Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.38 no.5 Coimbra jun. 2020
https://doi.org/10.4152/pea.202005331
ARTIGOS
Elaboration of Amorphous and Crystalline Titanium Dioxide on FTO; its Characterization and Photocatalytic Effect on Orange Methyl
Rahal Foudila and Abdi Djamilab*
a Laboratoire d’énergétique et d’électrochimie du solide. Département de génie des procédés, Faculté de Technologie, U. F.A. Sétif 1, 19000. Algérie
b Laboratoire d’énergétique et d’électrochimie du solide. Département de chimie, Faculté des Sciences, U. F.A. Sétif 1, 19000. Algérie
ABSTRACT
In this paper, we report on the preparation of an amorphous and nanocrystalline structure of titanium dioxide, by cathodic electrodeposition on fluorine doped tin dioxide (FTO) coated glass, from an aqueous peroxo-titanium complex solution. Structural X-ray analysis shows anatase phase for heated deposits, and an amorphous form for non-heated deposits. Scanning electron microscopy (SEM) allowed investigating the morphological aspect of the deposits which exhibited nano-particulate grain size. AFM results exhibited the different roughness values of both kinds of films. The crystalline deposits have been used as catalysts for the photocatalytic oxidation of methyl orange dye, under an irradiation source (UV lamp of 365 nm). The results revealed that the dye has undergone a slight degradation under UV illumination. Using spectrophotometer measurements, the decolourisation rate was estimated from residual concentration.
Keywords: titanium dioxide; electrodeposition; nanostructures; photocatalytic oxidation, methyl orange dye.
Introduction
Nanostructured semiconductor materials have been the prime focus of scientific research, due to their unusual optical, chemical, photo electrochemical and electronic properties. Since several years, nanocrystalline titanium dioxide (TiO2) is one of the most investigated oxide materials, due to its important applications in environmental cleanup, hydrophilic coating, gas sensors and dye-sensitized solar cell DSSC (1–3).
It is also used in photocatalytic processes, because, compared to other semiconductors, it is chemical and biologically inert, photocatalytically stable, relatively easy to produce, able to ef?ciently catalyze reactions, cheap, and without risks to the environment (4).
Dyes are widely used in many industries. Some of them are non-biodegradable, and have to be eliminated before being released into natural water streams. Studies have proven that most of them are toxic (5-6), and it is difficult to remove them from the water environment, as they are designed to be resistant to physical, chemical and microbial fading (7). However, as common wastewater treatments are not always effective, one of the new alternative biodegradation treatments consists of TiO2, which has the photocatalytic ability to cut down organic compounds (8-11). Colorants degradation in visible light or under artificial illumination using TiO2 has been earlier reported by Chatterjee and Mahata (12), and by a number of researchers who dealt with heterogeneous dye photocatalytic decomposition (13-16). Wang investigated MeO photocatalytic degradation with other commercial dyes in TiO2 suspension, under solar light (17). In fact, photo catalysis can be determined as a photo induced reaction that is accelerated by the presence of a catalyst (semiconductor), in which reactions are activated by the absorption of an energy photon (equal to or higher than the band-gap energy (Ebg) of the catalyst). The absorption leads to a charge separation, due to the promotion of an electron (e-) from the semiconductor catalyst’s valence band to the conduction band, thus generating a hole (h+). In order to favor the photo catalyst reaction, the recombination of the electron and the hole must be prevented as much as possible, leading the transfer of the activated electron to the oxidant, and producing a reduced product.
The research described by this paper included: the preparation of amorphous and crystalline titanium dioxide films on FTO, by electrodeposition, after cathodic deposition optimization; the study of their structural characterization by XRD, and of their morphological aspects by SEM and AFM; and the application and testing of their ability as catalysts under illumination, for methyl orange dye photocatalytic oxidation, under an irradiation source (UV lamp of 365 nm).
Experimental
Materials and instruments
Analytical grade chemicals of titanium salt (TiOSO4) and peroxide (H2O2) were purchased from Sigma-Aldrich; potassium nitrate (KNO3) and nitric acid (HNO3) are Biochem products.
Methyl orange (MeO) was obtained from Sigma Chemie GmbH (Germany), and used without further purification. MeO structure is C14H14N3SO3Na.
The photo catalyst used in this work was TiO2 crystalline films obtained after heat-treatment, to give them a better active surface. All deposits were prepared in a small three electrode cell. Optically transparent fluorine-tin-oxide coated glass plates (FTO, 20Ω/square) were used as cathodic substrates with an area of 1.5 cm2. A Pt plate (20×10×1 mm) was used as counter electrode and saturated Ag/AgCl as reference electrode. A Voltalab 340 potentiostat was employed. Prior to the film deposition, FTO substrates were sequentially cleaned in acetone, propanol and water, each one for 5 min, and, finally, the substrate was etched in 45% HNO3, for 2 min. FTO was used as a substrate for many reasons: it behaves as a TCO (transparent conductive oxide), thanks to its conductivity; TiO2 electrodeposition may be easily done; its high degree of transparency facilitates catalysts’ optical absorption measurements and favors the deposits’ optical properties investigation and their UV-Vis behavior before and after illumination, and it also permits both front and back-side illumination for the study of photo electrodes. Titanium dioxide films were cathodically electrodeposited from an aqueous peroxo-titanium solution. The deposition bath was mixed in a KNO3:TiOSO4:H2O2=1:0.2:0.3 ratio, and with an amount of HNO3, to adjust the pH at 1.4. The bath temperature was maintained at 20 ºC. H2O2 acts as generator of OH-, responsible for the increase in local pH that will react with Ti4+, to give titanium hydroxide on FTO.
Cathodic electrodeposition was carried out between 0 mV and -1.4 mV (versus saturated Ag/AgCl), which led to the formation of a TiO(OH)2.xH2O gel film on FTO. Subsequently, some films were subjected to heat-treatment in air, for 1 hour, at 450 ºC, to obtain a crystalline titanium dioxide thin film (18). The phenomenon of precipitation during TiO2 electrodeposition is a well-known problem for pH values greater than 2; however, the right precursor concentration and pH adjustment to a lower value, up to 1.4, allowed to avoid the precipitation, and, thus, the deposition only occurred at the surface.
The crystallization behavior of the fresh and heat-treated electrodeposited titanium dioxide films was analyzed at room temperature by X-ray diffraction (XRD), using a PHILIPS X'PERT diffractometer with Cu Kα radiation, and a 2θ scan rate of 0.01°/s. The surface morphological features of the films were observed using a LEO 982 scanning electron microscope (SEM). Images of the films were obtained using atomic force microscopic (AFM), and the scans were made on areas of the films with 1 μm x 1 μm.
Absorbance measurements
The absorbances of dye solutions, before and after degradation, were measured at different degradation times, under a UV light irradiation source. Measurements were carried out using a UV-1800 SHIMADZU UV–vis spectrophotometer and a UV-Probe software for the curves´ analysis. The percentage of degradation was calculated from the following equation:
Degradation % = (1-Ao/At) x 100 (1)
where At is the absorbance after time (t) and Ao is the dye initial concentration before degradation.
Results and discussion
Structural and morphological characterization
Fig. 1 (a and b) shows X-ray diffraction (XRD) of titanium dioxide films, as deposited and annealed at 450 ºC. Pattern a and b (Fig. 1) show the characteristics peaks of the as-deposited film and of the film heated at 450 ºC, for 1 hour, in air. The as-deposited film was found to be amorphous, as indicated by the absence of any diffraction peaks. Such results have been reported by (19-21), and a faint broad peak at about 2θ = 25 was observed. The heat-treated film at 450 ºC confirms the formation of an anatase structure of TiO2; the sharp peak detected at 25.77 can be attributed to its anatase phase, according to (22-24). The peaks indexed with an asterisk are related to the FTO substrate; the related JCPDS-file number is (21-1272).
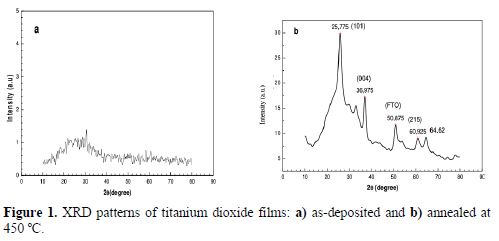
The average crystallite sizes of titanium dioxide deposits were estimated from the X-ray line broadening analysis by the Debye-Sherrer’s equation:
where θ is the Bragg angle, λ = 1.542Å is the wavelength of the X-ray radiation and β is full width at half maximum (FWHM). The calculation based on the peak at 2θ = 25.775 gave a value of 4.9 nm, which shows the nanoscale range of the grains.
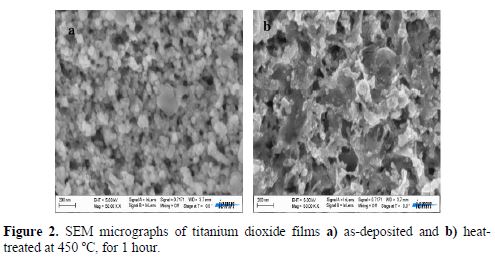
Fig. 2 shows scanning electron microscopy (SEM) images of as-deposited (Fig 2a) and heat-treated films, at 450 ºC, for 1 hour (Fig. 2b). It exhibits distinguished spherical morphology, and confirms the formation of nano particulate and nanoporous films. The as-deposited film is quite homogenous, clearly indicating that the particles are roughly spherical, and it shows areas richer in crystallites. Some cracks were observed on the heat-treated titanium dioxide, which was attributed to the film dehydration when subjected to heat-treatment, accordingly to the reaction:
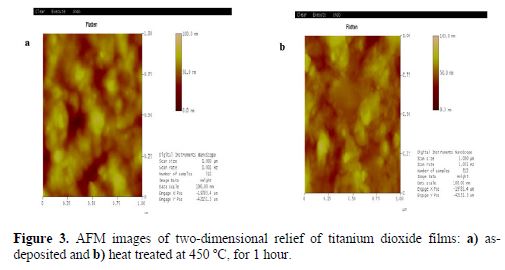
AFM technique was used to investigate surface morphology and topographic relief of our deposits. Figs. 3a and b show the AFM top-view of two-dimensional thin surface plots TiO2 films, as-deposited and annealed, at 450 ºC, for 1 hour, respectively. The surface morphology and roughness of the two films show the main differences between both deposits. Careful examinations of the images show that the heat-treatment at 450 ºC produced changes in the surface topography. The AFM image of the as-deposited titanium dioxide (Fig. 3a) indicates the formation of a rough surface, and the mean square roughness value is 8.81 nm. The appearance of the annealed film AFM image (Fig. 3b) seems to be quite similar to the as-deposited film, and the mean square roughness value decreased (6.79 nm), which was probably due to the formation of particles aggregates by heat-treatment. Such observations agree with our SEM pictures and XRD calculation peak size, and they are in accordance with previous studies (30-31).
Electrochemical characterization
The electrochemical characterization of the titanium dioxide thin films on as-deposited (crystalline) and sintered FTO, at 450 ºC, for one hour, in the air (amorphous), was carried out by a fast scan rate (100 mV/s) cyclic voltamperometry experiment, in order to establish the electrode active surface area of the titanium dioxide electrode in a 0.1 M K2SO4 solution Fig. 4 (a and b) .
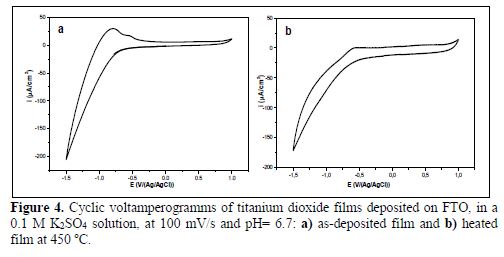
The cyclic voltamperograms present no significant currents at positive potentials, ca. -0.2 V versus Ag/AgCl, as expected for an n-type semiconductor (27). At potentials more negative than ca. -0.2 V versus Ag/AgCl, a cathodic peak and an ill-defined anodic hump or peak were observed; the former corresponding to the reduction of Ti(4) species at the surface in contact with the electrolyte to Ti(3), and the latter to the re-oxidation process obtained during the positive potential surface scan of Ti(3) species, giving rise to a couple of conjugated cathodic and anodic peaks, characterized by an irreversible system (32-33).
We also can observe that the as-deposited titanium film produced higher current values than those from the heated deposit, in the potential range from -0.5 to -1.5. The current of the as-deposited film is approximately 15 times higher than that corresponding to the heated film, which must be attributed to the high conductivity of the OH- of the as-deposited TiO(OH)2 film, on one hand, and to the decrease in the active surface site of the heated thin film, on the other. In the studied potential range, the hydrogen adsorption-desorption process also takes place (34-36).
Catalyst tests
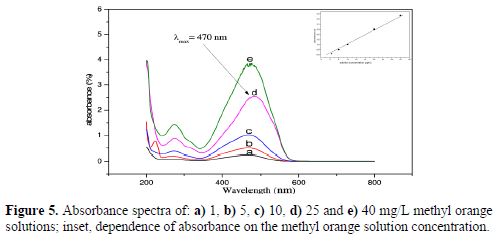
Besides its use as acid-base indicator (changing color from red to yellow, in the pH range from 3.1 to 4.4), methyl orange is also used in textiles, food stuffs, pulp, and paper and leather industry. Methyl orange measurements obtained in function of mass concentration, using a spectrophotometer, are presented in Fig. 5. MO solutions varied in concentrations from 1 to 40 mg/L (1, 5, 10, 25 and 40 mg/L); they were prepared by dissolving MO powders in ultra-pure water. A maximum of absorbance was obtained at l= 470 nm, which increased with MO concentrations, presented in Figs. 5a to 5e. We can also observe a secondary absorbance peak at 290 nm, which is less intense than the other (37).
In order to follow the dye degradation rate under irradiation, we have carried out a series of absorbance measurements of methyl orange solutions (MO) with 10 ppm, under a UV lamp of 365 nm, for a period which varied from 2 h, at ambient temperature (about 25 ºC), to 6 h, without stirring, on a TiO2 surface film. The following results were obtained as a function of the solutions’ mass concentration (Figs. 6 a, b, c and d). For better visualization values, results are gathered in table 1.
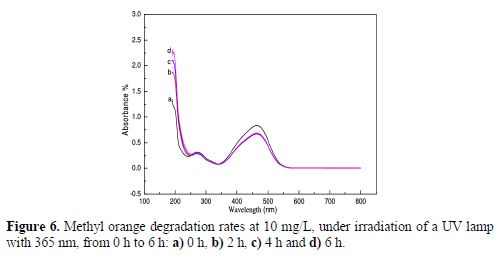
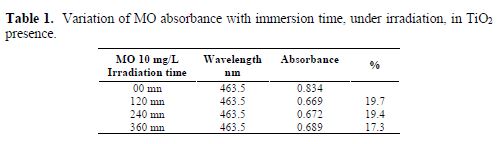
One can notice the decrease in the absorbance after 2 h; then, a quasi-stability for 4 h and 6 h. The decrease in absorbance intensities reveals the continuous dye degradation under increasing irradiation time. We also remark the increase in the absorbance intensity, in a regular way, with time, which leads us to say that one or more compounds were formed after MO degradation. In our case, we only have reached a partial degradation, as the whole absorption peak did not totally disappear. Maybe this is due to the small active area of our used photo catalyst. It is worth mentioning that, in all works, TiO2 has been used as a nano particulate powder, which possesses a high active adsorption surface. Nevertheless, these tests proved that photocatalytic degradation is an efficient way of dye removal from wastewaters, even with thin films, as also reported by (38).
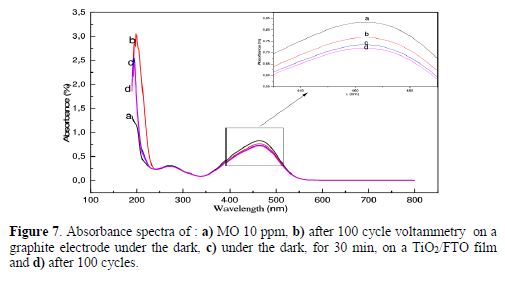
To better show TiO2 photo activity, we present, in Fig. 7, absorbances of several solutions comparatively measured. The first and the second are related to the 10 MO ppm solution; however, the second one underwent a series of cyclic voltamperograms (100 cycles), on non-irradiated carbon graphite, and the third one on a TiO2/FTO film obtained under darkness, during 30 min. One could observe here a small decrease in the absorbance value obtained with carbon graphite and TiO2; nevertheless, these differences are not well marked; they highlight the fact that, even in the absence of irradiation, our TiO2 film presents a catalyzing effect, in spite of its low thickness, and of the small exposed active surface area.
Conclusion
The electrodeposition route has been used to prepare nano-sized titanium dioxide on FTO, from a peroxo-titanium solution. The X-ray diffraction (XRD) shows that the as-deposited film was amorphous, and that the heat-treated film presented a crystalline anatase structure. The scanning electron microscopy (SEM) and atomic force microscopy (AFM) showed the homogeneity and little roughness of the films. The electrochemical behavior of such films shows the characteristic irreversible system, and more electric activity of the as-deposited titanium (non-heated) films, which can be attributed to their high OH conductivity. The tests of such films as photo catalysts gave good results by Spectro photochemical measurements; we have observed partial dye degradation on the TiO2 photocatalyst surface under illumination, and little decomposition also under the dark. The absorbance intensity decreased more in TiO2 presence than in that of carbon graphite. Such tests prove the value of electrodeposited TiO2 as a photo catalyst for dyes degradation.
References
- Carp O, Huisman CL, Reller A. Photoinduced reactivity of titanium dioxide. Prog Solid State Chem (Internet). 2004 Jan 1 (cited 2018 Apr 17);32(12):33–177. Available from: https://www-sciencedirectcom.www.sndl1.arn.dz/science/article/pii/S0079678604000123
- Grätzel M. Solar Energy Conversion by Dye-Sensitized Photovoltaic Cells. Inorg Chem (Internet). 2005 Oct (cited 2018 Apr 17);44(20):6841–51. Available from: http://pubs.acs.org/doi/abs/10.1021/ic0508371
- Longo C, De Paoli M-A. Dye-sensitized solar cells: a successful combination of materials. J Braz Chem Soc (Internet). 2003 Dec (cited 2018 Apr 17);14(6):898–901. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103- 50532003000600005 &lng=en&nrm=iso&tlng=en
- Akpan UG, Hameed BH. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J Hazard Mater (Internet). 2009 Oct 30 (cited 2018 Apr 17);170(2–3):520–9. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0304389409007699
- Kar A, Smith YR, Subramanian V (Ravi). Improved Photocatalytic Degradation of Textile Dye Using Titanium Dioxide Nanotubes Formed Over Titanium Wires. Environ Sci Technol (Internet). 2009 May (cited 2018 Apr 17);43(9):3260–5. Available from: http://pubs.acs.org/doi/abs/10.1021/es8031049
- Mahmoodi NM, Arami M. Degradation and toxicity reduction of textile wastewater using immobilized titania nanophotocatalysis. J Photochem Photobiol B Biol (Internet). 2009 Jan 9 (cited 2018 Apr 17);94(1):20–4. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S1011134408001929
- Wesenberg D, Kyriakides I, Agathos SN. White-rot fungi and their enzymes for the treatment of industrial dye effluents. In: Biotechnology Advances (Internet). 2003 (cited 2018 Apr 17). p. 161–87. Available from: https://www.sciencedirect.com/science/article/pii/S0734975003001344
- Honda H, Ishizaki A, Soma R, et al. Application of photocatalytic reactions caused by TiO2 film to improve the maintenance factor of lighting systems. J Illum Eng Soc (Internet). 1998 (cited 2018 Apr 17);27(1):42–9. Available from: http://ies.tandfonline.com/doi/pdf/10.1080/00994480.1998.10748209
- Linsebigler AL, Lu G, Yates JT. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem Rev (Internet). 1995;95(3):735–58. Available from: bbs.sciencenet.cn/blog/admin/images/upfiles/200710117325893655.pdf
- Stewart G, Fox MA. The effect of dark recovery time on the photoefficiency of heterogeneous photocatalysis by TiO2 suspended in non-aqueous media. Res Chem Intermed (Internet). 1995 Aug (cited 2018 Apr 17);21(8–9):933–8. Available from: http://link.springer.com/10.1163/156856795X00576
- Rashed MN, El-Amin AA. International journal of physical sciences. (Internet). Vol. 2, International Journal of Physical Sciences. Academic Journals; 2007 (cited 2018 Apr 17). 73-81 p. Available from: http://www.academicjournals.org/journal/IJPS/article-abstract/B251D2112957
- Chatterjee D, Mahata A. Demineralization of organic pollutants on the dye modified TiO2 semiconductor particulate system using visible light. Appl Catal B Environ (Internet). 2001 Sep 28 (cited 2018 Apr 17);33(2):119–25. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0926337301001709
- Chatterjee D, Dasgupta S. Visible light induced photocatalytic degradation of organic pollutants. J Photochem Photobiol C Photochem Rev (Internet). 2005 Oct 1 (cited 2018 Apr 17);6(2–3):186–205. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S1389556705000316
- Kiriakidou F, Kondarides DI, Verykios XE. The effect of operational parameters and TiO2-doping on the photocatalytic degradation of azo-dyes. Catal Today (Internet). 1999 Nov 26 (cited 2018 Apr 17);54(1):119–30. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0920586199001741
- Konstantinou IK, Albanis TA. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: A review. Appl Catal B Environ (Internet). 2004 Apr 20 (cited 2018 Apr 17);49(1):1–14. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0926337303005411
- Gonçalves MST, Oliveira-Campos AMF, Pinto EMMS, et al. Photochemical treatment of solutions of azo dyes containing TiO2. Chemosphere (Internet). 1999 Aug 1 (cited 2018 Apr 17);39(5):781–6. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0045653599000132
- Wang G-S, Liao C-H, Wu F-J. Photodegradation of humic acids in the presence of hydrogen peroxide. Chemosphere (Internet). 2001 Feb 1 (cited 2018 Apr 17);42(4):379–87. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0045653500001533
- Karuppuchamy S, Jeong JM. Super-hydrophilic amorphous titanium dioxide thin film deposited by cathodic electrodeposition. Mater Chem Phys (Internet). 2005 Oct 15 (cited 2018 Apr 17);93(2–3):251–4. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0254058405002579
- Zhitomirsky I. Cathodic electrosynthesis of titania films and powders. Nanostructured Mater (Internet). 1997 Jul 1 (cited 2018 Apr 17);8(4):521–8. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0965977397001840
- Zhitomirsky I, Kohn A, Gal-Or L. Cathodic electrosynthesis of PZT films. Mater Lett (Internet). 1995 Dec 1 (cited 2018 Apr 17);25(5–6):223–7. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/0167577X95001913
- Ge L, Xu M, Sun M, et al. Fabrication and characterization of nano TiO2 thin films at low temperature. Mater Res Bull (Internet). 2006 Sep 14 (cited 2018 Apr 17);41(9):1596–603. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S002554080600081X
- Karn RK, Srivastava ON. On the synthesis of nanostructured TiO2 anatase phase and the development of the photoelectrochemical solar cell. Int J Hydrogen Energy (Internet). 1999 Jan 1 (cited 2018 Apr 17);24(1):27–35. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0360319998000093
- Saini KK, Sharma SD, Chanderkant, et al. Structural and optical properties of TiO2 thin films derived by sol–gel dip coating process. J Non Cryst Solids (Internet). 2007 Jul 15 (cited 2018 Apr 17);353(24–25):2469–73. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0022309306013421
- An H-J, Jang S-R, Vittal R, et al. Cationic surfactant promoted reductive electrodeposition of nanocrystalline anatase TiO2 for application to dye-sensitized solar cells. Electrochim Acta (Internet). 2005 Apr 30 (cited 2018 Apr 17);50(13):2713–8. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0013468604011363
- Karuppuchamy S, Iwasaki M, Minoura H. Electrochemical properties of electrosynthesized TiO2 thin films. Appl Surf Sci (Internet). 2006 Dec 30 (cited 2018 Apr 17);253(5):2924–9. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0169433206008737
- Georgieva J, Armyanov S, Valova E, et al. Preparation and photoelectrochemical characterisation of electrosynthesised titanium dioxide deposits on stainless steel substrates. Electrochim Acta (Internet). 2006 Feb 1 (cited 2018 Apr 17);51(10):2076–87. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0013468605007449
- Chettah H, Abdi D, Amardjia H, et al. Electrosynthesis of TiO2 oxide film on ITO substrate and electrochemical comparative study of the oxide with its hydrated gel. Ionics (Kiel) (Internet). 2009 Apr 20 (cited 2018 Apr 17);15(2):169–76. Available from: http://link.springer.com/10.1007/s11581-008-0246-8
- Hachisu T, Shi K, Yokoshima T, et al. Preparation of anatase phase titanium dioxide film by non-aqueous electrodeposition. Electrochem commun (Internet). 2016;65:5–8. Available from: http://dx.doi.org/10.1016/j.elecom.2016.01.014
- Miyake M, Takahashi A, Hirato T. Electrodeposition and Anodization of Al-TiO2 Composite Coatings for Enhanced Photocatalytic Activity. Int J Electrochem Sci (Internet). 2017;12:2344–52. Available from: www.electrochemsci.org/papers/vol12/120302344.pdf
- Natarajan C, Nogami G. Cathodic Electrodeposition of Nanocrystalline Titanium Dioxide Thin Films. J Electrochem Soc (Internet). 1996 May 1 (cited 2018 Apr 17);143(5):1547. Available from: http://jes.ecsdl.org/cgi/doi/10.1149/1.1836677
- Kontos AI, Kontos AG, Tsoukleris DS, et al. Superhydrophilicity and photocatalytic property of nanocrystalline titania sol–gel films. Thin Solid Films (Internet). 2007 Jun 25 (cited 2018 Apr 17);515(18):7370–5. Available from: https://www-sciencedirect-com.www.sndl1.arn.dz/science/article/pii/S0040609007002362
- Utomo WB, Donne SW. Electrochemical behaviour of titanium in H2SO4–MnSO4 electrolytes. Electrochim Acta (Internet). 2006 Apr 10 (cited 2018 Apr 20);51(16):3338–45. Available from: https://www.sciencedirect.com/science/article/pii/S001346860501128X
- Hayden BE, Malevich DV, Pletcher D. Electrode coatings from sprayed titanium dioxide nanoparticles - Behaviour in NaOH solutions. Electrochem commun (Internet). 2001 Aug 1 (cited 2018 Apr 20);3(8):390–4. Available from: https://www.sciencedirect.com/science/article/pii/S1388248101001813
- Karuppuchamy S, Iwasaki M, Minoura H. Electrochemical properties of electrosynthesized TiO2 thin films. Appl Surf Sci (Internet). 2006 Dec 30 (cited 2018 Apr 20);253(5):2924–9. Available from: https://www.sciencedirect.com/science/article/pii/S0169433206008737
- Georgieva J, Armyanov S, Valova E, et al. Photoelectrochemical behaviour of electrodeposited tungsten trioxide and electrosynthesised titanium dioxide single component and bilayer coatings on stainless steel substrates. J Electroanal Chem (Internet). 2005 Nov 1 (cited 2018 Apr 20);585(1):35–43. Available from: https://www.sciencedirect.com/science/article/pii/S0022072805003712
- Zhang J, Yang C, Chang G, et al. Voltammetric behavior of TiO2 films on graphite electrodes prepared by liquid phase deposition. Mater Chem Phys (Internet). 2004 Dec 15 (cited 2018 Apr 20);88(2–3):398–403. Available from: https://www.sciencedirect.com/science/article/pii/S0254058404003827
- Meng F. Photocatalytic degradation of methyl orange by nano-TiO2 thin films prepared by RF magnetron sputtering ê. CHINESE Opt Lett. 2009;7(10):956–9. Available from: https://www.google.fr/url?sa=t&rct=j&q=&esrc=s&source=web&cd=4&cad=rja&uact=8&ved=0ahUKEwiqg6iJq8zaAhVCwxQKHaMWBcUQFgg2MAM&url=https%3A%2F%2Fvdocuments.site%2Fphotocatalytic-degradation-of-methyl-orange-by-nano-tio2-thin-films.html&usg=AOvVaw3zMhnbgmWLNK5pxN6tMBYS
- Du Y-B, Zhang L, Ruan M, et al. Template-free synthesis of three-dimensional porous CdS/TiO2 with high stability and excellent visible photocatalytic activity. Mater Chem Phys (Internet). 2018 Jun 15 (cited 2018 Apr 20);212:69–77. Available from: https://www.sciencedirect.com/science/article/pii/S0254058418301962.
* Corresponding author. E-mail address :naimadjam@hotmail.com
Acknowledgment
The authors are indebted to the University of Setif 1 (UFAS), who provided financial support for the conduct of this research.














