Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.20 no.4 Lisboa jul. 2013
https://doi.org/10.1016/j.jpg.2012.09.006
CLINICAL CASE
Trousseaus syndrome due to asymptomatic pancreatic adenocarcinoma
Síndrome de Trousseau por adenocarcinoma do pâncreas assintomático
António Murinelloa,∗, Pedro Guedesa, Gizela Rochab, Ana Serranoa, António Figueiredoa, Helena Damásioa, João Freireb, Fernando Cunhac, Liliana Alvesa
aServiço de Medicina Interna 1, Hospital de Curry Cabral, Lisboa, Portugal
bServiço de Oncologia Médica, Instituto Português de Oncologia de Lisboa de Francisco Gentil, Lisboa, Portugal
cLaboratório de Citologia Aspirativa do Serviço de Anatomia Patológica, Instituto Português de Oncologia de Lisboa de Francisco Gentil, Lisboa, Portugal
*Corresponding author
ABSTRACT
The authors report a case of Trousseaus syndrome presenting in a previously asymptomatic 58-year-old man diagnosed with pancreatic adenocarcinoma and liver metástases during a workup prompted by migratory venous thrombosis and pulmonary embolism. It was followed by an ischaemic stroke that occurred while the patient was just one day off anticoagulante therapy with low-molecular-weight heparin to allow for liver and pancreatic biopsies. Trousseaus syndrome is defined by recurrent or migratory venous thrombosis, arterial embolism caused by non-bacterial thrombotic endocarditis, or both, in patients with underlying malignancy. Treatment relies on the lifelong administration of heparin, and its interruption - however brief - may promote new thrombotic events.
Keywords: Trousseaus syndrome; Migratory thrombophlebitis; Heparin; Occult malignancy; Pancreatic adenocarcinoma
RESUMO
Os autores relatam um caso de Síndrome de Trousseau, manifestado por trombose venosa migratória e embolia pulmonar, num doente de 58 anos assintomático até à data de internamento. O estudo desencadeado revela adenocarcinoma do pâncreas com metástases hepáticas, e a situação torna-se ainda mais grave após a ocorrência de um acidente vascular cerebral isquémico, aparentemente em relação com a paragem por 24 horas da terapêutica anti-coagulante com heparina de baixo peso molecular para realização de biópsia hepática e pancreática guiadas por exame de imagem. A síndrome de Trousseau define-se por tromboses venosas recorrentes ou migratórias, embolias arteriais causadas por endocardite trombótica não-bacteriana, ou ambas, em doentes com neoplasia maligna subjacente. O tratamento implica a administração permanente de heparina, e qualquer interrupção −ainda que breve− pode proporcionar novo episódio de trombose.
Palavras-chave: Síndrome de Trousseau; Tromboflebite migratória; Heparina; Neoplasia maligna oculta; Adenocarcinoma pancreático
Introduction
Trousseaus syndrome (TS), named after the French physician Armand Trousseau who first described it in 1865, refers to recurrent or migratory spontaneous venous thrombosis, arterial embolism due to non-bacterial thrombotic endocarditis, or both, in a patient with known or occult malignancy which is usually difficult to diagnose and may even remain elusive until it is disclosed in an autopsy.1 Thrombosis can occur from months to years before cancer is known, and a negative thorough initial work-up does not forgo the need for continued evaluation that will ultimately allow an earlier diagnosis.2,3 Cryptogenic thrombosis prevented by heparin but not oral anticoagulants should prompt doctors to investigate the possibility of underlying malignancy. Patients with TS show persistent low-grade intravascular coagulation, thus accounting for the need to treat them with full large dose low molecular weight heparina on a lifelong basis.4
These patients show thrombotic diathesis that can be devastating when left untreated, and the most severe cases may lead to limb amputation in just a few hours, as a result of severe disseminated intravascular coagulation (DIC) that can happen before an actual thrombosis ensues. Particular forms of this syndrome are phlegmasia alba dolens and phlegmasia cerulea dolens,5 and a variant of classic TS has been identified, combining multiple arterial and venous thrombi with DIC prone to bleeding.6
Malignant neoplasms are pro-thrombotic, and anomalies are possible in each point of Virchows triad --- blood flow (stasis), components (hypercoagulability) or vessel Wall (endothelial injury). These surely are synergistic forces behind this, and many other factors such as concomitant diseases, medications and decreased motility have a role as contributing factors.7,8 TS involves marked changes in the clotting cascade, brought about by the production and release of procoagulant substances from tumour cells. Although it may be associated with any kind of neoplasm, TS is most often related to pancreatic, lung, prostate, gastric, colorectal, ovarian and breast cancer.9
Clinical report
Present illness
A 58-year-old man, electronics technician, was admitted in our Internal Medicine ward with deep venous thrombosis of the right lower limb. He presented to the Emergency Department with a 3-day course of right calf pain worsened by walking, followed by swelling and increased temperature in the same limb. Throughout the whole period he felt increasing fatigue and had an episode of fainting. Just four days before the current symptoms started he had arrived from a vacation in Ecuador, during which his right upper limb had become swollen, red and hot. He was diagnosed with right arm cellulitis and was started on antibiotic and antiinflammatory therapy, improving subsequently. He denied fever, sweating, weight loss or coughing, as well as any digestive, urinary or other musculoskeletal symptoms.
Past medical history
Past medical history was positive for some childhood infectious diseases (measles, mumps, chicken pox), grade I arterial hypertension (known for 21 years and without medication), smoking habits (20 pack-year units), mild alcohol intake (20 g daily), chronic lumbar disc disease, left varicocele surgery (at the age of 21) and benign prostatic hypertrophy.
Family diseases
His father deceased, with a history of chronic renal failure. There were no discernible accounts of cancer in close relatives.
Physical examination
His physical examination revealed great overall condition and stable vital signs (BP 113/70 mmHg, HR 70 bpm, RR 20 bpm, apyrexia); no skin lesions, lymphadenopathy or thyromegaly; normal cardiac and respiratory sounds; soft, nontender, nondistended abdomen with normal bowel sounds, no masses on abdominal examination, and no hepatosplenomegaly; no evidence of infection in his right upper limb; slight swelling and increased temperature in his right leg, with positive Homans sign; normal neurologic exam and fundus observation within normal limits.
Lab tests
Laboratory tests showed the following: haemoglobin 14.6 g/dl; WBC 10.9×109/l (68.1%N-20.3%L-7.1%M-4%E); platelets 258.0×109/l; ESR 13mm; CRP 3.5 mg/dl (N < 1); transferrin 195 mg/dl (N: 215-365); ferritin 344.9 ng/ml (26.0-388.0); glucose 84 mg/dl; creatinine 0.6 mg/dl; albumin 3.7 g/dl; normal serum electrophoresis; AST 42 U/l (17-59); ALT 65 U/l (21-72); GGT 168 U/l (N: 15-73); ALP 209 U/l (N: 38-126); total bilirubin 0.4 mg/dl; amylase 591 U/l (N: 30-110); lipase 6356 U/l (N: 23-300); LDH 704 U/l (N: 303-618); total cholesterol 180 mg/dl; triglycerides 122 mg/dl; total calcium 9.5 mg/dl; INR 1.1; aPTT 38.0; factor V 130.5%; factor VIII 152.2%; protein C 97%; protein S 92.8%; antithrombin III 107%; resistance to activated protein C 3.14 (within normal limits); Lupus anticoagulant 1.94 ratio (1.6-2.0), Silica clotting time 1.26 ratio (>1.16: positive); negative antinuclear antibodies and anti [1]2-glycoprotein; negative VDRL; normal TSH; negative serologies for both hepatitis B and C. Human chorionic gonadotropin 116 (N < 5) and CA 15.3 = 74.4 U/ml (N < 31); all other tumour markers (PSA, a-fetoprotein, CA 19.9 and CEA) within normal range. Normal urinalysis.
Cardiac tests
Cardiac tests showed the following: (1) EKG - normal; (2) cardiac ultrasound displaying good left ventricle global systolicfunction; diastolic dysfunction; no valve abnormalities; mild biatrial dilation; dilated right ventricle with preserved systolic function; IVC within normal limits, preserved inspiratory collapse; no intra-chamber thrombi or tumour.
Radiologic exams
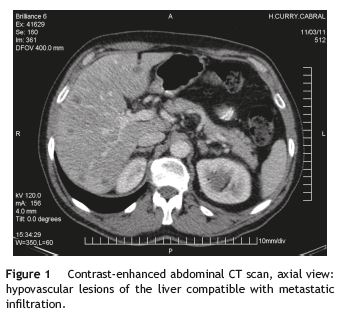
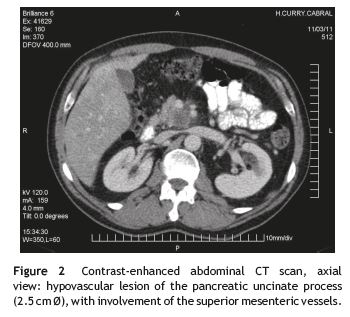
Radiologic exams revealed: (1) chest radiograph - normal; (2) venous ultrasound and Doppler of the lower limbs; (3) thoracic CT-angiogram and (4) abdominal and pelvic CT scan. The last three exams lead to the following diagnoses: (A) residual superficial venous thrombosis of the right basilic vein, maintaining deep venous (humeral and axillary) system permeability; (B) deep venous thrombosis of the right posterior tibial and calf veins, with normal popliteal, common femoral, superficial femoral vein, great saphenous and small saphenous vein permeability; left lower limb venous system with no lesions; (C) anterior segmental pulmonary embolism in the right upper lobe and the internal segmental branch of the ipsilateral inferior lobe; (D) enlarged liver with several images compatible with metastases (Fig. 1); and (E) infiltrative lesion of the pancreatic uncinate process, involving the superior mesenteric vessels and thus becoming inoperable (Fig. 2).
Treatment
He was treated with subcutaneous enoxaparin 60 mg bid, q12 h, with subsequent improvement.
Outcome
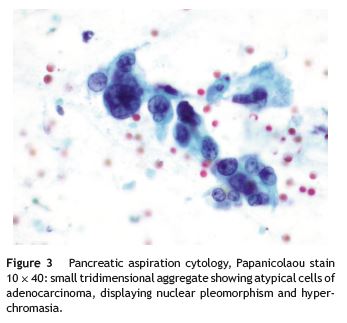
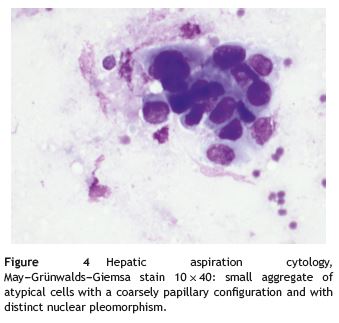
The patient was then transferred to the Lisbon Portuguese Oncology Institute, where he had an endoscopic ultrasound guided fine-needle aspiration biopsy of the liver and pancreas that confirmed a pancreatic adenocarcinoma (Fig. 3) with hepatic metastases (Fig. 4). In order to safely undergo these biopsies enoxaparin was withheld during 24 h. About 3 days after low-molecular-weight heparin (LMWH) was stopped the patient suffered a severe ischaemic stroke leaving him with right-side hemiplegia. Progressive deterioration in neurologic status quickly ensued and the patient eventually died a few days afterwards. No autopsy was made.
Discussion
The combination of conventional tumour markers, endoscopic methods and the most recent radiologic means including positron-emitting tomography (PET scan) allow us to correctly diagnose the malignancy behind TS in about 85-95% of cases.9 We stress the pivotal need - as we approach these patients in medical wards - to quickly and correctly identify the origin and histology of the underlying neoplasm, because TS is a quite serious clinical condition, and even though it is usually associated with advanced-stage cancer, there are also rare events when it helps to uncover cancer in an early phase and treat it, allowing for a better prognosis.10,11 Lifelong anticoagulation with heparin is mandatory, with an initial period of high dosage ranging from 15 to 90 days according to different authors.12,13 Abruptly interrupting heparin without previous tumour regression can be catastrophic, because procoagulating substances continue to be released by cancer cells, thus maintaining their prothrombotic effect. Withholding heparin for just a few hours can reactivate the clotting cascade and precipitate thrombotic events.2,14 Vitamin K antagonists are not effective in preventing these episodes, since the procoagulants released by neoplastic cells do not depend on this vitamin, and should not be used in this context.2
There are several explanations, probably true to some degree and most likely intertwined,15 to these prothrombotic effects in TS. Some of these are: (1) high sérum levels of tissue factor, a primary cellular initiator of blood clotting, primarily converting factor VII to its active form which then actives other proteases related to this process, particularly factor X; (2) intratumour secretion of a cystein-proteinase, activating factor X even in the absence of factor VII; (3) cancer cell hypoxia, the subsequente microenvironmental stress leading to the secretion of procoagulant and angiogenic factors, not only increasing expression of clotting-enabling genes but also correlating thrombotic processes and metastatic disease; (4) plateletrich microthrombotic processes; (5) activation of oncogenes that induce clotting; (6) toxicity from high environmental levels of iron, possibly contributing to the start and promotion of the tumour process while also instigating lipid oxidative lesions, resulting in an increased expression of tissue factor and a down-regulation of its inhibitory pathway; (7) indirect effect of inflammatory cytokines, encouraged by tumour cells, able to worsen TS by activating endotelial cells and subsequently increasing the expression of adhesion molecules including P-selectin; (8) putative action of mucines produced by some neoplasms, possibly connecting to P-selectin and L-selectin which then would promote the formation of platelet microthrombi.15
In a nutshell one might say that some structural or biochemical property of the tumour lesion that allows continuous exposure of blood to cancer cells and their procoagulant substances appears to be an essential componente of TS pathophysiology.
Conclusion
This reports goal was to relate a case of Trousseaus syndrome associated with pancreatic adenocarcinoma diagnosed during the patients stay in our Internal Medicine ward. Our patient displayed recurrent migratory venous thromboses, beginning in his extremities, and pulmonar embolism. He was placed on LMWH and subsequently showed clinical improvement regarding the thrombotic processes. The authors consider that the 24-h hiatus in heparin administration around the time of the liver and pancreatic biopsies may have triggered a boost of thrombotic activity, leading to the manifestation of an ischaemic stroke, which should alert us to the massive risk of suspending heparin in these settings.
References
1. Trousseau A. Phlegmasia alba dolens. Clinique Medicale de Hotel-Dieu de Paris, vol. 3. London: New Sydenham Society; 1868. pp. 695-727. [ Links ]
2. Bell WR, Starksen NF, Tong S, Poterfield JK. Trousseaus syndrome. Devastating coagulopathy in the absence of heparin. American Journal of Medicine. 1985;79:423-30. [ Links ]
3. Carrier M, Le Gal G,Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review. The Trousseau syndrome revisited. Should we screen extensively for cancer in patients with venous thromboembolism? Advances in Internal Medicine. 2008;149:323-33. [ Links ]
4. Callander N, Rapaport SI. Trousseaus syndrome. Western Journal of Medicine. 1993;158:364-71. [ Links ]
5. Hasegawa S, Aoyama T, Kakinoki R, Toguchida J, Nakamura T. Bilateral Phlegmasia Alba Dolens associated with Trousseaus syndrome: a case report. Archives of Physical Medicine and Rehabilitation. 2008;89:1187-90. [ Links ]
6. Santos VM, Rodrigues DB, Castro EC, Saldanha JC, Soares S, Teixeira VP, et al. Revista do Hospital das Clinicas; Faculdade de Medicina da Universidade de Sao Paulo. 2001;56:91-6 (in Portuguese). [ Links ]
7. Blann AD, Dunmore S. Arterial and venous thrombosis in cancer patients. Cardiology Research and Practice. 2011:11 (article ID 394740). [ Links ]
8. Sanon S, Lenihan DJ, Mouhayar E. Peripheral arterial ischemic events in cancer patients. Vascular Medicine. 2011;16:119-30. [ Links ]
9. Batsis JA, Morgenthaler TI. Trousseau syndrome and the unknown cancer: use of positron emission tomographic imaging in a patient with paraneoplastic syndrome. Mayo Clinic Proceedings. 2005:537-40. [ Links ]
10. Thrumurthy SG, Anuruddha AH, De Zoysa MI, Samarasekera DN. Unexpected outcome from Trousseau syndrome. BMC Surgery. 2011;11:1-3. [ Links ]
11. Womack WS, Castellano CJ. Migratory thrombophlebitis associated with ovarian carcinoma. American Journal of Obstetrics and Gynecology. 1952;63:467-9. [ Links ]
12. Masuda EM, Kessler DM, Kistner RL, Eklof B, Sato DT. The natural history of calf vein thrombosis: lysis of thrombi and development of reflux. Journal of Vascular Surgery. 1998;28:67-73. [ Links ]
13. Lautz TB, Abbas F, Walsh SJ, Chow C, Amaranto DJ, Wang E, et al. Isolated gastrocnemius and soleal vein thrombosis: should these patients receive therapeutic anticoagulation? Annals of Surgery. 2010;251:735-42. [ Links ]
14. Sack Jr GJ, Levin JK, Bell WR. Trousseaus syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine. 1977;56:1-37. [ Links ]
15. Varki A. Trousseaus syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723-9. [ Links ]
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflicts of interest
The authors have no conflicts of interest to declare.
*Corresponding author
E-mail addresses: amurinello@gmail.com, amurinello@iol.pt, antoniomurinello@yahoo.com (A. Murinello).
Received 7 August 2012; accepted 11 September 2012













