Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portugaliae Electrochimica Acta
Print version ISSN 0872-1904
Port. Electrochim. Acta vol.37 no.6 Coimbra Dec. 2019
https://doi.org/10.4152/pea.201906335
ARTIGOS
Inhibition of Aluminum Corrosion in 0.1 M Na2CO3 by Mentha pulegium
Hamdou Imane, Essahli Mohamed and Lamiri Abdeslam
Univ. Hassan 1, Laboratory of Applied Chemistry and Environment, Faculty of Science and Technology, Settat-Morocco
ABSTRACT
The corrosion inhibition of aluminum, in 0.1 M Na2CO3, by Mentha pulegium essential oil, was studied using both polarization and impedance methods. The results show that Mentha pulegium is an effective inhibitor, providing an inhibition rate of 94.16 %, with a concentration of 800 ppm. The polarization study shows that the Mentha pulegium essential oil acted as a mixed inhibitor in 0.1 M Na2CO3. The activation energy calculation has proved that the inhibitor molecules adsorbed onto the aluminum surface, according to the physisorption mechanism.
Keywords: Aluminum, corrosion, inhibition, Na2CO3, mentha pulegium.
Introduction
Aluminum is the second metal most used after iron, on an industrial scale [1-2], considering diversified advantages, such as low cost, light weight and good thermal and electrical conductivity [3]. The formation of the passivation layer onto the aluminum surface is the sole reason of its anti-corrosive immunity [2-6]; the latter is very soluble in aggressive media, with high concentrations of acids and bases [3-6]. The study of the aluminum behavior in aggressive media is required by virtue of these various industrial uses [4]. Several dangerous solutions have been adopted for aluminum protection, such as chromates, which are highly toxic and carcinogenic [4-10]. Therefore, the search for new green inhibitors to substitute chemical ones has become a requirement, because of their environmental friendliness and availability [11-15].
In this context, several works have been carried out on aluminum: N. Chaubey et al. [16] found that the papaya peel extract inhibits the aluminum corrosion in 1 M HCl, with an effectiveness of 95.5%. R. Nathiya et al. [17] compared the inhibitory efficacy of two extracts of Dryopteris cochleata leaves in 1 M H2SO4. The inhibitors were obtained by two different solvents (water and methanol). The results show that the methanol extract was more efficient (95.09%) than the aqueous extract (71.56%). A. Singh et al. [18] found that the Piper longum extract inhibits the aluminum corrosion in NaOH, with an efficiency of 89%. O. K. Abiola et al. [19] tested extracts of grains and leaves of Gossypium hirsutum on aluminum, in NaOH; both gave efficiencies that exceeded 90%. O. K. Abiola et al. [20] researched Cocos nucifera water to inhibit aluminum corrosion in HCl. J. Halambek et al. [21] evaluated the inhibitory power of Laurus nobilis oil in 3% NaCl, assuring an efficiency of 91.3%. K. Krishnaveni et al. [22] tested the aqueous extract of the leaves of Morinda tinctoria on aluminum in HCl, obtaining an efficiency of 96.72%, with a 2-hour immersion. N. O. Obi-Egbedi et al. [23] used the gravimetry technique to study the influence of the Spondias mombin extract on aluminum dissolution in H2SO4; the results show that this extract can protect aluminum against corrosion. D. Prabhu et al. [24] studied the effect of adding Coriandrum sativum grains extract to a phosphoric acid solution on the corrosion rate of aluminum; the obtained results ensure that this extract can be used as a green inhibitor.
The objective of our work was to join the topic of synthetic inhibitors substitution by the use of Mentha pulegium essential oil as an inhibitor of aluminum corrosion, in Na2CO3.
Experimental conditions
Steam distillation
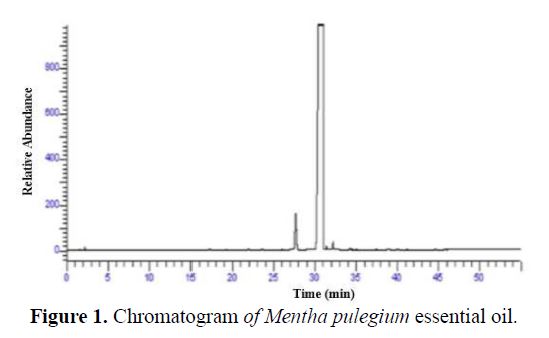
The Mentha pulegium essential oil was obtained by steam distillation for 3 hours, using a Clevenger apparatus consisting of a balloon, balloon heater, refrigerant and a glass collector, where the distillation extracts were collected [25]. One part of this oil was used for the analysis of the chemical composition, while the other was dedicated for the anticorrosive tests. The chromatogram which illustrates the chemical composition of this oil is given in Fig. 1.
Polarization and impedance
The electrochemical tests were performed in a three electrode cell: the working electrode was an aluminum disc with a surface of 1 cm2; and platinum and saturated calomel electrodes were respectively used as auxiliary and reference electrodes. These electrodes were connected to a PGZ100 potentiostat/galvanostat linked to a computer controlled by "Volta master 4" software.
The polarization curves were obtained by varying, in a controlled manner, the potential applied to the working electrode between 1600 mV and -1200 mV, with a scanning rate of 0.5 mV / sec. The study of the temperature effect on the polarization and impedance was carried out in a temperature range from 288 K to 318 K, in the absence and presence of different inhibitor concentrations. The effectiveness of corrosion inhibition by the polarization method is calculated by equation (1), where and Icor respectively represent the current density with and without inhibitor.
Electrochemical impedance spectroscopy (EIS) measurements were performed with the same electrochemical system by imposing a sinusoidal perturbation and recording the response. Frequencies between 100 KHz and 10 Hz were superimposed on the corrosion potential.
The Nyquist curves represent the opposite of the imaginary component as a function of the real component; these diagrams give an idea of the phenomenon that took place at the electrode / solution interface. The impedance inhibition efficiency is calculated by equation 2, where and respectively are the charge transfer resistance of aluminum in 0.1 M Na2CO3, with and without the presence of the inhibitor.
Preparation of the solution
The corrosive medium was a 0.1 M solution of sodium bicarbonate prepared by dissolution in distilled water. The test solutions were prepared before each experiment by adding the extract to be directly tested into the electrochemical cell.
Results and discussion
Analysis of Mentha pulegium essential oil
The analysis of the chemical composition of Mentha pulegium essential oil allows determining 99% of its constituents (Table 1).
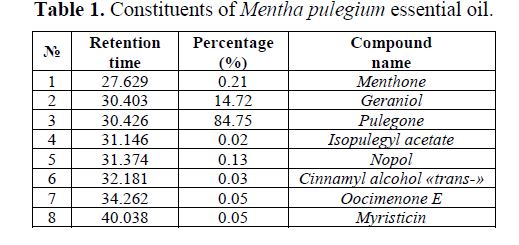
The major constituent of this oil is Pulegone (Fig. 2), with a percentage of 84.75%.
Effect of concentration
Polarization
The polarization curves of aluminum in 0.1 M Na2CO3, with and without inhibitor, are given in Fig. 3.
Table 2 lists the electrochemical parameters (corrosion potential, Ecor, corrosion current, Icor, inhibition efficiency, E, anodic Tafel slope, βa, and cathodic Tafel slope, βc).
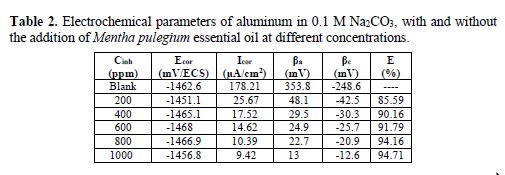
The decrease in the two Tafel slopes with the increase in the extract concentration shows that Mentha pulegium essential oil is a mixed inhibitor, acting on both anodic and cathodic reactions by the formation of a protective film onto the aluminum surface [26-27]. The sharp decrease of the corrosion current density from 178.21 μA / cm2 to 10.39 μA / cm2 reflects that Mentha pulegium essential oil is a good inhibitor, reaching a maximum inhibition of 94.16%, at a concentration of 800 ppm.
Electrochemical impedance spectroscopy
Fig. 4 shows the Nyquist diagrams of aluminum in 0.1 M Na2CO3, without and with the addition of the inhibitor at different concentrations. The electrochemical parameters of these diagrams are given in Table 3.
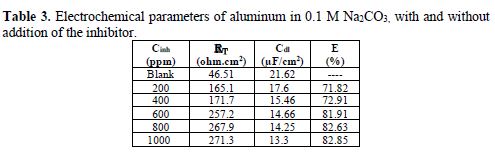
The semicircular shape of the loops in the Nyquist diagram (Fig. 4) indicates that the aluminum corrosion in 0.1 M Na2CO3 was controlled by a charge transfer phenomenon [28]. The diameter of the loops raised with increasing concentrations, reflecting an enhancement in the charge transfer resistance and, thereafter, an increase in the inhibitor efficiency [29].
The results in Table 3 show that the addition of the inhibitor led to an increase in RT values, which measure the electron transfer across the surface [30-31] and hence, the rise of inhibition efficiency. The decrease in Cd1 can be explained by the adsorption of the inhibitor molecules onto the aluminum surface, by forming an electronic double layer [32].
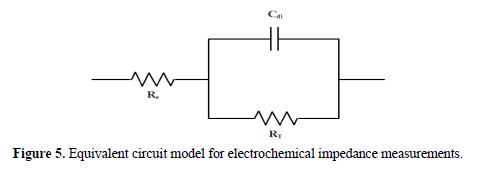
In the equivalent electrical circuit given in Fig. 5, Re is the resistance of the electrolyte, RT is the charge transfer resistor and Cdl is the double layer capacitance.
Effect of temperature
Polarization
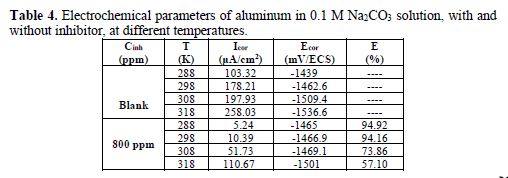
The polarization curves, with and without inhibitor, at different temperatures, are given in Figs. 6 and 7. The temperature influence on the electrochemical parameters is shown in Table 4. The increase in temperature makes the medium increasingly more corrosive, which results in an increase in the corrosion rate. The decrease in inhibitory efficiency (Table 4) can be explained by the hydrogen clearance on the metal surface, which causes desorption of the protective film [28].
Electrochemical impedance spectroscopy
The impedance curves obtained at different temperatures, with and without inhibitor, are presented in Figs. 8 and 9. The electrochemical parameters are grouped in Table 5.
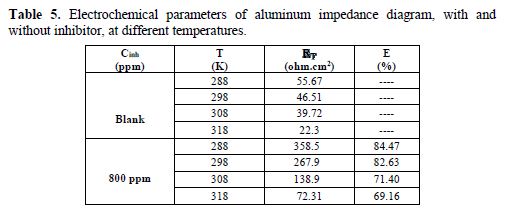
The increase in temperature led to a sharp decrease in RT and, consequently, to a reduction of the inhibition efficiency (Table 5). This was due to the fixation of the inhibitor molecules onto the aluminum surface, according to a physical adsorption mechanism [33].
Determination of activation energy
The Arrhenius equation (3), where K is the corrosion rate, A is the Arrhenius factor and Ea is the activation energy, was used to study the inhibition mechanism of aluminum corrosion. The Arrhenius diagrams of aluminum dissolution in 0.1 M Na2CO3, with and without inhibitor, are shown in Fig. 10.
The calculated values of the activation energy, with and without inhibitor, are, respectively, 68.57 KJ/mol and 21.82 KJ/mol. The activation energy increase in the presence of the essential oil of Mentha pulegium indicates that the inhibition was achieved by a mechanism of physisorption [34-35].
Conclusions
- The major constituent of Mentha pulegium essential oil is Pulegone, within a percentage of 84.75%.
- The essential oil of Mentha pulegium provides an inhibition efficiency of 94.16%, with a concentration of 800 ppm.
-The slowing down of the corrosion rate was manifested by the reduction of the anodic and cathodic Tafel slopes; with the progressive addition of the extract, the Mentha pulegium essential oil acted as a mixed inhibitor.
-The corrosion inhibition occurred by adsorption of the inhibitor molecules onto the aluminum surface, by forming weak bonds.
References
1. Prabhu D, Rao PJ. Environ Chem Eng. 2013;1:676. [ Links ]
2. Verma C, Singh P, Bahadur I, et al. J Mol Liq. 2015;209:767. [ Links ]
3. Awad MK, Metwally MS, Soliman SA, et al. J Ind Eng Chrm. 2014;20:796. [ Links ]
4. Oguzie EE. Corros Sci. 2007;49:1527. [ Links ]
5. El-Etre AY. Corros Sci. 2003;45:2485. [ Links ]
6. Li X, Deng S, Fu H. Corros Sci. 2011;53:2748. [ Links ]
7. Kartsonakis IA, Balaskas AC, Kordas GC. Corros Sci. 2011;53:3771. [ Links ]
8. Emregül KC, Aksüt AA. Corros Sci. 2003;45:2415. [ Links ]
9. Khaled KF. Corros Sci. 2010;52:2905. [ Links ]
10. Kwolek P, Kaminski A, Dychton K. Corros Sci. 2016;106:208. [ Links ]
11. El-Etre AY, Abdallah M, El-Tantawy ZE. Corros Sci. 2005;47:385. [ Links ]
12. Deng S, Li X. Corros Sci. 2012;55:407. [ Links ]
13. Mourya P, Banerjee S, Singh MM. Corros Sci. 2014;85:352. [ Links ]
14. Ji G, Anjum S, Sundaram S. Corros Sci. 2015;90:107. [ Links ]
15. Fuchs-Godec R, Zerjav G. Corros Sci. 2015;97:7. [ Links ]
16. Chaubey N, Kumar V, Quraishi MA. Ain Shams Eng J. 2016. [ Links ]
17. Nathiya RS, Raj V. Egypt J Pet. 2016. [ Links ]
18. Singh A, Ahamad I, Quraishi MA. Arab J Chem. 2012. [ Links ]
19. Abiola OK, Otaigbe JOE, Kio OJ. Corros Sci. 2009;51:1879. [ Links ]
20. Abiola OK, Tobun Y. Chinese Chem Lett. 2010;21:1449. [ Links ]
21. Halambek J, Berkovi K, Vorkapi J. Materials Chemistry and Physics Journal. 2013;137:788. [ Links ]
22. Krishnaveni K, Ravichandran J. Trans Nonferrous Met Soc China. 2014;24:2704. [ Links ]
23. Obi-Egbedi NO, Obot IB, Umoren SA. Arab J Chem. 2012;3:361. [ Links ]
24. Prabhu D, Rao P. Journal of Environmental Chemical Engineering. 2013. [ Links ]
25. Fadil M, Farah A, Ihssane B, et al. J Mater Environ Sci. 2015;6:2346. [ Links ]
26. Houbairi S, Lamiri A, Essahli M. Int J Eng Res Tech. 2014;3:698. [ Links ]
27. Abdallah M, Kamar EM, Eid S, et al. J Mol Liq. 2016;220:755. [ Links ]
28. Vengatesh G, Karthik G, Sundaravadivelu M. Egypt J Pet. 2016. [ Links ]
29. Liao LL, Mo S, Lei JL, et al. J Colloid Interface Sci. 2016;474:68. [ Links ]
30. Bensabah F, Essahli M, Lamiri A, et al. Port Electrochim Acta. 2014;32(6):381-393. [ Links ]
31. Badiea AM, Mohana KN. J Mater Eng Perform. 2009;18:1264. [ Links ]
32. Houbairi S, Lamiri A , Essahli M. Chem Sci Rev Lett. 2014;3:353. [ Links ]
33. Azzouyahar E, Bazzi L, Belkhaouda M. Rspublication Com. 2014;4:941. [ Links ]
34. Geetha S, Lakshmi S, Bharathi K. Int J Adv Sci Tech Res. 2013;3:258. [ Links ]
35. Zarrouk A, Al-Deyab SS. Int J Electrochem Sci. 2011;6:6261. [ Links ]
Received February 3, 2017; accepted October 8, 2018
ENDEREÇO PARA CORRESPONDÊNCIA E-mail address: imane.hamdou91@gmail.com














