Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.38 no.6 Coimbra set. 2020
https://doi.org/10.4152/pea.202006401
ARTIGOS
Influence of Zinc Sulphate on the Corrosion Resistance of L80 Alloy Immersed in Sea Water in the Absence and Presence of Sodium Potassium Tartrate and Trisodium Citrate
Grace Babya, Susai Rajendranb,*, V. Johnsirania and Abdulhameed Al-Hashemc
aPG Department of Chemistry, Sakthi College of Arts and Science for Women, Oddanchatram-624 619, India
bCorrosion Research Centre, Department of Chemistry, St Antony`s College of Arts and Science for Women, Dindigul-624005, India
cSenior Research Scientist, Petroleum Research Centre, Kuwait Institute for Scientific Research, Kuwait
ABSTRACT
In cooling water systems, seawater can be used. L 80 can be used as a pipeline for carrying sea water. However, this alloy will undergo corrosion. Corrosion can be prevented by the addition of inhibitors such as sodium potassium tartrate (SPT), trisodium citrate (TSC) and zinc sulphate. Corrosion resistance of L 80 alloy in sea water, in the absence and presence of the above inhibitors, has been evaluated by polarization study and AC impedance spectra. It was observed that SPT and TSC show better inhibition efficiency in the presence of Zn 2+. Further, it was found that the SPT-Zn system is better than the TSC-Zn system.
Keywords: corrosion inhibition, seawater, sodium potassium tartrate, trisodium citrate, polarization study, AC impedance spectra, L80 alloy.
Introduction
Seawater makes up the oceans and seas, covering more than 70 percent of Earth surface. Seawater is a complex mixture of 96.5 percent of water, 2.5 percent of salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates and a few atmospheric gases [1]. Almost anything can be found in seawater. This includes dissolved materials from Earth crust, as well as materials released from organisms. The most important components of seawater that influence life forms are salinity, temperature, dissolved gases (mostly oxygen and carbon dioxide) and nutrients [2].
Seawater can be used in cooling water systems, especially in ships and in marine environments. In these systems, L 80 alloy can be used to carry out seawater. Hence, knowledge of corrosion resistance of L80 alloy in seawater, in the presence of inhibitors, will be useful. Corrosion resistance of aluminium and its alloys [3, 4], zinc and its alloys [5, 6], copper and its alloys [7, 8], mild steel [9, 10], stainless steel [11, 12], nickel and its alloys [13, 14] in seawater has been evaluated. Corrosion inhibitors such as inorganic compounds [15, 16] and natural products [17, 18] have been used to prevent the corrosion of metals and alloys. In the present study, corrosion resistance of L 80 alloy in seawater, in the presence of sodium potassium tartrate (SPT) and trisodium citrate (TSC), has been evaluated by electrochemical studies, such as polarization study and AC impedance spectra. Influence of Zn2+ on the corrosion resistance of the above systems has also been evaluated.
Experimental
Electrochemical studies
In the present work, corrosion resistance of L 80 alloy immersed in various test solutions was measured by Polarization study and AC impedance spectra.
Polarization study
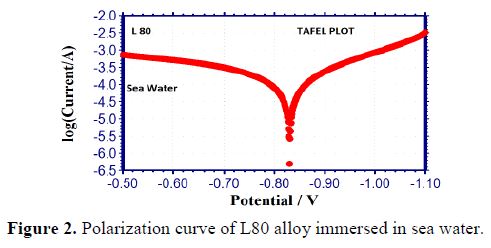
In the present study, polarization studies were carried out in a CHI Electrochemical work station/ analyzer, 660A model. It was provided with an automatic IR compensation facility. A three electrode cell assembly was used, as shown in Fig. 1.
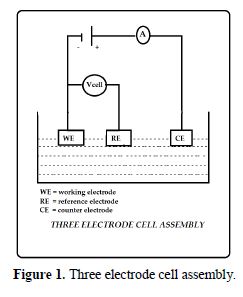
The working electrode was L 80 alloy. SCE was the reference electrode. Platinum was the counter electrode. A time interval of 5 to 10 min was given for the system to attain a steady state open circuit potential. The working electrode and the platinum electrode were immersed in sea water, in the absence and presence of the inhibitor. Saturated calomel electrode was connected with the test solution through a salt bridge. From the polarization study, corrosion parameters, such as corrosion potential (Ecorr), corrosion current (Icorr), Tafel slopes anodic = ba and cathodic = bc and LPR (linear polarization resistance) values, were considered. The scan rate (V/S) was 0.01. Hold time at (Efcs) was zero and quiet time (s) was two.
AC impedance spectra
In the present investigation, the same instrument and set-up used for the polarization study was used to record AC impedance spectra. A time interval of 5 to 10 min was given for the system to attain a steady state open circuit potential. The real part (Z’) and imaginary part (Z’’) of the cell impedance were measured in ohms at various frequencies. AC impedance spectra were recorded with initial E(v) = 0, high frequency (Hz = 1 x 105), low frequency (Hz = 1), amplitude (V) = 0.005 and quiet time (s) = 2. From Nyquist plot, the values of charge transfer resistance (Rt) and double layer capacitance (Cdl) were calculated.
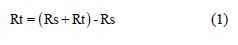
where Rs = solution resistance.
Cdl values were calculated using the relationship:
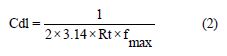
where fmax = frequency at maximum imaginary impedance.
Sea water
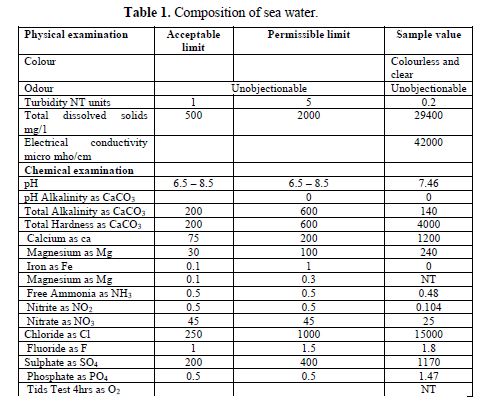
The composition of sea water used in this study is given in table 1. Sea water was collected in the Bay of Bengal, located at Kanampadi, East Coast Road, Chennai, India.
L80 alloy
L80 alloy was manufactured according to 5CT API specification. This is a controlled yield strength material with a hardness testing requirement.
L80 is usually used in wells with sour (hydrogen sulfide) environments. Chemical composition of L80 alloy is given in table 2.
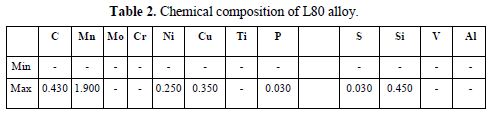
The remaining Wt % was iron.
Used inhibitors
Pure samples of Sodium Potassium Tartrate (SPT), Tri Sodium Citrate (TSC) and Zinc Sulphate were used as corrosion inhibitors.
Results and discussion
Electrochemical studies
In the present work, corrosion resistance of L80 alloy immersed in various test solutions was measured by polarization study and AC impedance spectra.
L80 alloy –sodiumpotassium tartrate (SPT) system
Polarization study
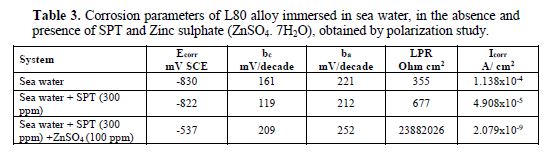
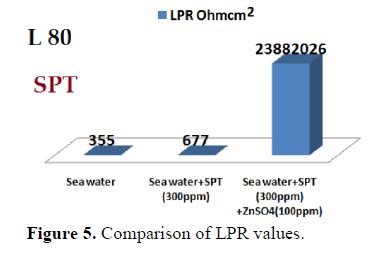
It is seen from table 3 that when SPT is added to sea water, the corrosion resistance of L80 alloy increases. This is revealed by the fact that when SPT is added to sea water, LPR values increases and Icorr value decreases, as shown in Figs. 2 to 5.
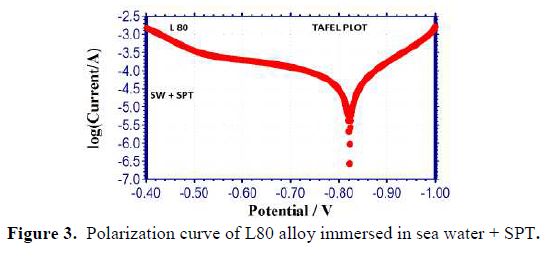
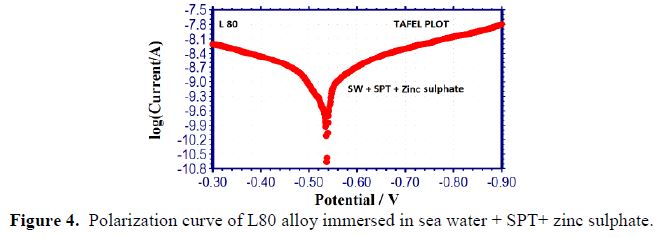
Influence of Zinc sulphate (ZnSO4.7H2O)
When ZnSO4.7H2O was added to the above system, LPR value increased to a great extent (Fig. 5) and Icorr decreased considerably. This indicates that, in the presence of SPT - ZnSO4.7H2O system, the corrosion resistance of L80 alloy in sea water increases to a great extent. A protective film is formed on the metal surface. This prevents transfer of electrons from anodic site to cathodic site. Hence, LPR value increases, which results in a decrease in the corrosion current value.
AC impedance spectra
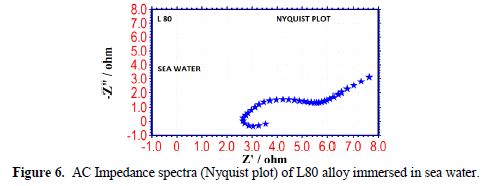
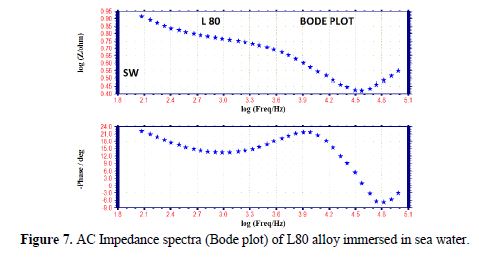
The AC impedance spectra (Nyquist plots, Bode plots) of L80 alloy immersed in various test solutions are shown in Figs. 6 to 11. The corrosion parameters are given in table 4. It is observed from table 4 that, when SPT is added to sea water, the corrosion resistance of L80 alloy increases. This is revealed by the fact that there is an increase in Rt value, impedance value and decrease in Cdl value.
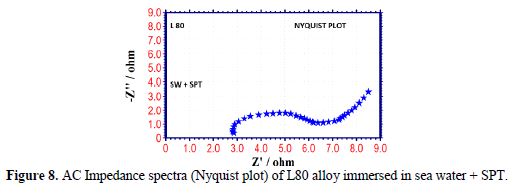
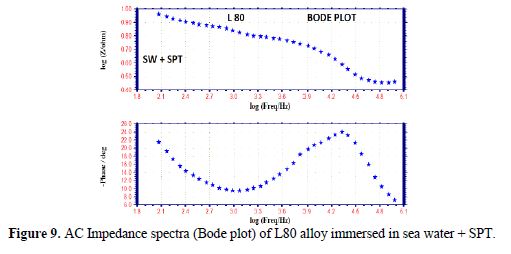
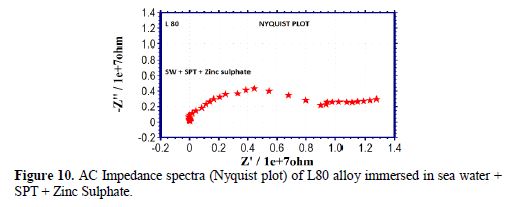
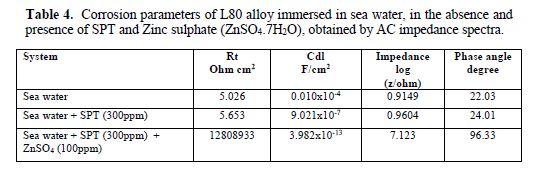
Influence of Zinc sulphate (ZnSO4. 7H2O)
When ZnSO4.7H2O is added to the above system, the corrosion resistance further increases (Fig. 12). This is revealed by the fact that there is an increase in Rt values and impedance value. There is a decrease in Cdl. Thus, electrochemical studies reveal that the corrosion resistance of L80 alloy in sea water decreases in the following order:
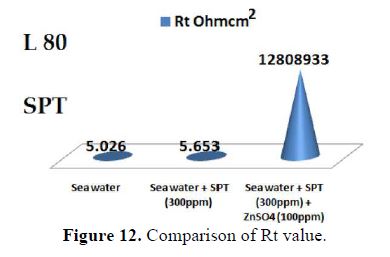
Sea water + SPT + ZnSO4 > Sea water + SPT > Sea water.
L80 alloy – TSC system
Polarization study
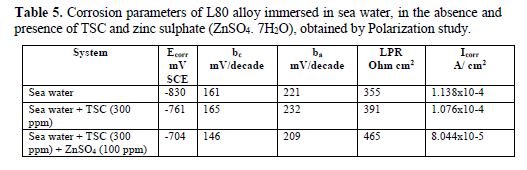
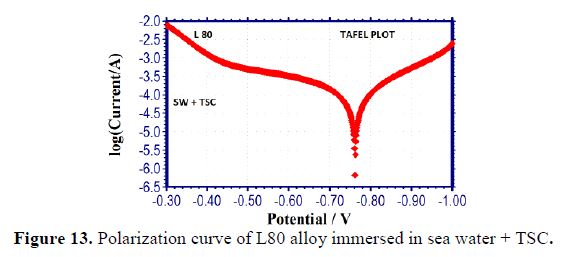
It is seen from table 5 that, when TSC is added to sea water, the corrosion resistance of L80 alloy increases. This is revealed by the fact that when TSC is added to sea water, LPR values increases and Icorr value decreases (Figs. 13 and 14). There is the formation of a protective film on the metal surface. So, the transfer of electrons from anodic site to cathodic site is restricted. Hence, LPR value increases and, correspondingly, corrosion current value decreases.
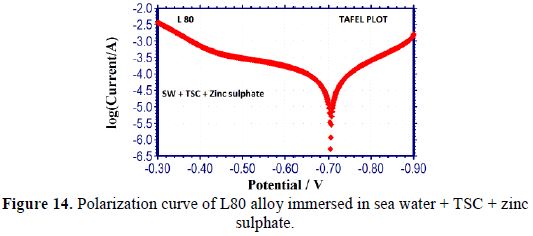
Influence of Zinc sulphate (ZnSO4.7H2O)
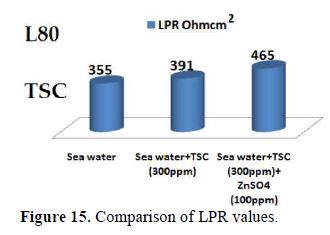
When ZnSO4.7H2O is added to the above system, LPR value increases tremendously (Fig. 15) and Icorr decreases very much. This indicates that, in the presence of SPT - ZnSO4.7H2O system, the corrosion resistance of L80 alloy in sea water increases to a great extent.
AC impedance spectra
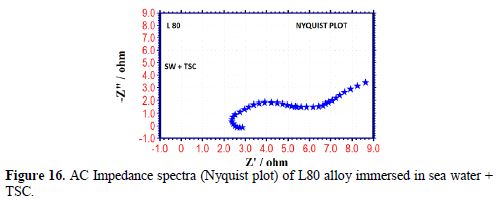
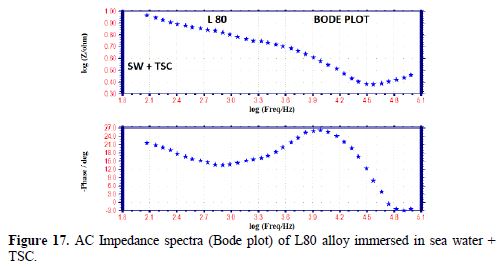
The AC impedance spectra (Nyquist plots, Bode plots) of L80 alloy immersed in various test solutions are shown in Figs. 16 to 19. The corrosion parameters are given in table 6. It is observed from table 6 that, when TSC is added to sea water, the corrosion resistance of L80 alloy increases. This is revealed by the fact that there is an increase in Rt value and impedance value and a decrease in Cdl value.
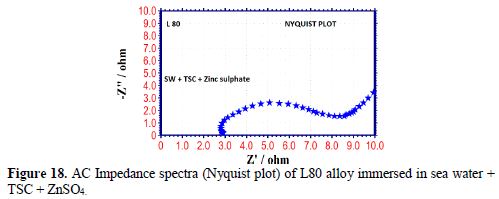
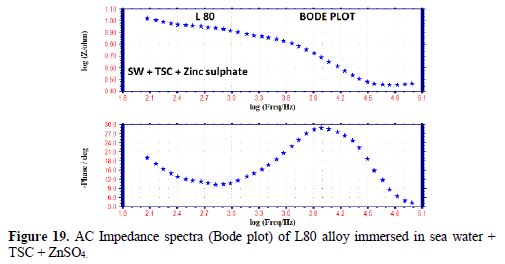
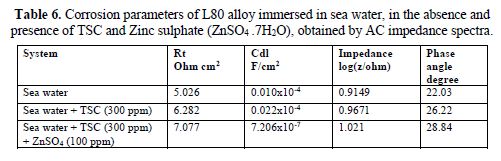
Influence of Zinc sulphate (ZnSO4 .7H2O)
When ZnSO4.7H2O was added to the above system, the corrosion resistance further increased. This is revealed by the fact that there is an increase in Rt values (Fig. 20) and impedance value. There is a decrease in Cdl. Thus, electrochemical studies [19-28] reveal that the corrosion resistance of L80 alloy in sea water decreases in the following order:
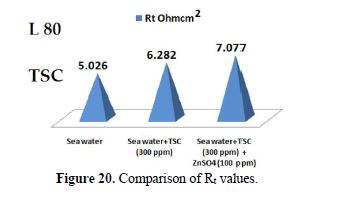
Sea water + TSC + ZnSO4 > Sea water + TSC > Sea water
Conclusion
The corrosion resistance of L80 alloy in sea water, in the absence and presence of sodium potassium tartate (SPT), trisodium citrate (TSC) and zinc sulphate (ZnSO4 . 7H2O), has been evaluated by electrochemical studies, such as polarization study and AC impedance spectra. The study led to the following conclusions:
L80 alloy – SPT system
Electrochemical studies revealed that the corrosion resistance of L80 alloy in sea water decreases in the following order:
Sea water + SPT + ZnSO4 > Sea water + SPT > Sea water
L80 alloy – TSC system
Electrochemical studies revealed that the corrosion resistance of L80 alloy in sea water decreases in the following order:
Sea water + TSC + ZnSO4 > Sea water + TSC > Sea water
Addition of Zinc sulphate improves the corrosion resistance of the TSC and SPT systems.
This study also led to the conclusion that the SPT-Zinc system offers better corrosion resistance than the TSC-Zinc system.
References
- Duxbury AC, Byrne RH, et al. Encyclopaedia Britannica. Available from: https://www.britannica.com/science/seawater.
- Genevieve (Genny) Aderson. Seawater Composition. Marine Science. Available from: http://www.marinebio.net/marinescience/02ocean/swcomposition.htm.2003; 2.1.1.
- Osman MM. Inhibition of aluminium-brass in 3.5% NaCl solution and sea water. Mater Chem Phys. 2001; 71:12-16.
- Berchmans LJ, Iyer SV, Sivan V, et al. 1,2,4,5 tetrazo spiro (5,4) decane-3 thione as a corrosion inhibitor for arsenical aluminium brass in 3.5% NaCl solution. Anti-Corros Methods Mater. 2001;48: 376-381.
- Aramaki K. Cerium (III) chloride and sodium octylthiopropionate as an effective inhibitor mixture for zinc corrosion. Corros Sci. 2002;44:1361-1374.
- Taha KK, Muhideen A. Characterization of anti-corrosion triazole film. J Sci Tech. 2009;10: 92-99.
- Aljinovic LJ, Gudic S, Smith M. Inhibition of CuNi10Fe in sea water by sodium-diethyl-dithiocarbamate: an electrochemical and analytical study. J Appl Electrochem. 2000;30: 973-979.
- Dafali A, Hammouti B, Aouniti A, et al. 2-Mercapto-1-methylimidazole as corrosion inhibitor of copper in aerated 3% NaCl solution. Annales Chimie Sci Matériaux. 2000;25: 437-446.
- Majumdar I, D’Souza F, Bhosle NB. Microbial exopolysaccharides: Effect on corrosion and partial chemical characterization. J Ind Inst Sci. 1999;79: 539-550.
- Rajendran S, Apparao BV, Palaniswamy N. Corrosion inhibition by phosphonic acid – Zn systems for mild steel in chloride medium. Anti-Corros Methods Mater. 2000; 47: 359-365.
- Dubey RS, Potdar Y. Corrosion inhibition of 304 stainless steel in sodium chloride by ciprofloxacin and norfloxacin. Ind J Chem Tech. 2009;16: 334-338.
- Aramide FO. Corrosion inhibition of AISI/SAE Steel in a marine environment. Leonardo J Sci. 2009;15: 47-52.
- Laamari MR, Derja A, Benzakourm J, et al. Calcium monofluorophosphate: a new class of corrosion inhibitors in NaCl medium. J Electroanal Chem. 2004;569: 1-6.
- Ozyilmaz AT, Erbil M, Yazici B. The influence of polyaniline (PANI) top coat on corrosion behavior of nickel plated copper. Appl Surf Sci. 2005; 252: 2092-2100.
- Aballe A, Bethencourt M, Botana FJ, et al. Electrochemical noise applied to the study of the inhibition effect of CeCl3 on the corrosion behaviour of Al-Mg alloy AA5083 in sea water. Electrochim Acta. 2002;47:1415-1422.
- Aramaki K. Cerium (III) chloride and sodium octylthiopropionate as an effective inhibitor mixture for zinc corrosion. Corros Sci. 2002;44:1361-1374.
- Rani PD, Selvaraj S. Inhibitive action of Vitis vinifera (Grape) on copper and brass in natural sea water environment. Rasayan J Chem. 2010;3: 473-482.
- Deepa Rani P, Selvaraj S. Emblica Officinalis (OMLA) leaves extract as corrosion inhibitor for copper and its alloy (Cu-27Zn) in natural sea water. Arch Appl Sci Res. 2010;2: 140-150.
- Rajendran S, Devi MK, Regis APP, et al. Electroplating using environmental friendly garlic extract, A case study. Zastita Materijala. 2009; 50: 131-140.
- Rajendran S, Chitradevi P, Johnmary S, et al. Corrosion behaviour of SS 316 L in artificial saliva in presence of electral. Zastita Materijala. 2010;51: 149-158.
- Rajendran S, Agasta M, Devi RB, et al.Corrosion inhibition by an aqueous extract of Henna leaves (Lawsonia Inermis L). Zastita Materijala. 2009;50: 77-84.
- Mary ACC, Rajendran S, Jeyasundari J. Influence of Coffee on the corrosion resistance of SS 316L, Ni-Ti alloy and thermoactive alloy in artificial saliva. Eur Chem Bull. 2017;6: 232-237.
- Sribharathy V, Rajendran S, Rengan P, et al. Corrosion Inhibition By An Aqueous Extract Of Aleovera (L) Burm F.(Liliaceae), Eur Chem Bull. 2013;2: 471-476.
- Epshiba R, Regis APP, Rajendran S. Inhibition Of Corrosion Of Carbon Steel In A Well Water By Sodium Molybdate–Zn 2 System. Int J Nano Corros Sci Eng. 2014;1: 1-11.
- Kavitha N, ManjulaCorrosion Inhibition of Water Hyacinth Leaves, Zn2+ and TSC on Mild Steel in neutral aqueous medium. Int J Nano Corros Sci Eng. 2014;1: 31 - 38.
- Nagalakshmi R, Nagarajan L, Joseph Rathish R et al. Corrosion Resistance Of SS316l In Artificial Urine In Presence Of D-Glucose. Int J Nano Corros Sci Eng. 2014;1: 39- 49.
- Angelin Thangakani J, Rajendran S, Sathiabama J, et al.Inhibition Of Corrosion Of Carbon Steel In Aqueous Solution Containing Low Chloride Ion By Glycine – Zn2+ Int J Nano Corros Sci Eng. 2014;1:50 - 62.
- Gowri S, Sathiyabama J, Rajendran S, et al. Tryptophan as corrosion inhibitor for carbon steel in sea water. J Chem, Biol Phys Sci. 2012; 2: 2223.
Received June 15, 2018; accepted August 20, 2020
* Corresponding author. E-mail address: susairajendran@gamil.com
Acknowledgements
The authors are thankful to their respective managements for their help and encouragements. Special thanks are due to the Congregation of The Immaculate Conception (CIC), Rev. Sr. Gnana Sundari, Provincial, Madurai Province of CIC, Rev. Sr. M Margaret Inbaseeli, the Secretary and Rev. Sr. Mary Pramila Shanthi, the Principal, St Antony`s College of Arts and Science for Women, Dindigul-624005, India.














