Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.34 no.6 Coimbra nov. 2016
https://doi.org/10.4152/pea.201606383
Improving the Corrosion Resistance of Lead in H2SO4 4 M by the Addition of Phosphoric and Phosphonic Compounds for Lead Grid Batteries
Salma Khatbi* , Youssef Gouale , Abdeslam Lamiri and Mohamed Essahli
University Hassan 1, Laboratory of Applied Chemistry and Environment, Faculty of Science and Technology, BP 5777, Settat, Morocco
Abstract
The effect of the addition of phosphoric acid ((HO)3P=O), potassium hydrogen phosphate ((HO)2P(O)(O-K+)), dimethyl vinylphosphonate (CH2=CH-P(O)(OCH3)2) and vinylphosphonic acid (CH2=CHP(O)(OH)2) on lead corrosion in 4 M H2SO4 was studied by potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). The results show that phosphoric acid and potassium hydrogen phosphate, added to an optimal concentration of 0.4 M, reduce the lead passivation current and increase its corrosion current, with charge transfer as the main reaction mechanism at the interface metal/electrolyte. They also increase PbO2 formation's potential when they are added at larger concentrations, while adding dimethyl vinylphosphonate and vinylphosphonic acid up to 0.3 M reduces corrosion current and lead passivation current. This last product appears to suppress the formation of PbO2. The parameters of potentiodynamic polarization are in good agreement with those of EIS.
Keywords: corrosion, inhibition, lead, phosphoric acid, potassium hydrogen phosphate, dimethyl vinylphosphonate, vinylphosphonic acid.
Introduction
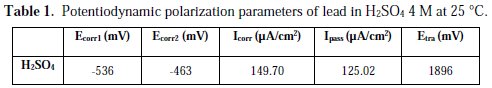
The study of lead corrosion in a sulfuric medium is mainly applied in the automotive batteries sector. These batteries suffer from severe corrosion of their anodic grills (lead plate) and from the phenomenon of sulphation (formation and deposition of PbSO4 on metallic plates). Addition of phosphoric acid and various phosphate compounds to the electrolyte has always been a very interesting and effective way to improve Pb-acid battery performance [1-5]. The majority of studies found that phosphoric acid addition reduces the sulphation by preventing the formation of an insulating PbSO4 layer, resulting in an increase of the cycle life and a decrease of the discharge [6-10]. Meanwhile, in some reports, phosphoric acid was found to improve the formation of the lead dioxide layer [11, 12], while others have claimed the opposite [13, 14]. However, the major disadvantage of the addition of phosphoric acid or its salts was found to be the loss of the cell capacity [15].
The motivation of our work is to improve the performance of the lead-acid battery by improving the corrosion resistance of lead in H2SO4 4 M, with the addition of phosphoric acid, potassium hydrogen phosphate, dimethyl vinylphosphonate and vinylphosphonic acid to the electrolyte, using potentiodynamic polarization and electrochemical impedance spectroscopy.
Experimental
Material preparation
For our studies, we used a working electrode formed with pure lead cut in a disk shape of 1 cm2 in size. To obtain reliable and reproducible results, before each experiment, the working electrode was polished with SiC paper 400, 600, 1200 and rinsed with distilled water.
Solutions preparation
The sulfuric acid 4 M solution was made by diluting H2SO4 96% (d = 1.84 and M = 98.08 g/mol) with distilled water.
The phosphoric acid solutions were made by diluting (HO)3P=O 85% (d = 1.69 and M = 98 g/mol) with distilled water, which were then added to the electrolyte at concentrations from 0.1 up to 0.6 M.
The potassium hydrogen phosphate solutions were made by dissolving (HO)2P(O)(O-K+) (M = 174.18 g/mol) in distilled water, which were then added to the electrolyte at concentrations from 0.1 up to 0.6 M.
The dimethyl vinylphosphonate solutions were made by diluting C2H3-P(O)(OCH3)2 98% (d = 1.13 and M = 136.09 g/mol) with distilled water, which were then added to the electrolyte at concentrations from 0.1 up to 0.3 M. The vinylphosphonic acid solutions were made by diluting CH2=CHP(O)(OH)2 97% (d = 1.37 and M = 108.03 g/mol) with distilled water, which were then added to the electrolyte at concentrations from 0.1 up to 0.3 M. The solutions were freshly made before each experiment.
Electrochemical studies
The electrochemical measurements were carried out using a three-electrode cell assembly comprised of a saturated calomel electrode (reference), a platinum electrode (counter) and lead coupon as working electrode. Before each experiment, an open circuit potential was measured for 5 minutes, in order to reach a steady state. Potentiodynamic polarization was performed with a sweep rate of 2 mV/s in the range of -1 to 2.5 V, with respect to the corrosion potential. Various corrosion kinetic parameters, such as corrosion current density (Icorr), corrosion potential (Ecorr) and passivation current (Ipass) were obtained. Corrosion current density was measured from the intersection point obtained by the extrapolation of Tafel lines. The impedance measurement was performed using a frequency range from 100 kHz to 10 Hz at corrosion potential. VoltaLab 10 electrochemical analyzer model (PGZ100) interfaced with HP computer along with VoltaMaster 4 and OriginLab softwares were used for data acquisition and analysis.
Results and discussion
Potentiodynamic polarization
The anodic and cathodic polarization curves for lead in H2SO4 solution, with and without the addition of phosphoric acid, potassium hydrogen phosphate, dimethyl vinylphosphonate and vinylphosphonic acid are shown in Figs. 1, 2, 3, 4 and 5, respectively.
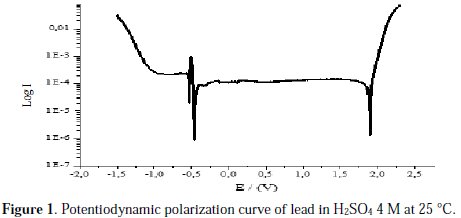
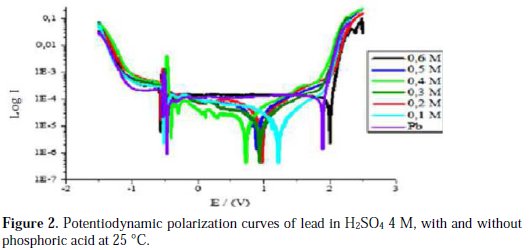
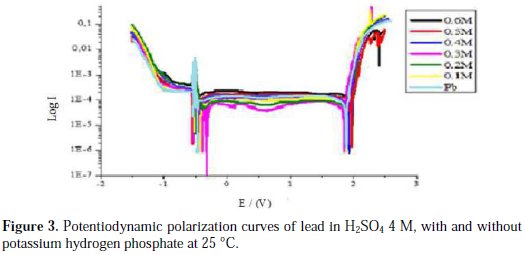
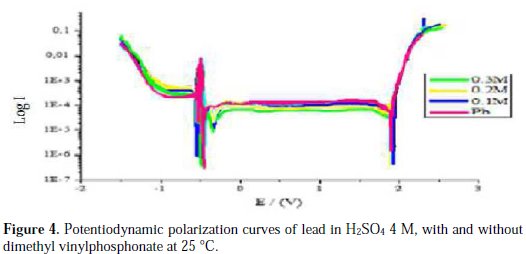
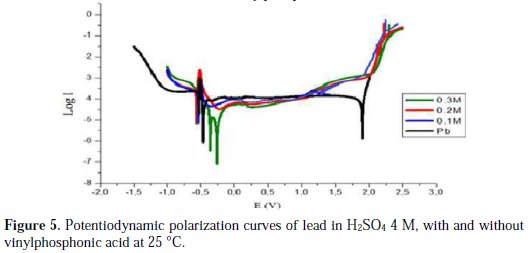
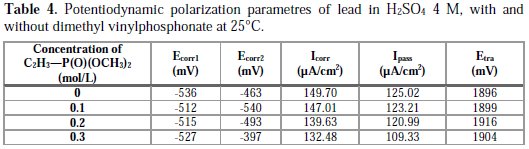
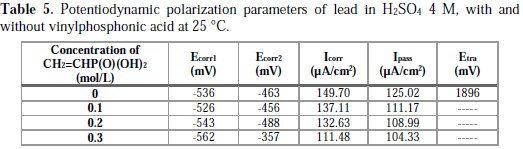
The intersection point of Tafel regions gives the corrosion current density (Icorr). The obtained polarization parameters Icorr, Ecorr, Ipass and Etra are given in Tables 1, 2, 3, 4 and 5.
Corrosion of lead in pure H2SO4 4 M medium
Examining the polarization curve shown in Fig. 1, we observe the evolution of hydrogen on the specimen represented by a high current that dropped with increasingly positive potential. The lead, at this range of potential, is at its active mode. Two pics of potential, observed at -536 mV and at -463 mV, correspond to the corrosion of lead and the formation of PbO and PbSO4, respectively, by the reaction of lead with water and H2SO4. Following these pics, the current drops to a low value (Ipass), indicating the attainment of passivity, and remains constant for a considerable range of potential. A layer comprised of lead sulfate PbSO4 and lead oxide PbO blocks the ion transfer from the electrolyte to the surface of the metal, inhibiting any further corrosion. Lead at this range shows passive characteristics. At 1896 mV, a pic was observed corresponding to the trans passive mode, where a layer of PbO2 is formed by the reaction between lead sulfate and sulfuric acid. Furthermore, the current increases again, representing the evolution of oxygen in the electrolyte [22].
Corrosion of lead with the addition of phosphoric acid
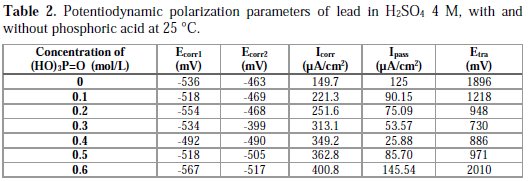
Fig. 2 and Table 2 show that the addition of phosphoric acid at concentrations up to 0.4 M makes the corrosion potential Ecorr1 shift towards less negative values from -536 to -492 mV, meaning that the metal was protected. This shifting is accompanied of a significant decrease of the passivity current Ipass from 125 to 25.88 μA/cm2. This indicates that the addition of phosphoric acid at this range of concentration increases the passivity of lead, by perhaps forming a porous layer of PbHPO4 [19]. After the reaction of lead with phosphoric acid, this layer is deposited along with PbSO4, leading to a better inhibition of ion transfer to the metal, and blocking any further corrosion. We have observed in our study that the main disadvantage of phosphoric acid is that the corrosion current Icorr increases with an increasing concentration, due to the fact that phosphoric acid is a strong acid that can increase the corrosion rate of lead. The trans-passive potential Etra decreases with an increasing concentration of phosphoric acid up to 0.4 M, indicating that phosphoric acid facilitates the formation of PbO2. On the other hand, by adding phosphoric acid at concentrations above 0.4 M, we notice that passivity current increases with an increasing concentration of phosphoric acid; this may be due to the competitive deposition of PbHPO4 with PbSO4, not possible at lower concentrations, and because PbHPO4 is more conductive than PbSO4, as it allows the passage of ions on the interface metal/electrolyte [20, 21]. In its turn, the trans-passive potential Etra increases with an increasing concentration of phosphoric acid, indicating that the addition of phosphoric acid, at this range of concentration, retards the formation of PbO2, perhaps through the formation of the complex Pb3(PO4)2, as an intermediate in the process of lead corrosion [10]; the oxidation of this complex into PbO2 is produced at higher potentials, due to its high stability.
Corrosion of lead with addition of potassium hydrogen phosphate
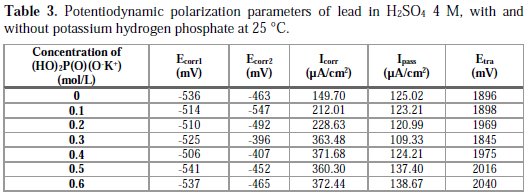
After analyzing Fig. 3 and Table 3, we conclude that the addition of potassium hydrogen phosphate at concentrations up to 0.3 M makes the corrosion potential Ecorr1 shift towards less negative values; this means that the metal is protected. Meanwhile, the passivity current decreases at this range of concentrations indicating an increase of the passivity of lead. This may be explained by the formation of a porous layer comprised of PbKPO4 that deposited together with PbSO4, forming a thick passive layer that blocks the passage of ions to the metal and increases its passivity. At low concentrations (< 0.3 M), the trans-passive potential does not shift towards a precise direction, indicating that potassium hydrogen phosphate does not influence the PbO2 formation. The corrosion current Icorr increases with an increasing concentration. Above 0.3 M of potassium hydrogen phosphate, we notice that the passivity current Ipass increases with an increasing concentration; this may be explained by the deposition of PbKPO4 (which is more conductive than PbSO4), in detriment of PbSO4, allowing the ion transfer at the interface lead/electrolyte, and thereby, decreasing the lead passivity. The trans-passive potential also increases at this range of concentration, indicating a retardation of PbO2 formation, possibly due to the formation of Pb3(PO4)2 that transforms into PbO2 at higher potentials, because of its stability.
Corrosion of lead with addition of dimethyl vinylphosphonate
Fig. 4 and Table 4 show us that the addition of dimethyl vinylphosphonate does not make the corrosion potentials shift towards a precise direction, indicating that dimethyl vinylphosphonate can be considered as a mixed inhibitor. The advantage of dimethyl vinylphosphonate is that the corrosion current Ecorr decreases with an increasing concentration of dimethyl vinylphosphonate up to 0.3 M, indicating a decrease in the corrosion rate, unlike phosphoric acid and potassium hydrogen phosphate. We also notice that the passivity current Ipass decreases with an increasing concentration, indicating an increase of the passivity of lead. This might be explained by the fact that dimethyl vinylphosphonate induces the formation of a passive layer that inhibits the corrosion of the metal by blocking the ion transfer. The trans-passive potential doesn't shift significatively, meaning that dimethyl vinylphosphonate has no influence on the PbO2 formation.
Corrosion of lead with the addition of vinylphosphonic acid
We have studied the addition of vinylphosphonic acid at concentrations up to 0.3 M, to see the difference between vinylphosphonic acid and the ester potassium hydrogen phosphate at this interesting range.
Fig. 5 and Table 5 show us that the addition of vinylphosphonic acid does not make the corrosion potentials shift towards a precise direction, indicating that vinylphosphonic acid can be considered as a mixed inhibitor. The advantage noticed for the addition of vinylphosphonic acid is that the corrosion current Ecorr decreases with an increasing concentration of vinylphosphonic acid, indicating a decrease in the corrosion rate, just like in the case of potassium hydrogen phosphate. We also have noticed that the passivity current Ipass decreases at low concentrations, indicating an increase of the passivity of lead. We also have noticed a significant decrease of the marge of the passivity, which may be due to a rapid degradation of PbSO4. After the addition of vinylphosphonic acid, we have noticed the complete absence of the Etra, indicating that there is no formation of PbO2, unlike previous cases.
Electrochemical impedance spectroscopy (EIS)
EIS was used to determine the action mode of the compound studied, and to evaluate the dielectric properties of the passive layer; it also helps explaining the electrochemical process that develops throughout the passive layer. Thus, we used this technique to study the reaction mechanisms during the lead corrosion in H2SO4 with the presence of phosphoric and phosphonic compounds.
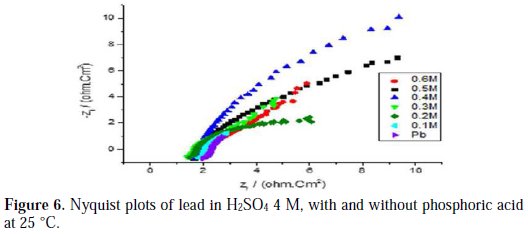
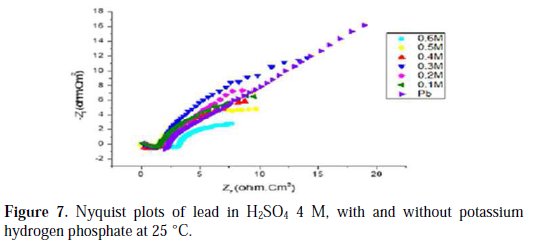
Figs. 6, 7, 8 and 9 show the Nyquist plots for pure lead in H2SO4 4 M, in the absence and presence of phosphoric acid, potassium hydrogen phosphate, potassium hydrogen phosphate and vinylphosphonic acid, respectively, generated at 25 °C.
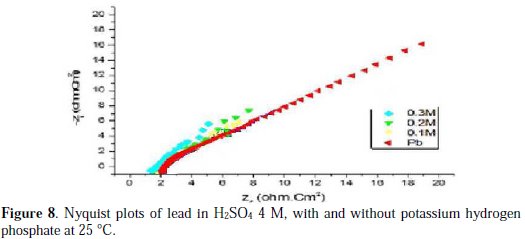
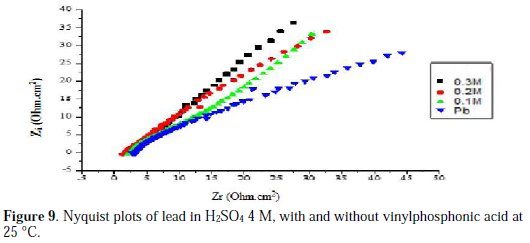
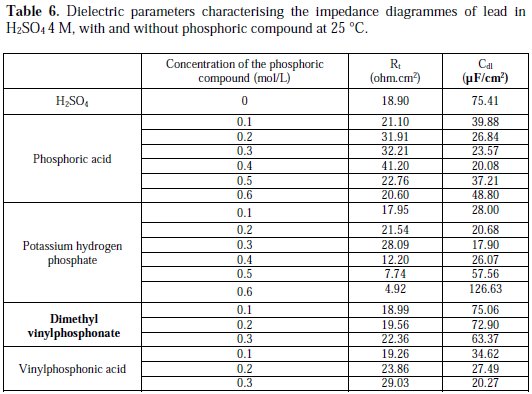
The values of the charge transfer resistance Rt, and those of the capacity of the double layer Cdl at Ecorr = -536 mV/SCE, are listed in Table 6.
The charge transfer resistance values Rt were calculated based on the difference between impedance values at lower and higher frequencies.
The Nyquist plots shown in Figs. 6, 7, 8 and 9 are in the shape of a unique semicircle, meaning that the charge transfer is the main reaction mechanism at the interface lead/electrolyte [16, 17]. At concentrations below 0.5 M for phosphoric acid, and 0.4 M for potassium hydrogen phosphate, and vinylphosphonic acid, the diameter of the semi-circles increases with an increasing concentration of phosphoric and phosphonic compounds, indicating the growth of a passive layer that protects the metal from corrosion. At higher concentrations, the diameter of the semi-circles decreases because of the degradation of the passive layer [18].
Analyzing Table 6, we can clearly see that, below a certain concentration of the phosphoric compound, the charge transfer resistance Rt increases, and the capacitance Cdl decreases with an increasing concentration. This indicates that the thickness of the passive layer increases with the addition of the compound, by formation and deposition of PbHPO4, after adding phosphoric acid to the electrolyte, and PbKPO4, after adding potassium hydrogen phosphate to the electrolyte, along with PbSO4 on the surface of lead, and by increasing the rate of PbSO4 formation, after adding potassium hydrogen phosphate and vinylphosphonic acid.
At higher concentrations of the three phosphoric and phosphonic compounds, Rt decreases and Cdl increases with an increasing concentration. This is attributed to the formation and deposition of PbHPO4 (for phosphoric acid) and PbKPO4 (for potassium hydrogen phosphate) in detriment of PbSO4, leading to a decrease in thickness of the passive layer.
Conclusions
With the addition of phosphoric acid or its salt potassium hydrogen phosphate at concentrations up to 0.4 M, the passivation current Ipass decreases, indicating an increase of the passivation rate of lead; the corrosion rate Icorr increases and trans- passive potential Etra decreases, meaning that these 2 compounds accelerate the formation of PbO2.
After increasing the concentration of potassium hydrogen phosphate and vinylphosphonic acid up to 0.3 M, the passivation current Ipass decreases, and the corrosion current Icorr also decreases, meaning that both products increase the lead passivation and decrease its corrosion rate. Our studies showed that potassium hydrogen phosphate has no effect on the PbO2 formation. Meanwhile, the addition of vinylphosphonic acid has the same effect on the corrosion and passivation of lead, but it suppresses the formation of PbO2.
We can conclude that phosphoric acid, potassium hydrogen phosphate, and vinylphosphonic acid, added to a certain concentration, create a better resistance of lead against corrosion in sulfuric acid, by increasing its passivation, and can improve the life time of the battery, by reducing the sulphatation phenomenon.
References
1. Venugopalan S. J Power Sources. 1994;48:371. [ Links ]
2. Bhattacharya A, Basumallick I N. J Power Sources. 2003;113:382. [ Links ]
3. Paleskaa I, Pruszkowska-Drachala R, Kotowskia J, et al. J. Power Sources. 2003;113:308. [ Links ]
4. Li S, Chen HY, Tang MC, et al. J. Power Sources. 2006;158:914. [ Links ]
5. Saminathan K, Jayaprakash N, Rajeswari B, et al. J Power Sources. 2006;160:1410. [ Links ]
6. Abd El-Rahman HA, Salih SA, Mokhtar AA. AFINIDAD LXVIII. 2011;351. [ Links ]
7. Badawy WA, El-Egamy SS. J Power Sources. 1995;55:11. [ Links ]
8. Stemberg S, Mateescu A, Branzoi V, et al. Electrochim Acta. 1987;32:349. [ Links ]
9. Doring H, Wiesener K, Garche J, et al. J Power Sources. 1992;381:261. [ Links ]
10. Bullock K. Phosphoric acid influence. Elsevier; 2009. [ Links ]
11. Visscher W. J Power Sources. 1976/77;1:257.
12. Doring H, Wiesener K, Garche J, et al. J Power Sources. 1992;38:261. [ Links ]
13. Sternberg S, Branzoi V, Apateanu L. J Power Sources, 1990;30:177. [ Links ]
14. Saminathan K, Jayaprakash N, Rajeswari B, et al. J Power Sources. 2006;160:1410. [ Links ]
15. Stemberg S, Mateescu A, Branzoi V, et al. Electrochim Acta. 1987;32:349. [ Links ]
16. Larabi L, Harek Y, Traisnel M, et al. J Appl Electrochem. 2004;34:833. [ Links ]
17. Li X, Deng S, Fu H. Corros Sci. 2010;52:2786. [ Links ]
18. Behpour M, Ghoreishi SM, Mohammadi N, et al. Corros Sci. 2010;52:4046. [ Links ]
19. Paleskaa I, Pruszkowska-Drachala R, Kotowskia J, et al. J Power Sources. 2003;113:308. [ Links ]
20. Li S, Chen HY, Tang MC, et al. J Power Sources. 2006;158:914. [ Links ]
21. Abd El-Rahman HA, Salih SA, Mokhtar AA. AFINIDAD LXVIII. 2011;349. [ Links ]
22. El Rahim A, El Halim AA, Fouad E. J Industrial Chem. 1999. [ Links ]
*Corresponding author. E-mail address: khatbisalma@gmail.com
Received May 24, 2016; accepted October 13, 2016














