up in the vast majority of lung cancer cases is based on biopsies and cytology samples, Immunohistochemical Bronchial Pulmonary Carcinoma Classification (IBPCC) is necessary to reveal the raft of characteristics available. This provides morphological support for the WHO’s 1999/2004 classification, in addition to an understanding of carcinogenesis.
The immunohistochemical panel clarifies the main morphology and cytology characteristics to maintain the leading histological types as squamous cell carcinoma (high weight molecular cytokeratins/HWMC), adenosquamous carcinoma (CK7, TTF1, HWMA), neuroendocrine carcinoma (Chrg, Syn, CD56, TTF1, Ki67), adenocarcinoma (CK7, CK20, TTF1) and bring the polymorphic and pleomorphic carcinomas under a single banner of pleomorphic carcinoma (Ck7, TTF1, HWMC, VMT, Desmin, Actin) which shelters large cell carcinomas and sarcomatoid carcinomas.
Lung cancer chemotherapy will still be based on platinum and gemcitabine for the near future and the IBPCC is a simple and efficient tool for streamlining the registration of lung cancer histological characteristics in biopsies and other reduced samples to support clinical evidence and trials.
Key-words: Bronchial-pulmonary carcinoma, immunohistochemistry.
Reclassificação do carcinoma broncopulmonar: Diferenciação do tipo histológico em biópsias por imuno–histoquímica
Resumo
Os conhecimentos actuais da patologia molecular do cancro do pulmão requerem outra caracterização histológica, para além de carcinoma de células pequenas e carcinoma não pequenas células para suporte da terapia personalizada e entendimento do valor real dos fármacos actualmente disponíveis.
Como o diagnóstico e seguimento clínico da maioria dos casos de cancro do pulmão se baseia em produtos de biópsia e citologia, a classificação imunoistoquímica do carcinoma broncopulmonar (IBPCC) é necessária para suporte morfológico da classificação da WHO 1999/2004, clarificando as características celulares das neoplasias e o entendimento da carcinogénese.
O painel imunoistoquímico reforça os tipos histológicos principais do carcinoma bronco – pulmonar: carcinoma epidermóide (queratinas de alto peso molecular – HWMC), carcinoma adenoscamoso (CK/TTF1, MWMC), carcinoma neuroendócrino (Chrg, Syn, CD56, TTF1, Ki67) e adenocarcinoma (CK7, Ck20, TTF1); as variantes do carcinoma de células grandes e do carcinoma sarcomatóide são englobados num único grupo de carcinomas pleomórficos (CK7, TTF1, HWMC, VMT, desmina, actina), onde cabe o polimorfismo e o pleomorfismo celular.
A quimioterapia do carcinoma broncopulmonar continuará baseada no platino e na gemcitabina no futuro próximo e a IBPCC será uma ferramenta simples e eficiente para o registo das características e tipos histológicos do carcinoma do pulmão presentes nas biopsias e amostras citológicas para suporte da evidência clínica e dos ensaios farmacêuticos.
Palavras-chave: Carcinoma broncopulmonar, imunoistoquímica.
Introduction
Pathology is far from what it can be considered as to have been its beginning in the XVIII century by the first descriptions of Morgagni and further by Virchow with the use of the microscope. What is important to have in mind is the fact that the survival age was in the thirties years of age, even from the time of Hippocrates, Celsius and Galen. The Egyptians left their papyrus, namely Ebers´ (Amenhotep Kingdom 1557 – 1501BC) with exhaustive reports of infections and tumours, although benign, but related to the different parts ofthe body. Medicine began at Cos School in Greece (500BC), from where the Corpus Hipocra ticus emanated, followed by the De Artibus of Aulus Cornelius Celsius from Verona – Italy. The Arabs had the possibility to ensemble the two knowledges while divagating around the Mediterranean during their struggles, making then a wealthy synthesis gathering also the Persian and Indian know ledge. Kanum is really a joint canon made then by Ibis Sina/Avicena (980-1037). By then confidence was achieved in recogni zing malignant tumours and metastasizing1.
The actual known scientific method comes in the catholic/medieval time with Galileo (1564-1634) and Kepler (1571-1635), succeeding Copernicus (1473) and having as starting point the greec-italian-arab-indu knowledge since the Salerno School (XI century).
The Magister Salernus as we get to know today was probably written by a Jew, an Arab, a Greek and a Christian. The Tumoribus Praeter Naturum written by Gabriel Falopius (1523-1562) is the first pathology book and the microscope used by Malpighi (1562-1682) helped in rooting the anatomy bases.
Histology encased in between by the eyes of Bichat (1771-1802) together with anatomy and pathology, reinforced by René Laennec (1781-1826). The expertise of Rudolf Virchow (1821-1826) with an acromatic microscope (1830) brought the zell/cell to the medical practice1-3.
This way we get to the contemporary medicine we can consider to have its beginning in 1953 with the DNA double helix of Watson and Creek and in fifty years we jumped into the molecular pathology and modern medicine with a huge advance in disease therapy, gathering all technical methodologies now available. At this point is the demanding diagnosis of bronco-pulmonary carcinoma made on small biopsies (or a couple of cells) submitted to the WHO 1999/2004 classification4-5.
We owe to the Sweden doctor Leiv Kreyberg the first histological characterization of bronchial-pulmonary carcinomas done in 1954 after studying carbon mines workers. These patients exhibited the illnesses dependent on mines environment: carbon lung and silicosis together with tuberculosis and developing lung cancer very frequently. Smoking accelerated the incidence of lung cancer as we know today. In 1967, the Organisation Mondiale de la Santé supported the first work directed to the standardization of tumoural nomenclature adapted to each organ to discipline Pathologists that frequently renamed differently the same lesion. Types Histologiques des Tumeurs du Poumon was the blue book number 1 of the WHO blue book series directed to the histological classifications of neoplasias in each organ2,6.
The incidence of lung cancer is far from diminishing its incidence although the habit of smoking is clearly decreasing and still 60 to 70% of new lung cancer cases are diagnosed in non surgical stages with 10 to 12 months probability of survival. This situation and the poverty of survival rates achieved with the possible therapy conducting to a high mortality raised the general histological reference of small cell carcinoma and non small cell carcinoma in Pathologists´ reports as the first histological type meant non surgical behaviour because of very poor prognosis compared with the other types. As molecular pathology is developing towards predictive therapy which means personalized choice dependent on carcinoma histological type and quite defined particularities, the diagnosis has to be made with approximate precision in the small biopsies obtained by endobronchial examination, transthoracic biopsies and even in groups of small number of neoplastic cells of cytological methods7-10.
The use of immunohistochemial antibodies and the experience acquired in reporting lung cancer in surgical specimens based in total inclusion in paraffin of tumours, have conducting either to WHO classification and to the recognition of the possible different patterns of bronchial-pulmonary carcinomas that can be explored in small biopsies11,12.
Lung cancer classifications till WHO 1999/2004
The morphological descriptions of lung carcinomas have been refined since Kreyberg and the OMS/1967 classification with maintenance of the histological types, firstly described in five groups, revealing a large heterogeneity recognized ab initio:
1. adenocarcinomas and epidermoid epitheliomas;
2. carcinoids;
3. solid tumours with or without mucus and giant cell and clear cell epitheliomas;
4. Small cell anaplastic epitheliomas and subtypes (and mixed tumours where combined carcinomas and neuroendocrine large cell tumours were reported);
5. Non-classifiable tumours neither by pattern nor by cell type. The following reorganizations of criteria reported in 1976 and 1981 preserved the initial nomenclature and the OMS/1981 written in French reports the actual histological types for malignant epithelial pulmonary tumours:
Epidermoid carcinoma
Small cell carcinoma
Adenocarcinoma
Large cell carcinoma
Adenosquamous carcinoma
Carcinoid
Bronchial glands carcinoma
In a special group – IV. Tumeurs Divers – carcinosarcoma and pulmonary blastoma were recognized. Spindle cell carcinoma and other neuroendocrine tumours were included in the groups of epidermoid carcinoma and small cell carcinoma respectively6.
The WHO 1999/2004 classifications maintained the same histological groups and basic knowledge in genetics was added to the new Blue Book format of the in English written WHO editions5.
Beyond establishing criteria for recognition of patterns and histological types, the actual classification has also commitment with prognosis and predictive studies of molecular pathology have definitely shown a correlation between morphology and prognosis outcome directing the choice of therapy13,14.
As the available molecular studies can not be forgotten when reporting small biopsies and cytological smears, the morphological criteria have to be applied as sharply as possible to neoplastic cells to be reliable and exclusive as this tumoural representation is kept in 60 to 70% of cases in non-surgical stages at the time of diagnosis, when therapy has to be decided15.
Small cell carcinomas, epidermoid carcinomas and adenocarcinomas offer no problems to be classified even in cases where differential diagnosis between primary and secondary adenocarcinomas has to be. The last situation is reasonably solved by applying morphological criteria and the currently used immunohistochemical antibodies CK 20 (digestive adenocarcinomas) and CK7 and TTF1 (pulmonary adenocarcinomas).
Meanwhile the recognition of more variants to be included in the WHO recognized groups reinforce the need of recurring to embryology and cellular type knowledge to decide rigorous classification and differential diagnosis.
Still in the actual classification, repetitions of cellular types under different histological types and the recognition of large cells, giant cells, spindle cells and inflammatory cells in small biopsies raise the utility of a new future classification with synthesis of language and grouping.
Differentiating histological types in biopsies by immunohistochemistry Squamous cell carcinoma
The variants of epidermoid carcinoma actua lly recognized include papillary, clear cell, small cell and basaloid morphologies.
Only the first one can be recognized in small biopsies without the use of immunohistochemistry (IHC) when clear basal membrane invasion is obvious together with the well differentiated pattern with extensive keratinisation16.
The other patterns and poorly differentiated epidermoid carcinoma – not exhibiting keratinisation and justifying the title of epidermoid carcinoma instead of squamous cell carcinoma – require use of IHC17,18. High-weight molecularcytokeratins(HWMC – LP34/34ßE12/…) are of daily use as adenocarcinomas of the bronchus or of the lung do not express those keratins that are expressed by metaplastic pavimentoid cells in respiratory epithelium, where normally a clear basal cell positive layer is distinctly defined till the epithelium of the bronchioles where it is absent (Fig. 1).
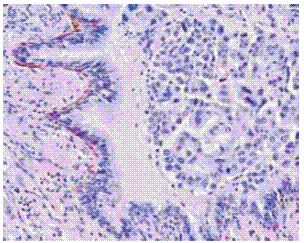
Fig. 1 – High weight molecular cytokeratins in basal bronchial cells. Mixed type adenocarcinoma. 34ß E12 X 200
Also TTF1 (Thyroid Transcription Factor 1) and CK7 are consistently negative in epidermoid carcinomas and if positive, the heterogeneity of cell types has to be considered and reported to make the diagnosis of combined small cell carcinoma or adenosquamous carcinoma and then validate therapy and prognosis19. The basaloid variant can only be reported as a pattern if no keratinisation is present as the basaloid carcinoma included in the large cell carcinoma group has poorer prognosis. CK7 may be expressed together with HWMC in these carcinomas and their rarity has also to be kept in mind.
Small cell variant of epidermoid carcinoma has become easily recognisable when TTF1 and Ki67 proliferation marker are searched together with HWMC. A Ki67 proliferation index higher than 80% together with clear expression of HWMC indicates a combined small cell carcinoma and epidermoid carcinoma; TTF1 may show the same quantity of nuclear expression or lower in this situation.
The WHO classification does not embrace the cellular capacities determined by IHC in small pieces of tissue as it has been done considering surgical specimens where poorly differentiated areas of carcinomas were not considered to be reported and not explored as it would have made a much more complex classification – the 10% law20.
Adenocarcinoma
Heterogeneity of primary bronchial-pulmonary adenocarcinomas and also of sarcomatoid and large cell carcinomas of the lung is related to embryology and multiple bronchial – pulmonary cell types with different functions are now under the explanation of the epithelial mesenchimal transition (EMT) theory21.
As referred for squamous cell carcinoma, HWMCs delineate the basal cell bronchial layer till bronchioles where ciliated cells continue to express CK7 and alveolar cells, CK7 and TTF1 focally. Cell types vary from cuboidal to columnar, with either variable mucinous cytoplasm and basal or hobnail nuclei22-24.
The expression of CK20 by adenocarcinomas has to be related with the common embryological origin of upper and lower airways together with anterior intestine as it does not occur in mature respiratory epithelium.
These primary carcinomas when expressing CK7 without TTF1, define intestinal carcinomas and having histogenetical dependence from respiratory epithelium might well be called bronchial adenocarcinomas25,26. The histogenesis of bronchial-pulmonary adenocarcinomas has long taken the attention of Pathologists and Researchers as firstly mature cells were not supposed to give origin to malignant cells in respiratory epithelium where epidermoid carcinoma develops from epidermoid metaplasia and small cell carcinoma, from one or various types of related cells with neuroendocrine variable expression not yet clearly defined beyond the high proliferation rate27,28.
Meanwhile bronchial and bronchiolar preinvasive lesions can be demonstrated for ade nocarcinoma as: basal cell hyperplasia, papillary hiperplasia, papillary metaplasia and bronchiolar columnar cell dysplasia (BCCD) (Fig. 2)29,30.
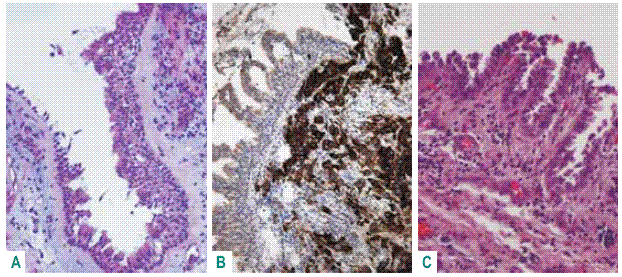
Fig. 2 – Bronchial preinvasive lesions: basal cell hyperplasia HE X 100; papillary hyperplasia CK20 X 100; papillary metaplasia HE X 100
Noguchi (Cancer 1995) called attention for small adenocarcinomas while in Europe still large tumours were diagnosed. Those small adenocarcinomas are of mixed type, with or without predominance of bronchioloalveolar (BAC) non-mucinous pattern with different prognosis, better when predominant non-mucinous BAC is present. Neoplastic cells express CK7 and TTF1, independently of the present patterns, even when concerning a pure acinar or papillary adenocarcinoma, this histological type long known to have the poorest prognosis among adenocarcinomas31-33.
Terminal respiratory unit adenocarcinoma described by Noguchi do not overtake 3cm diameter, has central desmoplastic stroma with tumoural acinar pattern, surrounded by papillary, micropapillary, acinar or nonmucinous BAC, keeping fidelity to CK7 and TTF1 nuclear expression and negativity to the other IHC markers (Fig. 3)34-35.
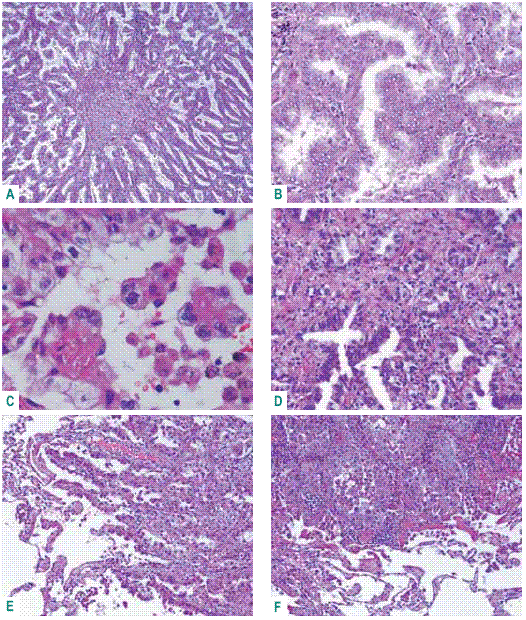
Fig. 3 – Terminal respiratory unit adenocarcinoma. A: central desmoplasia; B: true papillae; C: micropapillae; D: acinar pattern; E – F: peripheral acinar and papillary growth
The above referred theory is extended to larger adenocarcinomas where less organized patterns are present and then the TTF1 nuclear expression may be lost while CK7 is constant and this event has to be taken as very important when reporting bio psies as the less differentiated patterns determine a poorer prognosis and are often gathered in lymphatic vessels of bronchial biopsies36,37.
Differential diagnosis with mesothelioma keeps being a challenge when clinicians forget to refer pleural expansion without the presence of a definite mass and after reporting adenocarcinoma CAT does not show the tumour. The classical pseudo-mesotheliomatous adenocarcinoma has become rare.
Nowadays calretinin keeps being the most reliable antibody for malignant mesothelial cells together with cytokeratins (CK5/6 or CK7) to mark spindle mesotheliomatous cells. The use of anti-glicoproteins antibodies as CEA and Ber-EP4 is needed to discard adenocarcinoma and cytoplasmic membrane reinforcement by EMA in malignant mesothelial cells is reliable38-41.
BAC keeps being controversial as considered an in situ carcinoma in WHO classification and then reported with metastases in some series. Nonetheless atypical adenomatous hyperplasia is the preinvasive lesion of non-mucinous type that is also the pattern found in mixed type adenocarcinoma while pure mucinous BAC is multifocal and often bilateral. The mixed non-mucinous and mucinous type is seen in larger lesions when desmoplastic reaction is present with acinar invasion and then related to lymph node metastases. This last situation is no more a BAC in WHO classification but a mixed type adenocarcinoma42.
The IHC expression of BAC is also variable as limited to CK7 and TTF1 in non-mucinous type and revealing CK20 and scarcity of CK7 and no TTF1 in some cases of mucinous BAC. This IHC pattern can be related to an intestinal mucinous BAC that can be multifocal and distinct from bronchial adenocarcinoma (Fig. 4)43.
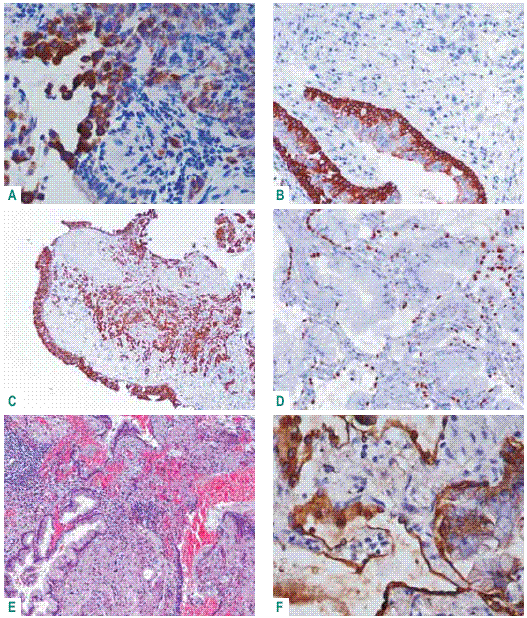
Fig. 4 – Bronchial adenocarcinoma – A: positive CK20 in malignant cells, not expressed in respiratory epithelium; B: CK 34ßE12 in current basal cells; C: CK7 revealing microacinar pattern (TTF1-). BAC – mucinous type – D: TTF1 -; E: intestinal glandular type; F: CK20 scarce positivity (CK7+)
No descriptions are available till now to distinguish gender patterns and it is well stated that pulmonary adenocarcinomas in women have a better prognosis. The feminine adenocarcinomas may show particular patterns as hepatoid pattern and oestrogen, progesterone and variable cytoplasmic membrane glicoproteins IHC expression.
When in small biopies an endometrioid pattern is suggested (Fig. 5). The immunohistochemical patterns of adenocarcinomas raise the possibility of defining a classification as si mple as the categories of IHC antibodies expressed, assumed in a small panel useful to define the platform for predictive therapy44-48.
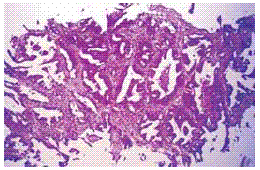
Fig. 5 – Adenocarcinoma - «endometrioid» acinar pattern. Female 62 years old; peripheral left lower lobe tumour (CAT) and bone metastases. HE X 100
Neuroendocrine tumours
Depending on patient age different small cell tumours raise differential diagnosis solved by IHC and complementary analysis to distinguish from small cell carcinoma. For this histological type, after nuclear crushing artefact, Ki67 proliferation index is the most reliable marker revealing more than 80% positive malignant nuclei. The alveolar cell maker TTF1 is also expressed by these tumours but not consistently. While chromogranin and synaptophisin expression are constant in carcinoids, CD56 is more prone to small cell carcinoma49,50.
Small cell carcinoma is keratin temperamental varying the expression from null till high expression of HWMC or CK7. In these cases a combined small cell carcinoma and squamous cell carcinoma or adenocarcinoma has to be taken in mind in small biopsies and careful attention given to bronchial epithelium that may reveal also positive TTF1 and Ki 67 dysplasia (Fig. 6)51,52.
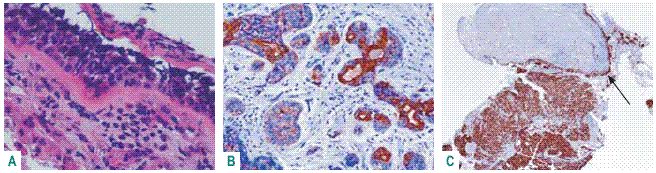
Fig. 6 – Combined small cell carcinoma and adenocarcinoma – A: neuroendocrine epithelial dysplasia; B: CK7 revealing acinar pattern; C: Ki67 100% positive malignant nuclei and characterizing neuroendocrine epithelial dysplasia (arrow)
Neuroendocrine carcinomas in the lung concern typical and atypical carcinoids and large cell neuroendocrine carcinoma beyond small cell carcinoma and histological criteria are clearly defined in the WHO 2004 classification. It is important to recognize typical carcinoid lymph node metastases as this may occur till 14% of the cases53,54.
The thyroid transcription factor 1 was also found in type II pneumocytes and connects an adenocarcinoma to the lung but also characterizes small cell carcinoma. Usually carcinoids do not express that factor that has also been understood as a proliferation marker. The proliferation index validated by Ki67 in small and artefactual biopsies is mandatory to distinguish typical and atypical carcinoid (<4-10%), large cell neuroendocrine carcinoma (20-50%) and small cell carcinoma (>80%) together with neuroendocrine markers usually55,56.
Adenosquamous carcinoma
Not many concern has been given to this pulmonary carcinoma but in small biopsies it is common to have HWMC and CK7 expression in neoplastic cells. When carefully observed it is possible to see in the pavementous/epidermoid cellular clusterings, luminal drafts with both different expression and patterns of those cytokeratins.
This way adenosquamous carcinoma may present in a spectrum from a well differentiated type to moderately differentiated and poorly differentiated types keeping the expression of CK7 and HWMC in separate and/or in the same cells, with combined patterns. Usually TTF1 is absent but if present the cell type has to be considered to raise the possibility of a combined small cell or a TRU adenosquamous carcinoma, depending on Ki67 nuclear rate57. It has to be kept in mind that salivary gland tumours have epidermoid and glandular patterns that are not mistaken for adenosquamous carcinomas. Also the cam 5.2 marker and androgen and oestrogen receptors are expressed in those tumours.
Pleomorphic carcinomas
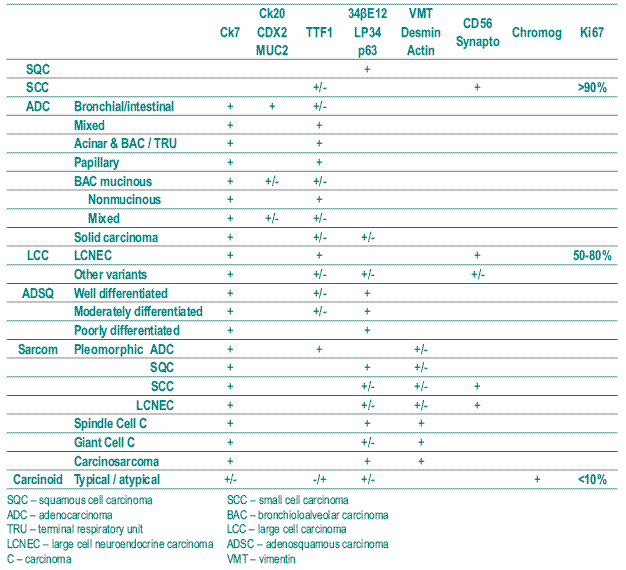
The confidence in IHC to classify bronchial – pulmonary carcinomas is defined in Table I (empty spaces mean negativity) based in the WHO 2004 classification. Then large cell carcinomas and sarcomatoid carcinomas have to be joined under the designation of pleomorphic carcinomas because of three reasons: the present approach is a result of working mostly in biopsies beyond surgical specimens; pleomorphism (different cell types) and polymorphism (one cell type with various forms) interface each other in the two groups of large cell and sarcomatoid carcinomas; lastly and to reinforce the two mentioned reasons, large cells and clear cells are seen everywhere in the previous histological types and need IHC (and mucinous stains PAS and Alcian blue) to allocate them.
Table I – Bronchial – Pulmonary carcinoma: Histological typing and immunohistochemistry
The bronchial-pulmonary carcinoma grouping is designed in Table II where morphology and adequate IHC panel are ensemble in order to be used in cytology, biopsies and surgical specimens reporting.
Table II – Bronchial-pulmonary carcinoma – Immunohistochemical classification

The utility of this classification can be tested in a biopsy of a poorly differentiated squamous cell carcinoma exhibiting large cells that keep expressing only HWMC confirming that grading carcinomas is not important. Also, as large cells may represent pleomorphic adenocarcinomas, only CK7 expression is expected; on the other hand, the expression of CK7 and HWMC toge ther point to poorly differentiated adenosquamous carcinoma. The TTF1 nuclear positive staining keeps a good marker to recognize pulmonary carcinomas with varia ble patterns.
It is important to verify that the columnar and/or cuboidal cells of an adenocarcinoma component in a pleomorphic carcinoma also express vimentin in an irregular way.
This may be important to recognize prognosis and histogenesis in future. By now, vimentin, desmin and smooth muscle actin have to be expressed (one positive antibody is sufficient) to define a pleomorphic carcinoma independently of the observed histological patterns58.
Discussion
The Pathologist concern is beyond the diagnosis of non-small cell or small cell carcinoma when reporting small biopsies or cytology specimens and IHC became an easy and useful tool. Cell morphology and histological types and patterns are leaders of reporting and rationalize the choice of limited IHC panel. This concern goes further than diagnosis as important therapeutic drugs are revealing prognostic value based in bronchial and pulmonary carcinoma histology59-62.
The IHC panel defined in Table I and quoted in the proposed immunohistochemical bronchial-pulmonary carcinoma grouping/classification – IBPCC – in Table II is widely used as the applied antibodies are reliable and easily validated. The usefulness of the presenting IBPCC relies in its simplicity of understanding cell types and cellular potentialities either in bronchial cylindrical and ciliated epithelium and also in carcinomas arising in the TRU unit or when presenting as solid tumours of heterogeneous patterns and cellularity. This work may be a refining of the attempt made by Dr Edwards in 1987 when ICM was not so accessible63.
The IBPCC raises from the knowledge acquired in reporting surgical specimens after observing loads of slides of the whole sectioned tumour. An adenocarcinoma of the lung does not express HWMC and even in the hilum where intestinal adenocarcinomas develop more frequently, CK20 and CK 7 are exclusive with a variability of the nuclear presence of TTF1. Also when acinar and/or papillary paterns are present in a small biopsy the expression of TTF1 and CK20 persist in the surgical specimen where complementing patterns of a mixed adenocarcinoma show up.
BAC is a challenging group of tumours whose macroscopy and histology has not yet been definitely corroborated. The mucinous type expressing CK20 and non-mucinous type with hobnail and Clara cells expressing CK7 and TTF1, with mixed types in between, as prone intestinal type with mucinous papillae or as gathering CK20, CK7 and TTF1 expression, have different therapeutical, staging and prognostic effects.
The first group known to be multifocal and with bilateral potentiality and the other types more often an unique mass, with or without central desmoplasia, defy the WHO criteria.
The above supposed purity changes when HWMC or other markers need to be included according with morphology beyond well differentiated squamous cell carcinoma.
When neoplastic cells express CK7 uniformly and TTF1 and HWMC in clusters, adenosquamous carcinoma is a correct diagnosis. Signet ring cells can be seen in this context. Whether scales or prickles are absent squamous cell carcinoma keeps being well characterized by expressing only HWMC.
Differential diagnosis of lymphoma, PNET, synovial sarcoma … have to be taken in account when dealing with small cell tumours and consider small cell carcinoma in the group of pulmonary neuroendocrine carcinomas leaving tumorlets behind as a preinvasive lesion in the development of carcinoid.
Again TTF1 plays an important role together with Ki67 as proliferation predictors in small cell carcinoma and large cell neuroendocrine carcinoma. Some small cell carcinomas present with lnger survival and still no explanation is adequate. It is fantasy admit that these cases might be poorly combined small cell carcinomas where CK7 and HWMC are expressed at cellular level without pertinent patterns as understood for ade nosquamous carcinomas64,65.
The mesenchimal antibodies more often used are vimentin, actin and desmin that are not expressed in adenocarcinomas, neuroendocrine carcinomas and epidermoid differentiating carcinomas. Better than considering two independent groups of large cell carcinoma and sarcomatoid carcinoma, still subdivided, or quote them together as poorly differentiated carcinomas, an unique group of pleomorphic carcinoma (Table II) shows to be either comprehensive and feasible as IHC is rationalized to cover all the listed histological types.
Grouping these carcinomas as poorly differentiated carcinomas might be minimalist as in these rare histological types may reside knowledge concerned with histogenesis and allowing the understanding of the actual theories of carcinogenesis meaning specific differentiation.
Pathologists fight with understanding where and why started a metaplasia or dysplasia to help in Preventive Medicine. In bronchial – pulmonary carcinoma some architectural alterations are coming to attention and being reported to help clinicians to follow patients with more expertise. In Table III a potential group of preinvasive lesions is recognized and data has to be gathered to recognize them in small biopsies.
Table III – Bronchial-pulmonary pre-invasive lesions

Embriogenesis may conduct our research to understand polarized epithelial phenotype together with highly motile mesenchimal phenotype as is observed in pleomorphic carcinomas, together when carcinomatous patterns are evident or independently by recognizing solid or sarcomatoid patterns that should not be called poorly differentiated.
This understanding can be again translated to cellular level and revealed by immunohistochemistry. Two good examples for illustrating this spectrum are lymphoepiteliomalike carcinoma and solid adenocarcinoma.
The first case might be considered a poorly differentiated squamous cell carcinoma because there is HWMC cellular expression and is known to have a better prognosis than the other histolical subtypes included in the large cell carcinoma. The second example fluctuates between a solid adenocarcinoma with mucin production and solid adenocarcinoma expressing TTF1 focally beside discrete large cells or small spindle cells with cytoplasmic vimentin39.
At the bottom of the group carcinosarcoma and blastoma polarize epithelial to mesenchimal transiton theory for carcinogenesis and embryogenesis potentiality respectively.
Spindle cell and giant cell patterns reinforce the cellular level capacities and the IBPCC already discussed67,68.
The IBPCC is a useful tool as molecular pathology is coming to the pathologist bench demanding accurate morphology recognition based in small tissue or cell samples dependent on an IHC panel that has to be reported having in mind reliable prognosis outcome69,70.
Predictive therapy is already a reality in some pulmonary pathology centres and it is a result of trials that gather therapy, gene expression and/or mutations and prognosis.
Still the commoner applied language is small cell carcinoma and non-small cell carcinoma with already cellular morphological and immunohistochemical bias. In the application and distinction between clinical outcomes from the applied therapies, first and second lines, a second biopsy may become mandatory to revalidate cellular characteristics to predict therapy answer39, 71, 72.
Still an enormous effort has to be done in order to understand the multidirectionality importance of each morphological pattern in each histological type of lung cancer when observing surgical specimens by descending to molecular level. After this laborious task more knowledge will be brought to cellular level characterization when diagnosing in small samples or neoplastic cells73-76. The complementary or primordial approach may reside in the neoplastic cells entourage concerning immune cells and mesenchymal cells wondering for stem cells and predictive therapy for these targets is already on the route by blocking angiogenesis and growing factors that make a wide net77-80.
Bibliography
1. Bernarda RA. Cancro: Cronologia histórica e génese de ideias. Faculdade de Medicina da Universidade de Coimbra. Gráfica da Lousã 1996.
2. Kreyberg L. Histological lung cancer types. Acta Pathologica e Microbiologica Scandinavica 1962; 157: 1 - 93.
3. Kreyberg L, Liebow AA, Uehlinger EA. Histological typing of lung tumours. Geneva, World Health Organization 1969. International Histological Classification of Tumours, n.º 1.
4. The World Health Organization histological typing of lung tumours . second edition 1981. Am J Clin Pathol 1982; 77:123 -136.
5. Travis W, Brambilla E, Muller-Hermelink HK, Harris C. Tumours of the lung, pleura, thymus and heart. Pathology and Genetics. World Health Organization Classification of Tumours, IARCPress, Lyon 2004.
6. Sakao Y, Miyamoto H, Oh S, et al. The impact of cigarette smoking on prognosis in small adenocarcinomas of the lung: the association between histologic subtype and smoking status. J Thorac Oncol 2008; 3: 958 -962.
7. Zhu CQ, da Cunha SG, Ding K, et al. Role of KRAS and EGFR as biomarkers of response to erlotinib in National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 2008; 26:4268-4275.
8. Hirsch FR, Herbst RS, Olsen C, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non -small -cell lung cancer patients treated with cetuximab and chemotherapy. Clin Oncol 2008; 26:3351-3357.
9. Carvalho L, Cardoso E, Nunes H, Baptista V, Gomes A, Couceiro P. Projecto de estadiamento do cancro do pulmão pela IASLC: Estudo comparativo entre a 6.ª edição TNM em vigor e a 7.ª edição proposta. Rev Port Pneumol 2009; 15(1):67-76. [ Links ]
10. Varella -Garcia M, Mitsudomi T, Yatabe Y, Kosaka T, et al. EGFR and HER2 genomic gain in recurrent non-small cell lung cancer after surgery. J Thorac Oncol 2009; 4(3):318-325.
11. Seve P, Lai R, Ding K, et al. Class III beta –tubulin expression and benefit from adjuvant cisplatin/vinorelbine chemotherapy in operable non-small cell lung cancer: analysis of NCIC JBR 10. Clin Cancer Res 2007; 13:994-999.
12. Seve P, Reiman T, Lai R, et al. Class III beta –tubulin is a marker of paclitaxel resistance in carcinomas of unknown primary site. Cancer Chemother Pharmacol 2007; 60:27-34.
13. Hewitt SM, Lewis FA, Cao Y, et al. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med 2008; 132:1929-1935.
14. Giovannetti E, Mey V, Nannizzi S, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small -cell lung cancer cells. Mol Pharmacol 2005; 68: 110-118.
15. Izbicki JR, Passlick B, Hosch SB, et al. Mode of spread in the early phase of lymphatic metastasis in non-small-cell lung cancer: significance of nodal micrometastasis. J Thorac Cardiovasc Surg 1996; 112: 623 -630.
16. Ceppi P, Volante M, Saviozzi S, et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006; 107:1589-1596.
17. Carvalho L. Wild type of exons 19 and 21 and polysomy of chromosome 7 were defined for EGFR gene in squamous cell carcinoma of the lung. Journal of Thoracic Oncology 2008; 3(4)S1:S35-S36.
18. Sousa V, Silva M, Alarcão A, Couceiro P, Gomes A, Carvalho L. Squamous cell carcinoma of the lung: polysomy of chromosome 7 and wild type of exon 19 and 21 were defined for EGFR gene. Virchows Archiv 2008; 452 (S1):S1.
19. Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond) 2009; 116:27-35.
20. Araujo A, Barata F, Parente B, et al. Pemetrexed in second line treatment of non -small cell lung cancer . The Portuguese experience. Rev Port Pneumol 2008; XIV (Supl 2): S9-S20. [ Links ]
21. Kerr KM. Pathologist and molecular biologist, ever the twain shall meet? Lung Cancer 2009; 63:161-163.
22. Oliveira AM, Tazelaar HD, Myers JL, Erickson LA, Lloyd RV. Thyroid transcription factor -1 distinguishes metastatic pulmonary from well -differentiated neuroendocrine tumors of other sites. Am J Surg Pathol 2001; 25:815-819.
23. Ramirez MI, Rishi AK, Cao YX, Williams MC. TGT3, thyroid transcription factor I, and Sp1 elements regulate transcriptional activity of the 1.3 -kilobase pair promoter of T1alpha, a lung alveolar type I cell gene. J Biol Chem 1997; 272:26 285-26 294.
24. Ngan ES, Lang BH, Liu T, et al. A germline mutation (A339V) in thyroid transcription factor -1 (TITF -1/NKX2.1) in patients with multinodular goiter and papillary thyroid carcinoma. J Natl Cancer Inst 2009; 101: 162-175.
25. Ninomiya H, Hiramatsu M, Inamura K, et al. Correlation between morphology and EGFR mutations in lung adenocarcinomas Significance of the micropapillary pattern and the hobnail cell type. Lung Cancer 2009; 63:235-240.
26. Nakazato M, Chung HK, Ulianich L, Grassadonia A, Suzuki K, Kohn LD. Thyroglobulin repression of thyroid transcription factor 1 (TTF -1) gene expression is mediated by decreased DNA binding of nuclear factor I proteins which control constitutive TTF -1 expression. Mol Cell Biol 2000; 20: 8499-8512.
27. Motoi N, Szoke J, Riely GJ, et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol 2008; 32: 810-827.
28. Kerr K. Pulmonary adenocarcinomas: classification and reporting. Histopathology 2009; 54: 12 . 27.
29. Ullman R, Bongiovanni M, Halbwedl I, Petzmann S, Gogg -Kammerer M, Sapino A, Papotti M, Bussolati G, Popper H. Bronchiolar columnar cell dysplasia-genetic analysis of a novel preneoplastic lesion of peripheral lung. Virchows Archiv 2003; 442(5):429-436.
30. Lantuéjul S, Salameire D, Salon C, Brambilla E. Pulmonary preneoplasia . sequential molecular carcinogenetic events. Histopathology 2009; 54:43-54.
31. Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995; 75: 2844-2852.
32. Silver SA, Askin FB. True papillary carcinoma of the lung: a distinct clinicopathologic entity. Am J Surg Pathol 1997; 21:43 -51.
33. Anagnostou VK, Syrigos KN, Bepler G, Homer RJ, Rimm DL. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol 2009; 27:271-278.
34. Noguchi M, Minami Y, Iijima T, Matsuno Y. Reproducibility of the diagnosis of small adenocarcinoma of the lung and usefulness of an educational program for the diagnostic criteria. Pathol Int 2005; 55:8 -13.
35. Sakurada A, Tsao MS. Predictive biomarkers for EGFR therapy. I Drugs 2009; 12:34 -38.
36. Goldstein NS, Mani A, Chmielewski G, Welsh R, Pursel S. Immunohistochemically detected micrometastases in peribronchial and mediastinal lymph nodes from patients with T1, N0, M0 pulmonary adenocarcinomas. Am J Surg Pathol 2000; 24:274-279.
37. Pohlenz J, Dumitrescu A, Zundel D, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest 2002; 109:469-473.
38. Parente B. Mesothelioma treatment. Rev Port Pneumol 2008; XIV (Sup.2):S35-S44 [ Links ]
39. Brock MV, Hooker CM, Ota-Machida E, et al. DNA methylation markers and early recurrence in stage I lung cancer. N Engl J Med 2008; 358: 1118 -1128.
40. Beasly MB. Immunohistochemistry of pulmonary and pleural neoplasia. Arch Pathol Lab Med 2008, 132:1062-1072.
41. Addis B, Roche H. Problems in mesothelioma diagnosis. Histopathology 2009; 54:55-68
42. Terasaki H, Niki T, Matsuno Y, et al. Lung adenocarcinoma with mixed bronchioloalveolar and invasive components: clinicopathological features, subclassification by extent of invasive foci, and immunohistochemical characterization. Am J Surg Pathol 2003; 27:937-951.
43. Eberhard DA, Giaccone G, Johnson BE. Biomarkers of response to epidermal growth factor receptor inhibitors in Non-small-cell lung cancer working group: standardization for use in the clinical trial setting. J Clin Oncol 2008; 26:983 -994.
44. Suehisa H, Toyooka S, Hotta K, et al. Epidermal growth factor receptor mutation status and adjuvant chemotherapy with uracil -tegafur for adenocarcinoma of the lung. J Clin Oncol 2007; 25:3952-3957.
45. Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med 2007; 356:800 -808.
46. Tsao MS, Viel -Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small -cell lung cancer. J Clin Oncol 2007; 25: 5240-5247.
47. Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006; 355: 983 -991.
48. Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol 2006; 24: 4731-4737.
49. Sica G, Wagner PL, Altorki N, et al. Immunohistochemical expression of estrogen and progesterone receptors in primary pulmonary neuroendocrine tumors. Arch Pathol Lab Med 2008; 132: 1889-1895.
50. Moran C, Suster S, Coppola D, Wick M. Neuroendocrine carcinomas of the lung. Am J Clin Pathol 2009; 131:206 -221.
51. Fellegara G, D’Adda T, Pilato FP, et al. Genetics of a combined lung small cell carcinoma and large cell neuroendocrine carcinoma with adenocarcinoma. Virchows Arch 2008; 453:107-115.
52. Wagner P, Kitabayashi N, Chen Y-T, Saqi A. Combined small cell lung carcinoma. Am J Clin Pathol 2009; 131: 376 -382.
53. Ferolla P, Daddi N, Urbani M, Semeraro A, et al. Tumorlets, multicentric carcinoids, lymph-nodal metastases and long term behaviour in bronchial carcinoids. Journal of Thoracic Oncology 2009; 4(3):383 -387.
54. Wurtz A, Benhamed L, Conti M, Bouchindhomme B, Porte H. Results of systematic nodal dissection in typical nad atypical carcinoid tumors of the lung. Journal of Thoracic Oncology 2009; 4(3):388 -394.
55. Gomez-Fernandez C, Jorda M, Delgado PI, Ganjei-Azar P. Thyroid transcription factor 1: a marker for lung adenoarinoma in body cavity fluids. Cancer 2002; 96: 289-293.
56. Miccadei S, De LR, Zammarchi E, Natali PG, Civitareale D. The synergistic activity of thyroid transcription factor 1 and Pax 8 relies on the promoter/enhancer interplay. Mol Endocrinol 2002; 16:837-846.
57. Rosell R, Felip E, Paz-Ares L. How could pharmacogenomics help improve patient survival? Lung Cancer 2007; 57(Suppl 2):S35-S41.
58. Wallace W. The challenge of classifying poorly differentiated tumours of the lung. Histopathology 2009; 54: 28 -42.
59. Hanauske AR, Eismann U, Oberschmidt O, et al. In vitro chemosensitivity of freshly explanted tumor cells to pemetrexed is correlated with target gene expression. Invest New Drugs 2007; 25:417-423.
60. Yendamuri S, Vaporciyan AA, Zaidi T, et al. 3p22.1 and 10q22.3 deletions detected by fluorescence in situ hybridization (FISH): a potential new tool for early detection of non -small cell lung Cancer (NSCLC). J Thorac Oncol 2008; 3:979 -984.
61. Hespanhol VP. Lung câncer treatment. Biology based decision, from gene to histology. Rev Port Pneumol 2008; XIV (Supl 2): S27 -S34. [ Links ]
62. Barata F. Pemetrexed in second line of non-small cell lung cancer. Rev Port Pneumol 2008; XIV(Supl 2):S21-S26. [ Links ]
63. Edwards C. Pulmonary adenocarcinoma: review of 106 cases and proposed new classification. J Clin Pathol 1987; 40:125 -135.
64. Rosai J. Evidence-based pathology and the pathologic evaluation of thymomas. Arch Pathol Lab Med 2008; 132: 1859.
65. Zhong L, Roybal J, Chaerkady R, et al. Identification of secreted proteins that mediate cell-cell interactions in an in vitro model of the lung cancer microenvironment. Cancer Res 2008; 68:7237-7245.
66. Seve P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin–binding agents? Lancet Oncol 2008; 9:168-175.
67. Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Busund LT, Bremnes RM. Prognostic impact of platelet-derived growth factors in non-small cell lung cancer tumor and stromal cells. J Thorac Oncol 2008; 3:963-970.
68. Crysam K, Lee J, Dohadwala M, Gardner B, et al. Inflammation, epithelial to mesenchimal transition and epidermal growth factor receptor tyrosine kinase inhibitor resistence. J Thorac Oncol 2008; 3(2):107-110.
69. Herbst RS, Lippman SM. Molecular signatures of lung cancer-toward personalized therapy. N Engl J Med 2007; 356:76-78.
70. Cappuzzo F, Ligorio C, Janne PA, et al. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trial. J Clin Oncol 2007; 25:2248-2255
71. Filipits M, Pirker R, Dunant A, et al. Cell cycle regulators and outcome of adjuvant cisplatin-based chemotherapy in completely resected non-small-cell lung cancer: the International Adjuvant Lung Cancer Trial Biologic Program. J Clin Oncol 2007; 25:2735 -2740.
72. Scagliotti GV, Parikh P, Von PJ, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26:3543 -3551.
73. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008; 359:1367 -1380.
74. Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung–cancer cells. N Engl J Med 2008; 359:366-377.
75. Lau SK, Boutros PC, Pintilie M, et al. Three–gene prognostic classifier for early-stage non small-cell lung cancer. J Clin Oncol 2007; 25:5562-5569.
76. Gazdar AF. DNA repair and survival in lung cancer – the two faces of Janus. N Engl J Med 2007; 356:771-773.
77. Togni M, Eber S, Widmer J, et al. Impact of vessel size on outcome after implantation of sirolimus–eluting and paclitaxel-eluting stents: a subgroup analysis of the SIRTAX trial. J Am Coll Cardiol 2007; 50:1123-1131.
78. Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncol 2008; 9:1048-1057.
79. Kawakami T, Nabeshima K, Hamasaki M, Iwasaki A, Shirakusa T, Iwasaki H. Small cluster invasion: a possible link between micropapillary pattern and lymph node metastasis in pT1 lung adenocarcinomas. Virchows Archiv 2009; 454:61-70.
80. Borgia J, Basu, S, Faber L, Kim A, et al. Establishment of a multy-analyte serum biomarker panel to identify lymph node metastases in non-small cell lung cancer. J Thorac Oncol 2009; 4(3):338-347.
1Professora de Anatomia Patológica
Chefe de Serviço dos Hospitais da Universidade de Coimbra
Lina Carvalho
Anatomia Patológica
Hospitais da Universidade de Coimbra
3000 Coimbra; Portugal
e-mail: lcarvalho@huc.min-saude.pt
Recebido para publicação/received for publication: 09.04.28
Aceite para publicação/accepted for publication: 09.05.19













