Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.20 no.4 Lisboa jul. 2013
https://doi.org/10.1016/j.jpg.2012.11.001
ENDOSCOPIC SPOT
Endoscopic resection combined with radiofrequency ablation for early adenocarcinoma in Barretts esophagus
Mucosectomia e radiofrequência na terapêutica de adenocarcinoma superficial em esófago de Barrett
Miguel Serranoa,∗, Susana Mão de Ferroa, Paula Chavesb, António Dias Pereiraa
aServiço de Gastrenterologia, Instituto Português de Oncologia de Lisboa Francisco Gentil, E.P.E., Lisboa, Portugal
bDepartamento de Patologia Morfológica, Instituto Português de Oncologia de Lisboa Francisco Gentil, E.P.E., Lisboa, Portugal
*Corresponding author
Barretts esophagus (BE) is a premalignant condition that results from the replacement of the normal squamous lining of the esophagus by a columnar epithelium containing intestinal metaplasia (IM) on biopsy.
A 53-year-old man was followed at our institution for long-segment BE (Prague classification C1 M4) since 2007. His past medical history was unremarkable. There were no visible nodules or ulcerations within the BE at endoscopy in 2007 and 2008. Biopsies, performed according to the Seattle protocol, were negative for dysplasia.
The patient returned in 2011 for surveillance endoscopy. At this exam a flat, slightly elevated, lesion (Paris classification 0-IIa) with 8mm of diameter was noted near the gastroesophageal junction (Fig. 1A). Targeted biopsies were compatible with intramucosal adenocarcinoma. Biopsy specimens of the remainder BE were negative for dysplasia.
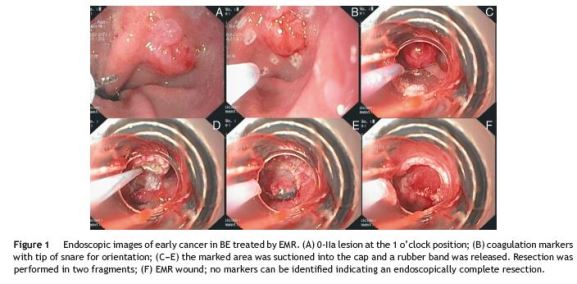
Endoscopic mucosal resection (EMR) was performed with the patient under deep sedation with propofol. We used the Duette Multiband Mucosectomy KitTM (Cook Medical, Limerick, Ireland), which consists of a modified variceal band ligator that allows passage of a hexagonal 1.5 cm×2.5 cm snare made of braided wire alongside the releasing wires for the bands. The area to be resected was previously delineated with coagulation markings (Fig. 1B). The lesion was first suctioned into the ligating barrel, and the rubber band was deployed creating a pseudopolyp.
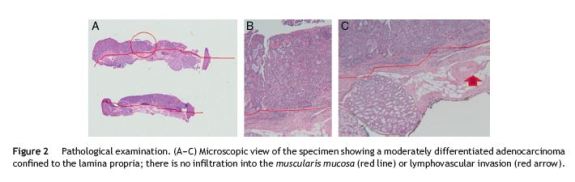
Resection was carried out, in two fragments, with the ESG-100 electrosurgical unit (Olympus Europe, Hamburg, Germany), using pure coagulation current (Fig. 1C-F). There were no early or delayed complications. Specimens were pinned on cork and fixed in formalin. Pathologic examination revealed a moderately differentiated adenocarcinoma limited to the lamina propria (Fig. 2A-C). Lateral margins were not evaluable given the piecemeal technique.
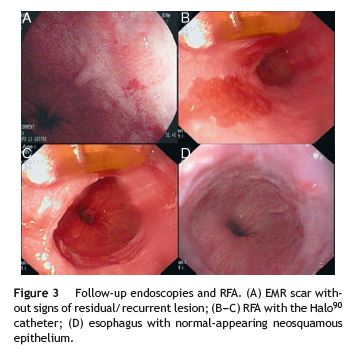
At 6-weeks follow-up endoscopy there were no signs of residual lesion (Fig. 3A). Biopsies of the resection scar and Barretts segment showed no dysplasia. Due to high risk of metachronous lesions ablation of the remaining BE was scheduled. Given the narrow tongue-like BE morphology we selected the focal ablation catheter to minimize potential stricture formation. At 12-weeks post EMR radiofrequency ablation (RFA) was carried out using the Halo90 catheter (BÂRRX Medical Inc., Sunnyvale, CA, USA) fitted on the tip of a standard endoscope (Fig. 3B-C). Barretts epithelium was positioned at the 12 oclock position in the endoscopic video image. Areas were ablated twice by using the double-double 15 J/cm2 regimen (2 consecutive ablations with 15 J/cm2 each, with cleaning of the ablated area after the first pass). The patient was kept on esomeprazole (40 mg BID for 2 months and 40 mg/day thereafter) and follow-up at 2, 6, 9 and 12 months after RFA showed a esophagus covered with normal-appearing neosquamous epithelium (Fig. 3D). Biopsies were negative for IM and dysplasia.
In recent years, endoscopic therapy of early BE neoplasia has become a safe and effective alternative to esophagectomy.1,2 Only patients with high-grade intraepithelial neoplasia or well and moderately differentiated intramucosal carcinoma without lymphatic involvement are eligible for curative endoscopic treatment.3,4 Lesions showing invasion of the submucosa are associated with a significant risk of lymph node metastases and therefore patients should be treated surgically.5 Due to the risk of synchronous and metachronous lesions in the remaining BE, complete ablation of the metaplastic epithelium should follow a successful resection of dysplastic lesions.
References
1. Pouw RE, Seewald S, Gondrie JJ, Deprez PH, Piessevaux H, Pohl H, et al. Stepwise radical endoscopic resection for eradication of Barretts oesophagus with early neoplasia in a cohort of 169 patients. Gut. 2010;59:1169-77. [ Links ]
2. Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency ablation in Barretts esophagus with dysplasia. N Engl J Med. 2009;360:2277-88. [ Links ]
3. Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-1. [ Links ]
4. Bennett C, Vakil N, Bergman J, Harrison R, Odze R, Vieth M, et al. Consensus statements for management of Barretts dysplasia and early-stage esophageal adenocarcinoma, based on a delphi process. Gastroenterology. 2012;143:336-46. [ Links ]
5. Westerterp M, Koppert LB, Buskens CJ, Tilanus HW, ten Kate FJ, Bergman JJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastroesophageal junction. Virchows Arch. 2005;446:497-504. [ Links ]
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflicts of interest
The authors have no conflicts of interest to declare.
*Corresponding author
E-mail address: mmserrano@gmail.com (M. Serrano).
Received 11 October 2012; accepted 22 November 2012













