Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.20 no.6 Lisboa dez. 2013
https://doi.org/10.1016/j.jpg.2012.11.004
ORIGINAL ARTICLE
Non-steroidal anti-inflammatory drugs and gastroprotection gap among Family Physicians: Results from a survey
Uso de anti-inflamatórios não esteroides e ausência de prescrição de gastroproteção por médicos de medicina geral e familiar: resultados de um inquérito
Miguel Areiaa,∗, António Dias Pereirab, António Banhudoc, Graça Coutinhod
a Gastroenterology Department, Portuguese Oncology Institute - Coimbra, Coimbra, Portugal
b Gastroenterology Department, Portuguese Oncology Institute - Lisbon, Lisbon, Portugal
c Gastroenterology Department, Hospital Amato Lusitano - Castelo Branco, Portugal
d Medical Department, Nycomed Portugal, Paço de Arcos, Oeiras, Portugal
*Corresponding author
ABSTRACT
Introduction: Use of non-steroidal anti-inflammatory drugs (NSAIDs) has increased over the last few years and identification of gastrointestinal risk factors is a key factor for prevention of its complications. Even after correct identification of those risk factors, only a small percentage of Family Physicians prescribe gastroprotection.
Aims: To knowledge gastrointestinal risk factors and gastroprotections prescription by Family Physicians in patients receiving NSAIDs.
Methods: Observational, cross-sectional, random sample study, using a survey among 300 Family Physicians, performed in 2007. Questions were asked about perceived patients rates or hypothetical scenarios and answers were valued on an intention-to-treat basis. The main outcome measure was the gastroprotections prescription rate among patients taking NSAIDs.
Results: The perceived proportion of patients receiving NSAIDs was 38% and from these, 40% were taking gastroprotective drugs. The main identified gastrointestinal risk factors were: complicated peptic ulcer (98%), age ≥ 65 years (96%), smoking habits and alcohol consumption (96%), dyspepsia (95%), high-doses of NSAIDs (94%), corticosteroids co-administration (91%) and consumption of two or more NSAIDs (90%). Gastroprotection would be prescribed in 82% of patients with history of complicated peptic ulcer; 60% if receiving two or more or a high dose of NSAIDs; 53% if with Helicobacter pylori infection and 51% if aged ≥ 65 years. For all risk factors, gastroprotection use would be only of 47.3% (95% confidence interval: 45.6-49.0%).
Conclusions: Family Physicians are aware of NSAIDs gastrointestinal toxicity but risk estimation seems inadequate since they will not prescribe gastrointestinal protection in more than half the cases.
Keywords: Nonsteroidal/adverse effects; Aspirin/adverse effects; Peptic ulcer/prevention & control; Risk factors; Primary health care
RESUMO
Introdução: O uso de Anti-Inflamatórios Não Esteroides (AINE) tem aumentado nos últimos anos e a identificação de fatores de risco gastrointestinal é fundamental para a prevenção de suas complicações. Mesmo após a correta identificação desses fatores de risco, apenas uma pequena percentagem dos médicos de Medicina Geral e Familiar efetuam gastroproteção.
Objetivos: Conhecimento dos fatores de risco gastrointestinais e prescrição de gastroproteção por médicos de Medicina Geral e Familiar em pacientes que tomam AINE.
Métodos: Estudo observacional, transversal, por amostra aleatória, por meio de um inquérito a 300 médicos de Medicina Geral e Familiar, realizado em 2007. Foram efetuadas perguntas sobre as taxas de pacientes esperadas ou cenários hipotéticos e as respostas foram avaliadas com base na intenção de tratar. A principal variável de resultado foi a taxa de prescrição de gastroproteção em pacientes medicados com AINE.
Resultados: A proporção de pacientes medicados com AINE foi de 38% e, destes, 40% estavam a efetuar gastroproteção. Os principais fatores de risco gastrointestinais identificados foram: úlcera péptica complicada (98%), idade ≥ 65 anos (96%), tabagismo e consumo de álcool (96%), dispepsia (95%), alta dose de AINE (94%), coadministração de corticosteroides (91%) e uso simultâneo de 2 ou mais AINE (90%). Seria prescrita gastroproteção em 82% dos pacientes com história de úlcera péptica complicada, 60% se recebessem 2 ou mais AINE ou um AINE mas em dose elevada, em 53% se houvesse infeção por Helicobacter pylori e em 51% se idade ≥ 65 anos. Para todos os fatores de risco, o uso de gastroproteção seria de apenas 47,3% (intervalo de confiança a 95%: 45,6-49,0%).
Conclusões: Os médicos de Medicina Geral e Familiar estão conscientes da toxicidade gastrointestinal dos AINE, mas a sua estimativa do risco parece inadequada, uma vez que não planeiam prescrever proteção gastrointestinal em mais da metade dos casos necessários.
Palavras-chave: Anti-inflamatórios não esteroides/efeitos secundários; Aspirina/efeitos secundários; Úlcera péptica/prevenção e tratamento; Fatores de risco; Cuidados de Saúde Primários
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) use, including acetylsalicylic acid (ASA), has been increasing over the last years, being amongst the most commonly prescribed and used drugs. A study conducted in Portugal showed that the most prescribed therapeutic class by Family Physicians was NSAIDs totalling 8.2%, while ASA and derivatives represented 1.3% of all medicines.1 Other studies in Portugal showed that NSAIDs, analgesics and antipyretic drugs rank as fifth among the chronically used medicines, being used by 12-15% of the studied users.2
NSAIDs are highly effective agents; however, its use is associated to adverse events, especially gastrointestinal. NSAIDs-related adverse events accounted for 11% of the reports received by the Portuguese Drug Prescription Vigilance System between 1993 and 2002 and gastrointestinal complications represented 19% of the overall reports. Severe adverse reactions to NSAIDs, which represented more than 50% of the reports, caused hospitalization in 31% of the cases.3
NSAIDs induced gastrointestinal complications are a public health concern4 and the appropriate identification and evaluation of gastrointestinal risk factors associated to NSAIDs use in each and every patient as well as the concomitant use of gastrointestinal protective agents, if appropriate, are highly effective preventive strategies.5 To the best of our knowledge, there are no published clinical studies carried out in the Portuguese population, evaluating both the prescription of gastroprotective agents in patients receiving NSAIDs and the influence of gastrointestinal risk factors in this prescription at a Primary Care setting, with only one published study that evaluated the gastroprotection use among NSAIDs admissions using hospital records in a Tertiary Care setting.6
The aim of this study was to feature Family Physicians clinical practice in Portugal, regarding both the identification of gastrointestinal risks and the prevention of NSAIDs complications, namely the recognition of gastrointestinal complications risk factors and the impact of those risk factos in the decision of prescribing gastroprotective therapy.
Materials and methods
Study sample
Observational, cross-sectional study, conducted according to methods generally used for research interview-based studies using a random sample. The study population consisted of Family Physicians registered in Districts from the north (Porto), centre (Coimbra), south (Faro/Portimão) and the capital city of Portugal (Lisbon). Prime Focus (Lisbon, Portugal), a specialized company in Market Research Studies, provided the database used for the sample selection.
The sample size (estimated to ensure a 5% error margin and a 95% confidence interval) was 300 interviews; 300 randomly selected Family Physicians from the above-cited regions were included, stratified in a non-proportional way, based on the variable Region, to ensure a minimum basis of 30 responders in Coimbra and in Faro/Portimão.
Interview and questionnaire
The measuring tool used was a non-validated questionnaire developed by the authors of the manuscript on a consensos base and consisted of open questions about perceived rates of patients medications, complaints, symptoms and gastroprotection use and also spontaneous and pre-specified answers about knowledge on gastrointestinal risk factors.
The questionnaire was applied on a personal interview basis, by well-trained professionals. The questionnaire was fulfilled by the interviewer according to the physicians answers, with mean interview duration of 20 min. After three unsuccessful phone contacts, another randomized doctor, under the same conditions as those used for the remaining sample, replaced the former doctor. Participation in the interview was voluntary, confidential and anonymous and there was no financial compensation as a result of the participation in the study. All variables analyzed were valued on their perceived existence or intention-to-treat by the Family Physician. As this is an observational study based on the results of a survey and not a study on human subjects, no formal approval from any type of committee was demanded.
Statistical analysis
A descriptive analysis of the results obtained was performed. For continuous variables the Students t-test was used and the One-way analysis of variance (ANOVA) for the assessment of the regional differences. The adopted significance level was set at 0.05. SPSS software v17.0 (Chicago, IL, USA) was used for data analysis.
Results
Interviews were carried out between June and July 2007 in Portugal (mainland). To achieve the pretended 300 interviews, 957 Family Physicians were needed to contact by phone as 657 (69%) refused to participate. Three hundred interviews were performed, stratified by region as follows: 140 in the district of Lisbon; 100 in Porto and 30 in Coimbra and Faro/Portimão. From the inquired physicians, 45% were women, 75% had more than 20 years of clinical practice and 79% worked also in emergency units.
Perceived patients receiving non-steroidal anti-inflammatory drugs and acetylsalicylic acid
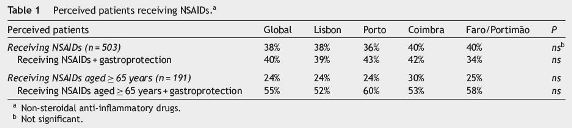
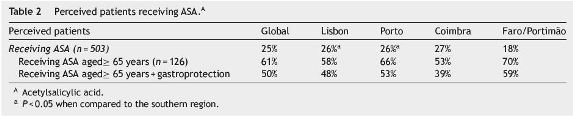
Five hundred and three patients were, in average, followed per month per doctor with no significant diferences by region. The proportion of perceived patients receiving NSAIDs was 38%, from whom 24% were aged≥65 years old; from this last group, 55% were receiving gastroprotective agents (Table 1). Twenty five per cent of perceived patients were receiving ASA, from which 61% were aged≥65 years old (Table 2).
Gastrointestinal symptoms and impact in quality of life
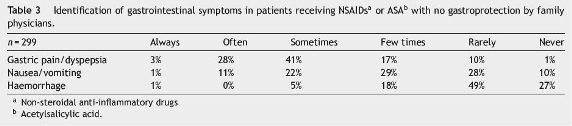
Physicians referred that around 57% of their patients had gastrointestinal symptoms. In the rating scale used (values ranging from 1 - never to 6 - always), the mean value obtained was 3.6. The main NSAIDs-related gastrointestinal adverse events were dyspepsia or gastric pain (Table 3).
Also 69% referred that gastrointestinal symptoms had a negative impact in the quality of life of their patients. In the rating scale used (values ranging from 1 - no impact to 6 - great impact) the mean value obtained was 4.1.
Pharmacologic prevention of gastrointestinal complications in patients receiving non-steroidal anti-inflammatory drugs
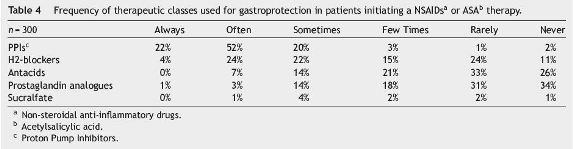
Proton Pump Inhibitors (PPIs) were the most commonly used drugs for gastroprotection in patients receiving NSAIDs: 74% of the respondents referred that they would always or often use PPIs in their patients if they were initiating a NSAIDs therapy, while 28% referred the use of H2-blockers (Table 4).
Risk factors and prevention of gastrointestinal complications
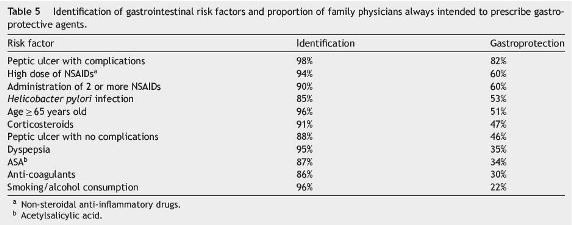
Risk factors for gastrointestinal complications identified by the respondents are described in Table 5. All these risk factos were identified by more than 85% of the respondents, first spontaneously and afterwards by being specifically asked about those not previously reported. However, only in the case of complicated peptic ulcer, more than 80% of the respondents would always prescribe gastroprotective agents, while the administration of high doses or the administration of two or more NSAIDs only motivated such gastroprotection in 60% of the physicians. For the remaining risk factors identified the gastroprotective prescription intention would be only around 50% or even lower. For all gastrointestinal risk factors identified, gastroprotections prescription would be used in only 47.3% of cases (95% confidence interval: 45.6-49.0%).
Helicobacter pylori eradication in patients receiving non-steroidal anti-inflammatory drugs
The presence of Helicobacter pylori infection influenced 78% of the physicians in their decision for prescribing gastroprotective agents; however, diagnostic testing was performed in only 38% of cases mainly due to lack of symptoms, not indicated/recommended and related costs. From those assessing H. pylori status in patients receiving NSAIDs, 91% would prescribe eradication therapy for positive cases.
Importance of gastrointestinal protection and national recommendations
Of the responding physicians, 81% considered this as a very important or extremely important matter. In the analogue scale used (ranging from 1 - not important at all to 6 - extremely important), a mean value of 5.2 was achieved. Additionally, the existence of national recommendations on this subject was deemed extremely important or very importante by 76%.
Discussion
In the published literature, gastroprotective agents use ranges between 7 and 42% in patients receiving NSAIDs.6-12 In this study, the perceived use of NSAIDs referred by the Family Physicians in their patients was high (38%). From the patients receiving NSAIDs, a high proportion (40%) was somehow receiving gastroprotection and this rate increased to 55% when only patients aged ≥ 65 years old were considered. Regarding prescription of gastroprotective agents to patients receiving ASA for cardiovascular prevention, given its chronic use and the older age of most users, only 61% of patients receiving ASA and aged ≥ 65 years old were taking gastroprotective drugs. Our result (40%) is higher than the one reported by Couto et al. (15%) but while our grade is a result of an interview perception on an intention-to-treat basis and might be an overestimation, the other grade comes from a retrospective analysis of hospital databases involving only admissions from NSAIDs complications and might be an underestimation of the real gastroprotection use.6
Our results are consistent with others in which 50% of the patients, ≥ 65 years old taking NSAIDs, were not receiving gastroprotection8 while in patients treated with ASA only 23% of patients presenting at least one risk factor and 56% with a history of complicated peptic ulcer were receiving gastroprotection.13-15 This low use of gastroprotective agents is in accordance with the fact that the physicians only recalled haemorrhage to occur always or often in 1% of cases, eventually due to an inadequate feedback from Tertiary Care centres reports on complications, but this issue was not addressed in this study. The low use of gastroprotective agents in patients receiving ASA may be related to na inappropriate recognition of the gastrointestinal risks associated to this drug, either by the patients or the healthcare professionals themselves and this is a worldwide problem, again eventually related to an underreporting feedback of complications from tertiary centres to the primary care physicians.16,17
This study also shows that most of the physicians inquired seem to be aware of the gastrointestinal adverse effects associated to NSAIDs use and also seem to be aware of the main gastrointestinal risk factors, as most of them were appropriately identified, spontaneously or after being specifically asked about. However, although the risk is recognized, its magnitude is undervalued. As result, the proportion of physicians that would always prescribe gastroprotective agents to patients with gastrointestinal risk factors is low, except for patients with previous history of complicated peptic ulcer, achieving 82%. Our results suggest that more than half of the patients receiving NSAIDs with indication for gastroprotection (presence of one or more risk factors), would not receive it. These results reveal na incomplete compliance with the existing clinical practice recommendations.5,15,18-23
Several observational studies carried out within the scope of Primary Care, with a different methodology compared to the one used in this study, have confirmed this low use of gastroprotection strategies in patients receiving NSAIDs with gastrointestinal risk factors with prescription rates of only 10-39% in patients with at least one risk factor.10,11,24-27
Concerning the use of gastroprotective medicines, although PPIs were the most efficient and commonly used drugs, 28% of the respondents always or often used H2-blockers, even though at the time the study was conducted, the use of these drugs was already considered inappropriate.15,19 This use of a less efficient drug might be explained by the fact that, still in recent national recommendations, its use is suggested as an alternative to PPI with no explanation on the different efficiency rates and safety profiles.28 Also, although 85% of the Family Physicians recognized H. pylori infection as a gastrointestinal risk factor, 62% did not screened for the infection in patients receiving NSAIDs in clinical practice.
The Maastricht Consensus as well as consensus statements issued by other professional organizations recommend both screening and eradication therapy for positive cases, before initiating long-term treatment with NSAIDs and for patients on NSAIDs therapy who developed gastroduodenal ulcers.29-31 These guidelines also establish that in NSAIDs chronic users with high gastrointestinal risk (history of complicated peptic ulcer), eradication therapy alone is not enough to prevent recurrences of gastrointestinal complications; therefore, an additional maintenance therapy with PPIs is necessary. The complexity of this subject and the continuous information update on the infection approach in patients receiving NSAIDs may have influenced the answers of the physicians.19
The main limitation of this study is that all answers are based on the physicians perception and intention-to-treat rather than on their own clinical practice records and this fact might result in an overestimation of the real gastroprotection use. Only a prospective study could produce a more realistic estimation of the Family Physicians prescription profile. Nevertheless the observed consistency across the different regions provides some internal validation of the results and the sample size gives us a narrow confidence interval to allow some confidence on the results obtained. Another limitation was the random sample selection by phone contact, which is influenced by the availability both of the telephone line and of the respondent. In addition a relatively high participation refusal rate (69%) was observed and the database used does not provide us with the demographic features of the physicians included, preventing us from establishing a comparison between respondents and non-respondents. This might have resulted in more answers from Family Physicians more aware of the problem and, again, bias the results in favour of better gastroprotection rates. A potential inquirer-related bias was minimized by a careful selection and training of the inquirers and a close supervision of the fieldwork.
The results of this study allow us to say that clinical recommendations on gastrointestinal protection are not fully implemented and that this is an area that should be more valued. In this study, the Family Physicians confirm the need to elaborate national clinical recommendations on this topic. A full collaboration between Family Physicians and Gastroenterology Societies in promoting joined updates by conferences or lectures in their national meetings, showing the two perspectives of the same problem, could be a nice way to improve better implementation of gastroprotection use.
Conclusions
In conclusion, we found that although most of the inquired Family Physicians were aware of NSAIDs induced gastrointestinal toxicity and were able to appropriately identify the main gastrointestinal risk factors, the risk magnitude estimate seemed to be inappropriate, since Family Physicians would not prescribe gastrointestinal protective agents in more than half the patients with associated gastrointestinal risk factors.
References
1. Medeiros A, Costa A, Magalhães A, Luzia E, Gonçalves H. Terapêutica em cuidados de saúde primários numa população rural do distrito de Faro. Rev Port Clin Geral. 2000;16:279-90. [ Links ]
2. Ferreira R. Consumo crónico de medicamentos na população de um Centro de Saúde. Rev Port Clin Geral. 2007;23:125-32. [ Links ]
3. Bragança F, Prisca S, Araújo A, Pinheiro L, Carmona R. Reacções adversas aos inibidores selectivos da COX-2 notificadas ao Sistema Nacional de Farmacovigilância. Bol Farmacovigilância. 2004;8:1. [ Links ]
4. Higham J, Kang JY, Majeed A. Recent trends in admissions and mortality due to peptic ulcer in England: increasing frequency of haemorrhage among older subjects. Gut. 2002;50:460-4. [ Links ]
5. Targownik LE, Thomson PA. Gastroprotective strategies among NSAID users: guidelines for appropriate use in chronic illness. Can Fam Physician. 2006;52:1100-5. [ Links ]
6. Couto G, Macedo G, Ribeiro F. Upper gastrointestinal bleeding associated with acetylsalicylic acid and non-steroidal antiinflammatory drugs in Portugal Results from PARAINES study. GE - J Port Gastrenterol. 2010;17:200-6. [ Links ]
7. Helin-Salmivaara A, Huupponen R, Virtanen A, Lammela J, Klaukka T. Frequent prescribing of drugs with potential gastrointestinal toxicity among continuous users of non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol. 2005;61:425-31. [ Links ]
8. Clinard F, Bardou M, Sgro C, Lefevre N, Raphael F, Paille F, et al. Non-steroidal anti-inflammatory and cytoprotective drug coprescription in general practice. A general practitioner-based survey in France. Eur J Clin Pharmacol. 2001;57:737-43. [ Links ]
9. Carvajal A, Arias LH, Vega E, Sanchez JA, Rodriguez IM, Ortega PG, et al. Gastroprotection during the administration of nonsteroidal anti-inflammatory drugs. A drug-utilization study. Eur J Clin Pharmacol. 2004;60:439-44. [ Links ]
10. Smalley W, Stein CM, Arbogast PG, Eisen G, Ray WA, Griffin M. Underutilization of gastroprotective measures in patients receiving nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2002;46:2195-200. [ Links ]
11. Sturkenboom MC, Burke TA, Dieleman JP, Tangelder MJ, Lee F, Goldstein JL. Underutilization of preventive strategies in patients receiving NSAIDs. Rheumatology (Oxford). 2003;42 Suppl. 3:iii23-31. [ Links ]
12. Keys J, Beardon PH, Lau C, Lang CC, McDevitt DG. General practitioners use of non-steroidal anti-inflammatory drugs in Tayside and Fife regions. J R Soc Med. 1992;85:442-5. [ Links ]
13. Chey WD, Eswaren S, Howden CW, Inadomi JM, Fendrick AM, Scheiman JM. Primary care physician perceptions of non-steroidal anti-inflammatory drug and aspirin-associated toxicity: results of a national survey. Aliment Pharmacol Ther. 2006;23:655-68. [ Links ]
14. Balakrishnan S, Jhaj R. Prophylactic use of gastro-protective agents in patients on low-dose aspirin. Br J Clin Pharmacol. 2008;65:621-2. [ Links ]
15. Dubois RW, Melmed GY, Henning JM, Laine L. Guidelines for the appropriate use of non-steroidal anti-inflammatory drugs, cyclo-oxygenase-2-specific inhibitors and proton pump inhibitors in patients requiring chronic anti-inflammatory therapy. Aliment Pharmacol Ther. 2004;19:197-208. [ Links ]
16. Targownik LE, Metge CJ, Leung S. Underutilization of gastroprotective strategies in aspirin users at increased risk of upper gastrointestinal complications. Aliment Pharmacol Ther. 2008;28:88-96. [ Links ]
17. Lanas A, Garcia-Rodriguez LA, Arroyo MT, Gomollon F, Feu F, Gonzalez-Perez A, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal antiinflammatory drugs, aspirin and combinations. Gut. 2006;55:1731-8. [ Links ]
18. Lanza FL. A guideline for the treatment and prevention of NSAID-induced ulcers members of the Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2037-46. [ Links ]
19. Wilcox CM, Allison J, Benzuly K, Borum M, Cryer B, Grosser T, et al. Consensus development conference on the use of nonsteroidal anti-inflammatory agents, including cyclooxygenase-2 enzyme inhibitors and aspirin. Clin Gastroenterol Hepatol. 2006;4:1082-9. [ Links ]
20. Lee JH, Lee YC, Jeon SW, Kim JW, Lee SW. Guidelines of prevention and treatment for NSAID-related peptic ulcers. Korean J Gastroenterol. 2009;54:309-17. [ Links ]
21. Burmester G, Lanas A, Biasucci L, Hermann M, Lohmander S, Olivieri I, et al. The appropriate use of non-steroidal anti-inflammatory drugs in rheumatic disease: opinions of a multidisciplinary European expert panel. Ann Rheum Dis. 2011;70:818-22. [ Links ]
22. Lanas A, Garcia-Tell G, Armada B, Oteo-Alvaro A. Prescription patterns and appropriateness of NSAID therapy according to gastrointestinal risk and cardiovascular history in patients with diagnoses of osteoarthritis. BMC Med. 2011;9:38. [ Links ]
23. Rostom A, Moayyedi P, Hunt R. Canadian consensus Guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks. Aliment Pharmacol Ther. 2009;29:481-96. [ Links ]
24. Thompson PW, Tee L, McBride J, Quincey D, Strat Liddiard G. Long-term NSAID use in primary care: changes over a decade and NICE risk factors for gastrointestinal adverse events. Rheumatology (Oxford). 2005;44:1308-10. [ Links ]
25. Abraham NS, El-Serag HB, Johnson ML, Hartman C, Richardson P, Ray WA, et al. National adherence to evidence-based Guidelines for the prescription of nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005;129:1171-8. [ Links ]
26. Vonkeman HE, Fernandes RW, van de Laar MA. Under-utilization of gastroprotective drugs in patients with NSAID-related ulcers. Int J Clin Pharmacol Ther. 2007;45:281-8. [ Links ]
27. Price-Forbes AN, Callaghan R, Allen ME, Rowe IF. A regional audit of the use of COX-2 selective non-steroidal anti-inflammatory drugs (NSAIDs) in rheumatology clinics in the West Midlands, in relation to NICE guidelines. Rheumatology (Oxford). 2005;44:921-4. [ Links ]
28. DGS. Anti-inflamatórios não esteróides sistémicos em adultos: orientações para a utilização de inibidores da COX-2. Norma da Direção Geral de Saúde. 2011;013:1-13. [ Links ]
29. Malfertheiner P, Megraud F, OMorain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-81. [ Links ]
30. Malfertheiner P, Megraud F, OMorain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection - the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-64. [ Links ]
31. Hunt R, Fallone C, Veldhuyzan van Zanten S, Sherman P, Smaill F, Flook N, et al.Canadian Helicobacter Study Group Consensus Conference: update on the management of Helicobacter pylori - an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H. pylori infection. Can J Gastroenterol. 2004;18:547-54. [ Links ]
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Funding
This work was partially supported by Nycomed Portugal (implementation and translation phases) but there was no involvement in data analysis or publication decisions.
Conflicts of interest
The authors have no conflicts of interest to declare.
*Corresponding author
E-mail addresses: miguel.areia@netcabo.pt, miguel.areia75@gmail.com (M. Areia).
Received 29 May 2012; accepted 6 November 2012













