Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Jornal Português de Gastrenterologia
versão impressa ISSN 0872-8178
J Port Gastrenterol. vol.20 no.1 Lisboa jan. 2013
https://doi.org/10.1016/j.jpg.2012.04.015
Autoimmune pancreatitis and ulcerative colitis: A clinical challenge of a true association
Pancreatite auto-imune e colite ulcerosa: um desafio clínico de uma associação real
Pedro Barreiroa,∗, Pedro Pinto Marquesb, Gilberto Coutob, David Serraa,b, Cristina Chagasa, Leopoldo Matosa
a Department of Gastroenterology, Egas Moniz Hospital, Lisbon, Portugal
b Digestive Disease Center, Luz Hospital, Lisbon, Portugal
*Corresponding author.
ABSTRACT
Autoimmune pancreatitis is emerging as a well-defined clinical entity, yet its diagnosis and therapeutic approach still constitute a clinical challenge. Its association to other autoimmune diseases, namely ulcerative colitis, is known although the exact relationship between the two entities is not completely clarified.
We present the case of a patient who developed obstructive jaundice with later onset of blood-stained diarrhea, leading to a final diagnosis of autoimmune pancreatitis and ulcerative colitis.
We make a brief revision of autoimmune pancreatitis, its relationship with ulcerative colitis and therapeutic approaches of the same, namely the eventual necessity of immunosuppressive therapy.
KeywordsAutoimmune pancreatitis; Chronic pancreatitis; Ulcerative colitis; Inflammatory bowel disease; Immunosuppression
RESUMO
A pancreatite auto-imune é compreendida cada vez mais como uma entidade clínica bem definida, contudo o seu diagnóstico e abordagem terapêutica constitui ainda um desafio clínico. A sua associação com outras doenças auto-imunes, nomeadamente com a colite ulcerosa é conhecida porém a verdadeira relação entre as duas entidades não está totalmente esclarecida.
Apresentamos o caso clínico de um doente que iniciou quadro de icterícia obstrutiva chegando-se ao diagnóstico final de pancreatite auto-imune. Durante a investigação clínica o doente apresenta quadro de diarreia sanguinolenta diagnosticando-se colite ulcerosa extensa associada.
Fazemos uma breve revisão da pancreatite auto-imune, da sua relação com a colite ulcerosa e abordagem terapêutica das mesmas nomeadamente perante a eventual necessidade de terapêutica imunosupressora.
Palavras-ChavePancreatite auto-imune; Pancreatite crónica; Colite ulcerosa; Doença inflamatória intestinal; Imunosupressão
Introduction
For decades, different cases of chronic pancreatitis associated with an important immunological component have been recognized. In 1961, Sarles et al. used the term primary inflammatory pancreatitis to describe a group of patients with pancreatitis, until then of unknown aetiology, who presented little or no abdominal pain, cholestasis, increased serum immunoglobulins and severe pancreatic inflammatory infiltrate with fibrosis.1 Since then many terms were employed to describe cases of pancreatitis with similar characteristics until 1995 when, for the first time, the term autoimmune pancreatitis (AIP) was applied.2 From this date, many advances in the understanding of this entity have been recorded.
At the same time, an increased incidence of pancreatic diseases in patients with inflammatory bowel disease (IBD) has been reported, namely with ulcerative colitis (UC). This may be drug-related or due to the increased incidence of cholelithiasis among IBD patients.3 However rarer forms of chronic pancreatitis are described, and its association with AIP is underlined by different case reports, although the true incidence is still unknown.3-5
Clinical case
We present the case of a 34-year-old white man with no past medical history who developed malaise, fatigue, persistente epigastric discomfort and one month later jaundice. There was no history of alcohol intake, drug abuse or medication. The physical exam was unremarkable except for jaundice and epigastric pain. Laboratory evaluation was remarkable for abnormal liver function tests with cholestasis and slight hepatic cytolysis (alkaline phosphatase, 340 UI/L; gammaglutamyl transferase, 191 UI/L; total bilirubin, 5.57 mg/dl; aspartate aminotransferase, 86 UI/L; alanine aminotransferase, 102 UI/L). Abdominal ultrasound was consistente with extra-hepatic cholestasis and an abdominal computed tomography (CT) documented common bile duct (CBD) narrowing at the pancreatic level, which was described as normal. The endoscopic retrograde cholangiopancreatography (ERCP) confirmed the intra-pancreatic regular CBD stenosis without further changes of the extra-pancreatic bile structures (Fig. 1A). Biliary citology was negative for malignancy. Pancreatic duct canulation was unsuccessful and a 10 Fr biliary stent was placed (Fig. 1B).
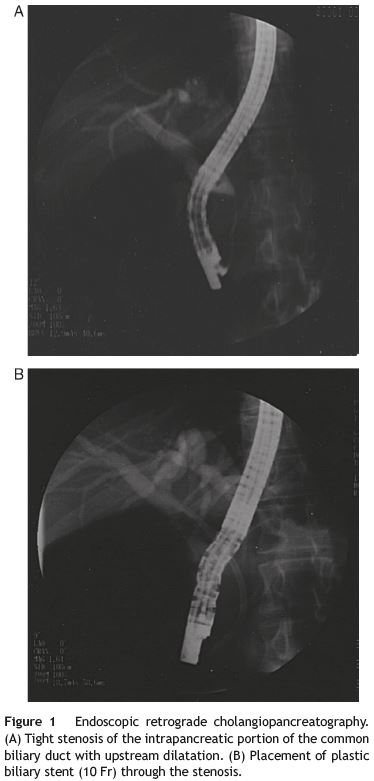
For further evaluation a magnetic resonance imagingcholangiopancreatography (MRI-CP) was ordered, which revealed discrete pancreatic head heterogeneity, with no main pancreatic duct (MPD) abnormalities. An endoscopic ultrasound (EUS) showed an abnormal pancreatic head, overall hypoechoic, heterogeneity and slightly increased, with no MPD visualization (Fig. 2). This was felt suggestive of AIP and fine needle aspiration with a 19 g Trucut needle (Cook) at the pancreatic neck was performed. Histology showed extensive pancreatic fibrosis, marked ductopenia, diffuse lymphocytic infiltration predominantly periductal as well as peri-venular lymphocytic infiltrates (Fig. 3). These findings were felt to support the diagnosis of AIP.
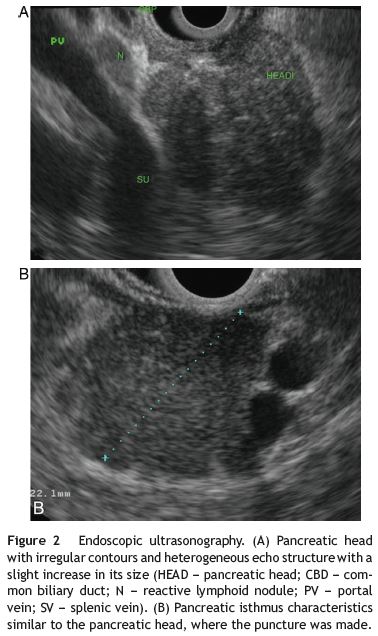
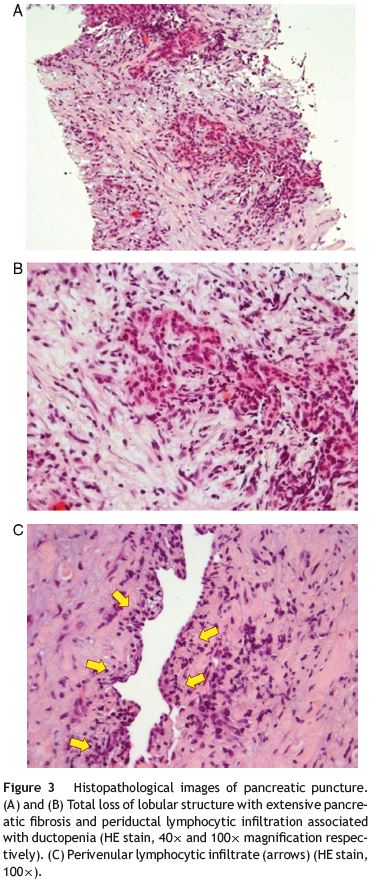
Additional laboratory evaluation showed increase of IgG4 (212 mg/dl). The autoantibodies studied (ANA, AMA, Ac Anti-DNA, ASMA, ANCA e ASCA) and the rheumatoid factor were normal.
One week after the initial diagnostic workup, the patient presented with blood-stained diarrhea and abdominal pain. Stool culture, stool evaluation for ova and parasites and Clostridium difficile toxin assay were negative. Colonoscopy showed diffuse continuous superficial erosions and ulcerations throughout the entire colon and rectum with loss of the vascular pattern. Histology supported the hypothesis of active UC diagnosis.
The clinical, analytical, imaging and histological evaluation of the patient therefore allowed for establishing the diagnosis of AIP associated with UC.
The patient was started on prednisolone 40 mg qd for 2 weeks combined with messalazine 3 gr qd. Rapid remission of all symptoms was noted as well as decreased inflammatory parameters, including Ig G4.
Although EUS after 4 weeks of treatment was identical to the initial procedure; the biliary stent was removed and no cholestasis recurrence was noted.
At 5-month the patient is in complete remission without evidence of auto-immune pancreatic activity (i.e., without signs or symptoms of pancreatic insufficiency or cholestasis).
Discussion
The diagnosis of AIP is a clinical challenge, not only due to its rarity, but also due to the need of integrating clinical, laboratory, imaging and histology data for confirmation.6,7 Because of that, AIP patients are frequently submitted to multiple exams, and some of which are invasive, until a definitive diagnosis can be reached. The clinical case presented here is an example of that, much because of the absence of characteristic imaging (such as the lack of the sausage-like aspect of the pancreas on the CT or the identification of focal pancreatic lesions) and the inability to obtain a pancreatography by ERCP, which in case of AIP typically reveals focal segmental or diffuse stenosis, with little or no dilatation of the amount of segments.6-9 Therefore, EUS proved fundamental in this case. Although no imaging criteria can be considered pathognomonic, morphology on EUS raised the suspicion which lead to the decision of obtaining pancreatic tissue,8,10,11 underscoring the fact that histological evaluation by an experienced pathologist could be considered the gold standard.6,7,12-14
The association of AIP with other autoimmune illnesses can be identified in more than half of the cases.11,13 They can precede the pancreatic illness diagnosis or present later during the natural course of the disease.9 Among these, the association with IBD, and more specifically with UC, has been described, being the most common in an Italian series (35% of analysed cases).9,10,13 Overall, however, the true dimension of the relationship between these two entities is still not totally clear. This is likely due to the fact that only recently AIP has been considered a proper nosological entity with well defined diagnostic criteria. Indeed, we believe that previous case reports referring to chronic pancreatitis with indeterminate aetiology associated to UC probably were cases of autoimmune pancreatitis based on current diagnosis criteria.15
In fact, the observed pancreatic alterations of patients with UC are more frequent than initially expected. Although UC patients present an increase incidence of gallbladder lithiasis and are administered drugs that can potentially be pancreato-toxic, these factors alone are probably not enough to explain the great incidence of pancreatic alterations among UC patients.16 Some studies demonstrate insufficient levels of pancreatic exocrine in 21-80% of IBD patients and autopsy studies register pancreatic alterations, macroscopic or microscopic, in 14-53% of UC patients. Pancreatic duct changes, such as irregularities or short-segment stenosis of the main pancreatic duct, were observed in 8.4-10.8% of IBD patients independently of prior history of pancreatitis or exocrine insufficiency.4,17 It seems that predominantly asymptomatic pancreatic alterations of indolente development might exist in these patients, albeit the fact that the exact aetiology and pathogenesis are still poorly understood. We believe that a large spectrum of pancreatic changes can be documented in IBD patients, from symptomfree cases (likely the majority) to clinically exuberant forms such as the case of our patient. The aetiopathogenesis could be related to an abnormal immunological response leading to pancreatic inflammation such as Ectors et al. previously suggested.18
The association between AIP and UC presents a clinical challenge concerning the treatment strategy. UC patients need immunosuppressive treatment in up to 30% of cases.19 Thiopurins (azathioprine and 6-mercaptopurine) continue to be the most widely used. However, potential pancreatic adverse effects are well established, raising concerns of its use in patients with AIP. In this setting other therapies (e.g. methotrexate or biological therapy) could step-in as first line options.20 There are some authors who advise against the use of thiopurins in AIP, although its use has been described as presenting good results in cases of relapse of AIP with a low level of adverse effects.21-23 Albeit more studies are needed, its use can be justified to avoid long-term treatment with corticosteroids, under close monitoring for pancreatic toxicity.
In respect to corticotherapy, a good clinical response is considered by some groups as a diagnostic criterion for AIP.7 In our case, a clinical and analytical improvement was seen, with no cholestasis relapse after biliary stent removal. Pancreatic morphology improvement on EUS was not observed after corticotherapy, supporting the idea of an irreversible extensive fibrotic process.11
A word of caution is in order, concerning the uneventful evolution of the presented case. A long-term follow-up strategy is mandatory, namely to maintain a low threshold for future associated autoimmune illnesses.
References
1. Sarles H, Sarles JC, Muratoren R, Guien C. Chronic inflammatory sclerosis of the pancreas - an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688-98. [ Links ]
2. Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-8. [ Links ]
3. Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol. 2010;44:246-53. [ Links ]
4. Barthet M, Lesavre N, Desplats-Jego S, Panuel M, Gasmi M, Bernard JP, et al. Frequency and characteristics of pancreatitis in patients with inflammatory bowel disease. Pancreatology. 2006;6:464-71. [ Links ]
5. Barthet M. Acute pancreatitis: an emerging presentation for autoimmune pancreatitis in patients with inflammatory bowel disease. Gastroenterol Hepatol (NY). 2009;5:431-3. [ Links ]
6. Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626-31. [ Links ]
7. Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, et al. Diagnosis of autoimmune pancreatitis: The Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-6. [ Links ]
8. Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, Mueller PR, et al. Autoimmune pancreatitis: imaging features. Radiology. 2004;233:345-52. [ Links ]
9. Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99: 1605-16. [ Links ]
10. Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670-6. [ Links ]
11. Quereda LA. Chronic autoimmune pancreatitis. Rev Esp Enferm Dig. 2008;100:490-502. [ Links ]
12. Kim KP, Kim MH, Kim JC, Lee SS, Seo SK. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006;12:2487-96. [ Links ]
13. Pearson RK, Longnecker DS, Chari ST, Smyrk TC, Okazaki K, Frulloni L, et al. Controversies in clinical pancreatology. Autoimmune pancreatitis: does it exist? Pancreas. 2003;27:1-13. [ Links ]
14. Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, et al. Histopathological features of diagnostic and clinical revelance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchow Arch. 2004;445:552-63. [ Links ]
15. Barthet M, Hastier P, Bernard JP, Bordes G, Frederick J, Alio S, et al. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94:2141-8. [ Links ]
16. Bhatt SP, Makharia GK. An unusual association between chronic pancreatitis and ulcerative colitis. J Pancreas. 2008;9:74-5. [ Links ]
17. Heikius B, Niemela S, Lehtola J, Karttunen T, Lahde S. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1996;31:517-23. [ Links ]
18. Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41:263-8. [ Links ]
19. Faubion WA, Loftus EV, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255-60. [ Links ]
20. Floyd A, Pederson L, Nielsen GL. Risk of acute pancreatitis in uses of azathioprine: a population-based case---control study. Am J Gastroenterol. 2003;98:1305-8. [ Links ]
21. Matsushita M, Ikeura T, Fukui T, Uchida K, Okazaki K. Refractory autoimmune pancreatitis: azathioprine or steroid pulse therapy? Am J Gastroenterol. 2008;103:1834, author reply 1834-1835. [ Links ]
22. Church NI, Pereira SP, Deheragoda MG, Sandanayake N, Amin Z, Lees WR, et al. Autoimmune pancreatitis: clinical and radiological features and objective response to steroid therapy in a UK series. Am J Gastroenterol. 2007;102:2417-25. [ Links ]
23. Ghazale A, Chari ST. Optimising corticosteroid treatment for autoimmune pancreatitis. Gut. 2007;56:1650-2. [ Links ]
Conflicts of interest
The authors have no conflicts of interest to declare.
*Corresponding author.
E-mail address: pedrobarreiro@msn.com (P. Barreiro).
Received 21 July 2011; accepted 5 October 2011













