Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.36 no.4 Coimbra jul. 2018
https://doi.org/10.4152/pea.201804271
Cationic Surfactant - Zn2+ Systems as Mixed Corrosion Inhibitors for Carbon Steel in a Sodium Chloride Corrosive Medium
Reda S. Abdel Hameeda,b,*
a Faculty of Science, Department of Chemistry, Al-Azhar University,11884 ,Cairo, Egypt
b Department of Chemistry, Faculty of Science, Hail University, 14560, Hail, KSA
Abstract
Benzyl dimethyl dodecyl ammonium chloride-zinc ion system, as quaternary ammonium salt (QA-Zn2+), was investigated as corrosion inhibitor for carbon steel in a 2.0 M sodium chloride solution, by different techniques such as weight loss, open circuit potential, potentiodynamic polarization and electrochemical impedance spectroscopic techniques. The inhibition efficiency of the used system (QA-Zn2+) increases with increasing mixed inhibitor concentrations and with rising temperatures. A synergistic effect exists between QA and Zn2+. Potentiodynamic polarization curves indicate that the used system mainly acts as an anodic mixed inhibitor. The polarization resistance values were (Rp) increased, and the interface capacitance (Cdl) was decreased in the mixed inhibitor system (QA- Zn2+) presence, more than in the case of individual inhibitors. The corrosion inhibition is due to the adsorption of (QA-Zn2+) onto the metal surface, and the formation of a barrier film that separates the metal from the corrosive medium. The maximum inhibition efficiency of 98% was obtained at 200 QA and 50 ppm Zn2+ of the mixed inhibitor system, due to a synergistic effect.
Keywords: steel corrosion inhibitors; surfactant; quaternary ammonium salt; synergistic effect; EIS.
Introduction
Corrosion inhibition of metals and alloys is of technical, economical, and environmental importance. Steel is the backbone of many industries. Corrosion inhibitors are commonly used to reduce corrosion attack on the steel surface. The presence of the inhibitors leads to a decline in steel corrosion rate, increasing its lifetime [1-5]. Most of the effective acid inhibitors are organic compounds containing nitrogen, oxygen and /or sulfur, which adsorb onto the steel surface, and hence, slow down the corrosion rate [1-5]. The strength of adsorption depends on several factors, such as the chemical structure of the inhibitors, the presence of electro donating or electro repelling groups, molecular weight of the inhibitor, and temperature and electrochemical potential at the metal/solution interface [6, 7]. Surfactant inhibitors have many advantages, such as high inhibition efficiency, low price, low toxicity and easy production [8]. The adsorption of the surfactant onto the metal surface can markedly change the corrosion resisting property of the metal [9-11]; therefore, studying the relationship between adsorption and corrosion inhibition is of great importance. Cationic surfactant compounds show a good corrosion inhibition of carbon steel [12]. Several surfactants that function as corrosion inhibitors have biocidal properties; so, they can be used as inhibitors in a petroleum field. As gemini surfactants have got an excellent antimicrobial activity, they are great corrosion inhibitors for carbon steel in acidic environments, due to the presence of nitrogen and oxygen atoms in their chemical structure [13]. In the work of Abdel Hameed et al. [14], nonionic surfactants derived from plastic waste have been studied as corrosion inhibitors for mild steel in 2.0 M NaCl solutions. Weight loss, potentiodynamic polarization techniques and open circuit potential measurements have been used. The aim of the present work is to evaluate the corrosion inhibition efficiency of benzyl dimethyl dodecyl ammonium chloride, as a cationic surfactant towards steel corrosion in sodium chloride, by determination of its inhibition efficiency in the absence and presence of Zn2+, using chemical (weight loss) and electrochemical techniques, such as open circuit potential, potentiodynamic polarization, and electrochemical impedance spectroscopic techniques. The effect of concentrations and temperatures was studied, and thermodynamic parameters were calculated and discussed.
Experimental
Materials
Benzyl dimethyl dodecyl ammonium chloride and zinc acetate were purchased from Aldrich Chemical Co. Ltd., UK. Sodium chloride (NaCl) was obtained from Shinyo Pure Chemicals (99.99%). Carbon steel used in this study had the composition (weight %) of 0.26 C, 1.35 Mn, 0.04 P, 0.05 S, 0.005 Nb, 0.02 V, 0.03 Ti and the remainder Fe.
Weight loss techniques
For weight loss measurements, a coupon of carbon steel, with the dimensions of 2 cm × 2 cm × 0.2 cm, was used. The electrode surface was polished with different grades of emery papers, degreased with acetone, and rinsed with distilled water. Pure sodium chloride was used for preparing the corrosive solution. To calculate the accurate values of the percentage inhibition efficiency, three parallel experiments were performed for each test, and the mean value was calculated.
Open circuit potentials
The steel electrode potential was measured against a saturated calomel electrode (SCE) in a 2.0 M NaCl solution, in the absence and presence of different concentrations of the QA-Zn2+ system at 25 °C. All measurements were carried out using a multi-tester, until the steady-state potentials would be reached.
Potentiodynamic polarization measurements
The working electrode was made from a carbon steel rod that had the same composition as mentioned in the subsection ''Materials''. The rod was axially embedded in araldite holder to offer an active flat disc shaped surface with an area of 1 cm2. Prior to each experiment, the working electrode was successively polished with fine emery paper, rinsed with acetone, washed with double distilled water, and finally dried before dipping into the electrolytic cell. A platinum wire was used as counter electrode, and a three compartment cell with a saturated calomel electrode (SCE) was used as reference electrode. The electrochemical experiments were performed using a Voltamaster (PGZ 301, Dynamic Els Voltammetry) analytical radiometer. Under stirring, with a scan rate of 1 mV/s, potential was scanned in the range of -1800 to 0 mV.
Synergism parameters
Synergism parameter (SI) indicates the synergistic effect existing between inhibitors, if SI value is more than one [15-17]. SI can be calculated from the following relation [16, 17].
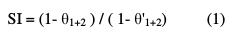
where θ1+2 = (θ1 + θ2) - (θ1 × θ2), θ1 = surface coverage of the QA inhibitor, θ2 = surface coverage of the Zn2+ inhibitor, and θ'1+2 = combined surface coverage of the saturated calomel inhibitor system (QA-Zn2+).
Electrochemical impedance spectroscopy (EIS)
Electrochemical impedance was obtained using a Voltalab 40 for all EIS measurements, with a frequency range of 100 kHz to 50 mHz, with a 4 mV sine wave as the excitation signal at open circuit potential. If the real part is plotted on the X-axis, and the imaginary part is plotted on the Y-axis of a chart, we get a Nyquist plot. The charge transfer resistance values (Rct) were calculated from the difference in impedance at lower and higher frequencies.
Results and discussion
Weight loss measurements
Effect of inhibitor concentrations
Weight loss measurements were used to evaluate the surfactant–Zn2+ system (QA-Zn2+), the corrosion inhibitor for carbon steel, in a 2.0 M sodium chloride solution, at different concentrations (50, 100, 150 and 200 ppm), with and without 50 ppm of zinc acetate (Zn2+). The value of corrosion rate, Rcorr, was calculated from the following equation:

where ΔW is the average weight loss of steel coupons in the corrosive solution, S is the total surface area of the specimen, and t is the immersion time. The percentage inhibition efficiency (% IE) and the surface coverage (θ), which represents the part of the metal surface covered by the inhibitor molecules, were calculated using the following equations:
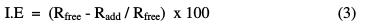
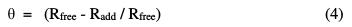
where Rfree and Radd are the weight losses of carbon steel, without and with addition of inhibitors, respectively [6].
The percentage values of inhibition efficiency (I.E.) and surface coverage (θ) of the prepared compounds are listed in Table 1.
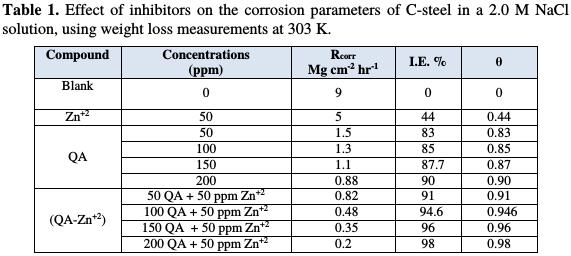
Inspection of this table reveals that the QA surfactant compound acts as good inhibitor, but the inhibition efficiency enhanced in the presence of 50 ppm of Zn2+ ion. The values of inhibition efficiency increase with increased concentrations of the QA surfactant compound. The value of inhibition efficiency is 98% in the presence of (200 QA + 50 ppm Zn2+) as an inhibitor system. Therefore, the mixture of inhibitors shows better inhibition efficiency than the individual inhibitors. In the presence of Zn2+ ions, a large amount of QA surfactant is directed towards the metal surface. (Fe+2-QA) complex is formed on the anodic sites of the steel surface. Thus, the anodic reaction is controlled. The cathodic reaction is the generation of OH - ion, which is controlled by the formation of Zn(OH)2 on the cathodic sites of the steel surface. Thus, both anodic and cathodic reactions are effectively controlled. This is due to the synergistic effect existing between Zn2+ and the QA surfactant [18, 19].
Synergism parameters
For mixed inhibitors, it is known that, when SI values are less than one, the negative interaction of the inhibitors prevails (i.e., the corrosion rate increases). But when SI value is greater than one, the positive interaction between the inhibitors compounds is called synergistic effect; in this case, the corrosion rate decreases and the inhibition efficiency increases. The values of synergism parameters (SI) that are calculated from the surface coverage equation (1) are shown in Table 2.
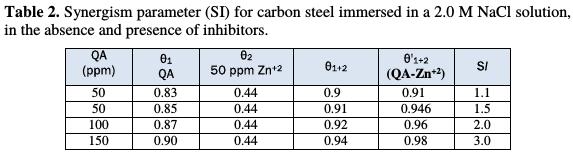
It is clear from the table that the values of SI are higher than one, suggesting that a synergistic effect exists between the QA surfactant and Zn2+ ion. SI increases with increased QA concentrations. The synergistic combination of 50 ppm of Zn2+ and 200 ppm of QA offered the maximum inhibition efficiency of 98 %.
Effect of temperature
To determine the kinetic parameters of the corrosion process, and to elucidate the mechanism of inhibition, weight loss measurements were performed at different temperatures: 303, 313, 323 and 333 K. The effect of temperature on the corrosion inhibition efficiency of carbon steel, in the inhibitor presence, is graphically represented in Fig. 1.
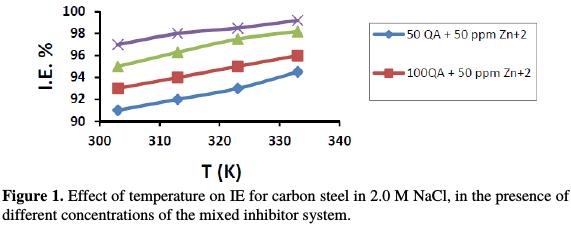
The inhibition efficiency increases with an increase in temperature, leading to the conclusion that the protective film of these compounds formed on the carbon steel surface is stable at higher temperatures; this indicates that the inhibitor system ( QA- Zn2+) produces chemical adsorption. The apparent activation energy (Ea) of metal corrosion in corrosive media can be calculated from the Arrhenius equation [6]:
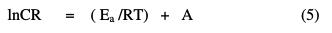
where Ea is the apparent activation energy for the corrosion of steel, R is the universal gas constant, A is Arrhenius pre exponential factor and T is the absolute temperature. Fig. 2 depicts the plot of log CR vs. 1/T, and the values of Ea obtained from the plot slope are given in Table 3.
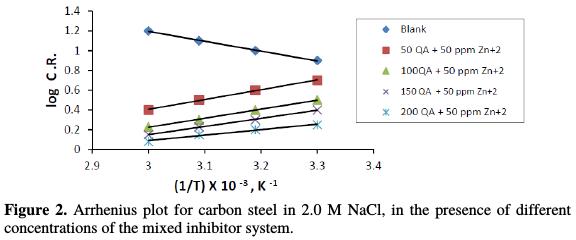
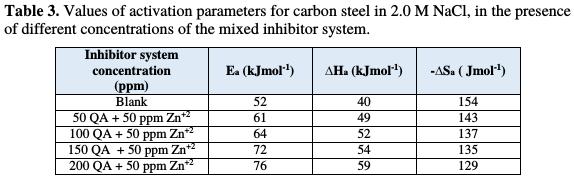
The higher value of activation energy (Ea) in the inhibitor presence is higher than in its absence, which is attributed to its chemical adsorption [25-26].
In the present study, the higher value of Ea for carbon steel, in the inhibitor system presence, compared to that in its absence, is attributed to its chemical adsorption. An alternative form of Arrhenius equation is the transition state equation [26-28]:
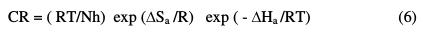
where h is the Plank's constant, N is the Avogadro's number, ΔSa is the entropy of activation, and ΔHa is the enthalpy of activation. A log plot (CR/T) vs. 1/T gave a straight line, as shown in Fig. 3, with a slope of (-ΔH /2.303R) and an intercept of [log(R/Nh) + (ΔS /R)], from which the values of ΔHa and ΔSa were calculated and listed in Table 3.
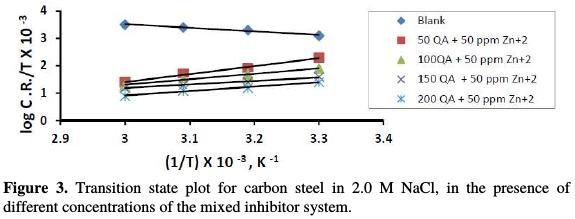
The positive values of ΔHa for carbon steel corrosion, in the presence and absence of the inhibitor, reflect the endothermic nature of the metal dissolution process. The increase in ΔHa with increasing inhibitor system concentrations for carbon steel corrosion reveals that the decrease in carbon steel corrosion rate is mainly controlled by kinetic parameters of activation [25-28]. The entropy of activation values is less negative for inhibited solutions than that for uninhibited solutions. This suggests that an increase in randomness occurred while moving from reactants to the activated complex [25-26].
Open circuit potential measurements
In open circuit, steel potential, as a function of exposure time in the absence and presence of different concentrations of the QA-Zn2+ inhibitor system, was measured against SCE reference electrode for 50 min; the obtained data are presented in Fig. 4.
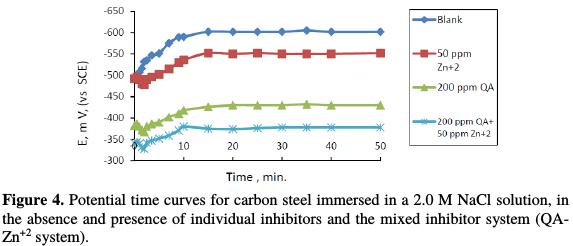
The used concentration ranges are 50 ppm of Zn2+, 200 ppm of QA, and 200 of QA + 50 ppm of the Zn2+ system, at 25 °C. As seen from Fig. 4, in an uninhibited 2.0 M NaCl solution, the E value of steel tends towards more negative potentials, although it slightly shifted to a positive direction. After that, it was kept at a nearly stable value. The same behavior appeared at the addition of individual inhibitors and the mixed inhibitor system (QA-Zn2+ system), but a steady state potential, which was produced from the addition of inhibitors, shifted to more nobler values. This shift increased more in the case of the mixed inhibitor system (QA-Zn2+ system) than with individual inhibitors, due to the synergistic effect. The inhibitors raised the free corrosion potential of steel (shifted it to more noble values) compared to the blank solution. This initially indicates that the studied inhibitors and inhibitor system act as anodic inhibitors [20-24]. In all curves, the steady-state values are always more negative than the immersion potential, suggesting that before the steady state condition is achieved, the steel oxide film has to dissolve [29].
Potentiodynamic polarization measurements
Polarization studies have been used to evaluate the corrosion inhibition efficiency of the inhibitors, by studying the formation of a protective film on the metal surface. The potentiodynamic polarization curves of carbon steel immersed in the test solutions are shown in Fig. 5.
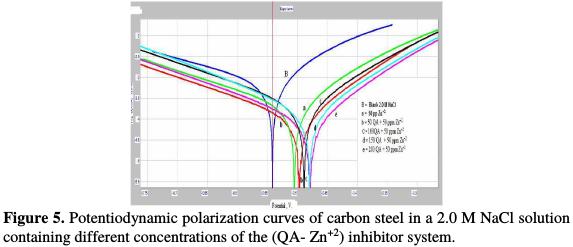
Tafel corrosion parameters and inhibition efficiency values are given in Table 4.
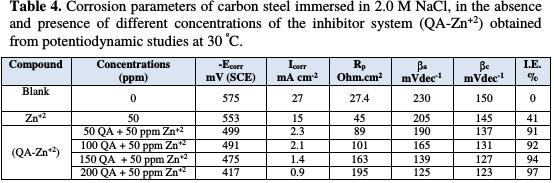
As shown from the table, the inhibitors and inhibitor system shifted the corrosion potential to less negative values versus SCE. This indicates that the anodic current reaction is predominantly controlled. The corrosion current potential values for carbon steel in a 2.0 M NaCl solution decreased in the presence of inhibitors, and the corrosion resistant values increased. This indicates that a protective film has been formed on the metal surface. In case of Zn2+ alone, the corrosion current (Icorr) is slightly reduced. A higher shift is observed in the presence of QA alone, but the highest shift is in Icorr. Respective values were observed in the case of the inhibitor system (QA-Zn2+ system) and therefore, the corrosion inhibition efficiency increases, indicating that a synergistic effect exists amidst the mixed inhibitor system (QA -Zn2+ system) [20-24]. From Table 4, the addition of 50 ppm of Zn2+ to 200 ppm of QA enhances the corrosion inhibition efficiency. The shift in anodic Tafel slope (βa) is greater than the shift in cathodic Tafel slope (βc), in the case of the inhibitors system, indicating that the inhibitors act as mainly anodic mixed inhibitors [20-24]. The inhibition efficiency is given by the following equation [25]:
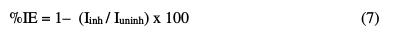
where Iuninh and Iinh are the corrosion current densities in the inhibitor absence and presence, respectively.
Electrochemical impedance spectroscopy (EIS) measurements
Impedance spectra have been used to detect the formation of a film on the metal surface. The impedance spectra (Nyquist plots) of carbon steel in 2.0 M NaCl, in the inhibitors and inhibitor system (QA- Zn2+) absence at 30 °C, are shown in Fig. 6.
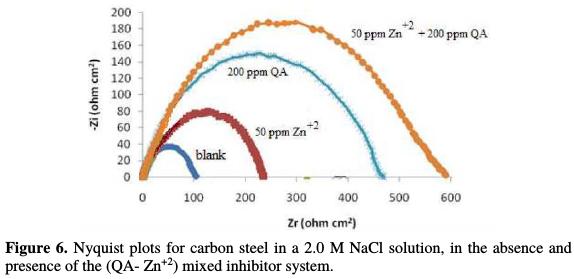
Nyquist plots contain a depressed semicircle with its center under real axis. The semicircle size increases more in the case of the mixed inhibitor system (QA- Zn2+) than in the case of each individual inhibitor. This confirms the formation of a protective film on the metal surface, and the occurrence of the synergistic effect between the QA surfactant and zinc acetate inhibitors. Impedance parameters, such as charge transfer resistance (Rt) and double layer capacitance (Cdl) values, are given in Table 5.
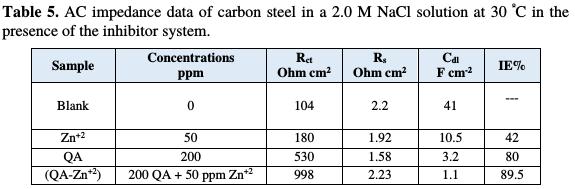
The values of polarization resistance (Rp) were increased, and the interface capacitance (Cdl) was more decreased in the mixed inhibitor system (QA- Zn2+) presence than in the case of individual inhibitors. This is most probably due to the decrease in local dielectric constant and/or the increase in thickness of the electrical double layer. This suggests that the mixed inhibitor system acts via adsorption at the metal/solution interface [29].
The decrease in Cdl values is caused by the gradual replacement of water molecules by the adsorption of the inhibitor molecules onto the electrode surface, which decreases the extent of metal dissolution [26-29]. The inhibition efficiency is given by the following equation [30-32]:
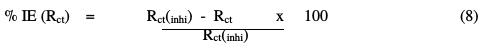
where Rct is the polarization resistance without inhibitor, and Rct(inhi) is the polarization resistance with inhibitor. The proposed equivalent circuit is represented in Fig. 7.
The role of zinc in increasing the inhibition efficiency
The inhibition efficiency increases due to the co-adsorption of Zn2+ ions and the cationic surfactant, which may be either competitive or co-operative [33-35]. In competitive adsorption, the anions and cations are adsorbed at different sites on the metal surface. In co-operative adsorption, the anion is chemisorbed onto the surface, and the cation is adsorbed onto the anion. The co-adsorption of Zn2+ ions onto the steel surface forms an oriented dipole with their negative ends towards the solution, thus increasing the adsorption of organic molecules [33]. Besides the nature of the anions present in the NaCl solutions, they play a significant role in influencing the extent of adsorption. This, in turn, may favor a greater adsorption of cations on the surface, resulting in a stronger inhibition.
Conclusions
1. All the measurements showed that the (QA-Zn2+) mixed inhibitor system has excellent inhibition properties for carbon steel corrosion in a 2.0 M NaCl corrosive medium.
2. The synergistic effect exists between QA surfactants and Zn2+. 3. The inhibitors raise the free corrosion potential of steel (shifted it to more noble values) compared to the blank solution, indicating that the inhibitors and inhibitor system act as anodic inhibitors.
4. Potentiodynamic polarization measurements showed that the (QA-Zn2+) mixed inhibitor system acts as a mixed-type inhibitor of predominantly anodic nature.
5. The semicircle size increases more in the case of the mixed inhibitor system (QA-Zn2+) than in the case of each individual inhibitor; this confirms the formation of a protective film on the metal surface, and the occurrence of the synergistic effect between the QA surfactant and zinc acetate inhibitors.
6. The values of polarization resistance were (Rp) increased, and the interface capacitance (Cdl) was more decreased in the mixed inhibitor system (QA-Zn2+) presence than in the case of individual inhibitors.
7. The maximum inhibition efficiency of 98 % was obtained at 200 ppm QA and 50 ppm Zn2+ of the mixed inhibitors system, due to the synergistic effect.
8. The data obtained from different techniques are in good agreement.
References
1. Golestani Gh, Shahidi M, Ghazanfari D. Appl Surf Sci. 2014;308:347. [ Links ]
2. Hosseini MG, Ehteshamzadeh M, Shahrabi T. Electrochim Acta. 2007;52:3680. [ Links ]
3. Abdallah M, Zaafarany I, Khairou KS, et al. Int J Electrochem Soc. 2012;7:1564. [ Links ]
4. Fouda AS, Abdallah M, Medhat M. Prot Met Phys Chem Surf. 2012;48:477. [ Links ]
5. M. Abdallah, H.M. Al-Tass, B.A. Al Jahdaly, A.S. Fouda, J Mol. Liq., 2016;216:590. [ Links ]
6. Abdelhameed RS. Adv Appl Sci Res. 2016;7,2:92. [ Links ]
7. Hanane H, Douadi T, Al-Noaimi M, et al. Corros Sci. 2014;88:234. [ Links ]
8. Abdallah M, Asghar BH, Zaafarany I, et al. Prot Met Phys Chem Surf. 2013;49:485. [ Links ]
9. Abdallah M, Al Jahdaly BA, Al-Malyo OA. Int J Electrochem Sci. 2015;10:2740. [ Links ]
10. Abdallah M, Al Jahdaly BA, Sobhi M, et al. Int J Electrochem Sci. 2015;10:4482. [ Links ]
11. Sobhi M, El-Sayed R, Abdallah M. Chem Eng Comm. 2016;203:758. [ Links ]
12. Malik MA, Hashim MA, Nabi F, et al. Int J Electrochem Sci. 2011;6:1927. [ Links ]
13. El-Sadek BM. Der Chemica Sinica. 2011;3:125. [ Links ]
14. Hameed RSA, Alshafy HI, Farghaly O. Research Rev Electrochem. 2012;3:41. [ Links ]
15. Kanimozhi SA, Rajendran S. Int J Electrochem Sci. 2009;4:353. [ Links ]
16. Manivannan M, et al. Int J Eng Sci Tech. 2011;3,1:8048. [ Links ]
17. Johnsirani V, Ragendran S, Sathiyabma J, et al. Bulg Chem Comm. 2012;44:41. [ Links ]
18. Abdel-Fatah HTM. Anti-corrosion Methods Mater. 2012;59,1:23. [ Links ]
19. Gowri S, Sathiyabama J, Rajendran S. Int J Chem Eng. 2014;2014:1. [ Links ]
20. Gonzalez Y, Lafont MC, Pebere N, et al. J Appl Electrochem. 1996;26:1259. [ Links ]
21. Pech-Canul MA, Chi-Canul LP. Corrosion. 1999;55:948. [ Links ]
22. To XH, Pebe re N, Pelaprat N, et al. Corros. Sci. 1997; 39:1925. [ Links ]
23. Rajendran S, Apparao BV, Palaniswamy N. Bull Electrochem. 2001;17:171. [ Links ]
24. Pech-Canul MA, Bartolo-Perez P. Surf Coat Technol. 2004;184:133. [ Links ]
25. Abdel-Hameed RS, Shamroukh AH. Int J Corros Scale Inhib. 2017;6:333. [ Links ]
26. Abdel-Hameed RS. J Mater Environ Sci. accepted 07/ 2017, in press.
27. Abd El-Hameed RS. Port Electrochim Acta. 2011;29:273. [ Links ]
28. Abdel Hameed RS. Adv Appl Sci Res. 2011;2:483. [ Links ]
29. Abdel Hameed RS, Al-Shafey HI, Ismail EA, et al. Int J Electrochem Sci. 2015;10:2098. [ Links ]
30. Abdel Hameed RS, Al Shafey HI, Abu-Nawwas AH. Int J Electrochem Sci. 2014;9:6006. [ Links ]
31. Abdel Hameed RS, El-Zomrawy AA, Abdallah M, et al. Int J Corros Scale Inhib. 2017;6:196. [ Links ]
32. Alshafey HI, Abdel Hameed RS, Ali FA, et al. Int J Pharm Sci Rev Res. 2014;27:146. [ Links ]
33. Abdallah M, Atwa ST, Zaafarany IA. Int J Electrochem Sci. 2014;9:4747. [ Links ]
34. Abdallah M, Hazzazi OA, Saad AF, et al. Protec Metals Phys Chem Surf. 2014;50:659. [ Links ]
35. Abdallah M, Atwa ST, Salem MM, et al. Int J Electrochem Sci. 2013;8:10001. [ Links ]
*Corresponding author. E-mail address: mredars2@yahoo.com
Received May 13, 2017; accepted September 15, 2017














