Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portugaliae Electrochimica Acta
Print version ISSN 0872-1904
Port. Electrochim. Acta vol.34 no.4 Coimbra July 2016
https://doi.org/10.4152/pea.201604287
Modeling the Corrosion Inhibition of Mild Steel in HCl Medium with the Inhibitor of Pawpaw Leaves Extract
M. Omotiomaa,* and O. D. Onukwulib
a Department of Chemical Engineering, Enugu State, University of Science and Technology, P.M.B. 01660, Enugu, Nigeria
b Department of Chemical Engineering, Nnamdi Azikiwe University, Awka, Nigeria
Abstract
Modeling the corrosion inhibition of mild steel in HCl medium with inhibitor of pawpaw leaves extract is presented. The extract was analyzed using gas chromatography-mass spectrometry. Thermometric and gravimetric methods were employed in the corrosion inhibition study. The inhibition efficiency was modeled and optimized using response surface methodology (RSM). It was observed that the free energy of adsorption (ΔGads) was negative and less than the threshold value of -40 kJ/mol. The adsorption of the extract was spontaneous, and occurred according to the mechanism of physical adsorption. A quadratic model was generated, with optimum inhibition efficiency of 80.29% obtained. The extract was highly efficient in the corrosion control process. It is effective for surface treatment of mild steel in the acid medium. Therefore, it is recommended that pawpaw leaves extract should be employed as corrosion inhibitor in oil well acidizing and surface treatment of mild steel.
Keywords: Corrosion, HCl, Mild Steel, Pawpaw Leaves.
Introduction
It has been acknowledged that mild steel is widely employed in most industries, due to its low cost and availability for the fabrication of various reaction vessels, such as cooling tower tanks and pipelines [1]. Mild steel corrodes as a result of an electrochemical reaction with its environment. To prolong the life span of mild steel structures, inhibitive additives are needed in pickling and other related maintenance operations. In view of environmental concern, efforts are geared towards applications of plant extracts as corrosion inhibitors in oil well acidizing and inhibition additives for surface treatment of metals. In the development of an eco-friendly corrosion inhibitor of plant origin, pawpaw leaves extract is of great interest. Pawpaw leaves produce several alkaloids and other phytochemicals with important pharmaceutical and industrial applications.
Experimental methods
Sheets of mild steel with P (0.02%), Mn (0.11%), Si (0.02%), S (0.02%), Cu (0.01%), C (0.23%), Ni (0.02), Cr (0.01%) and Fe (99.56 %) composition were cut into coupons (5 cm × 4 cm). Leaves of pawpaw (Carica papaya) were collected from Akpugo, Enugu State, Nigeria. In the extraction of the pawpaw leaves extract and surface preparation of the mild steel, the method used in the previous study was adopted [2]. Standard method was used for the chemical analysis of the extract [3, 4]. It was carried out using a gas chromatography-mass spectrometer (GCMS-QP2010 PLUS, SHIMADZU). An acid medium of 1.0 M HCl was used for the study.
Thermometric and gravimetric measurements were used in the corrosion inhibition study. The inhibition efficiency was optimized using response surface methodology (RSM). For the thermometric measurements, the method used by previous authors was adopted with slight modifications [5, 6]. The inhibitor efficiency was determined using Equation (1),
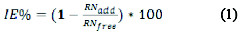
where RNfree and RNadd are the reaction numbers for the metal dissolution in free and inhibited corrosive medium, respectively. The reaction number (RN) was evaluated using Equation (2),

where Tm and Ti are the maximum and initial temperatures (in °C) respectively, and t is the time in minutes elapsed to reach Tm.
For the gravimetric measurements, the weight loss (Δw), corrosion rate (CR), inhibition efficiency (IE) and degree of surface coverage were determined using Equations (3), (4) and (5), respectively.


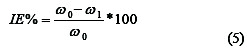
The surface coverage was obtained using the following Equation (6) [7],

where wi and wf are the initial and final weight of mild steel samples, respectively; w1 and w0 are the weight loss values in presence and absence of the inhibitor, respectively. A is the total area of the mild steel sample and t is the immersion time.
Considering the corrosion rates of the metal at T1 and T2 as CR1 and CR2, the activation energy, Ea, was obtained using Equation (7) [8-10].
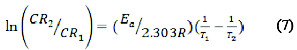
The heat of adsorption Qads (kJmol-1) was calculated using Equation (8) [9, 11],
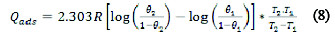
where R is the gas constant, θ1 and θ2 are the degree of surface coverage at temperatures T1 and T2, respectively.
Different adsorption isotherms were used to determine the adsorption mechanism of the extract on the metal surface. Langmuir, Frumkin, Temkin and Flory- Huggins isotherms are shown in Equations (9), (10), (11) and (12), respectively [9, 12, 13, 14].

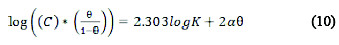
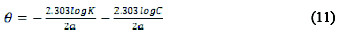
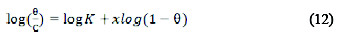
where C is the concentration of the inhibitor, K is the adsorption equilibrium constant, θ is the degree of surface coverage, α is the lateral interaction term describing the interaction in adsorbed layer, a is the attractive parameter, and x is the size parameter and a measure of the number of adsorbed water molecules substituted by a given inhibitor molecule.
The free energy of adsorption (ΔGads) was calculated according to Equation (13) [9, 12].
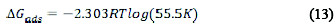
where R is the gas constant, and T is the temperature.
Results and discussions
Analysis of the pawpaw leaves extract
In Fig. 1, the GC MS chromatogram of the pawpaw leaves extract shows various peak levels.
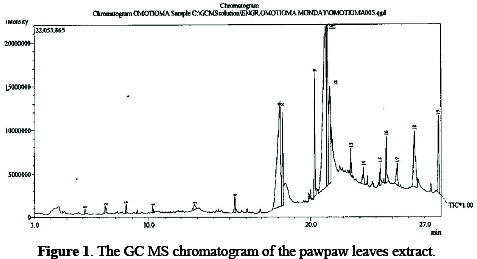
Each of the peaks represents compounds, as determined by the GC MS. The analysis revealed the presence of C8H7N (117g/mole: Benzyl nitrile; Benzyl cyanide; Benzene acetonitrile; Phenylacetonitrile; Phenyl acetyl nitride); C9H9NO3 (179 g/mole (Benzene propanoic acid); C8H7N (117 g/mole: 2,4,6Cycloheptatriene- 1-carbonitrile; 1,3,5-Cycloheptatriene-7-carbonitrile; 7-Cyano1,3,5- cycloheptatriene; 7-Cyanocycloheptatriene; 1-Cyano-2,4,6-cycloheptatriene; 7-cyanotropilidene); C8H8O2 (136 g/mole: Benzaldehyde, 2-Hydroxy-4methylbenzaldehyde). It also contains C5H10N2O3 (146 g/mole: Levoglutamide; l-Glutamine; Glutamic acid amide) C7H14O6 (194 g/mole: Methyl beta.-dgalactopyranoside); C16H30O (238 g/mole: cis-9-Hexadecenal; 9-Hexadecenal); C14H26O (210 g/mole 9-Tetradecenal); C18H34O2 (282 g/mole: Oleic Acid, 9Octadecenoic acid; cis-9-Octadecenoic Acid; cis-.delta.9-Octadecenoate); C16H30O2 (254 g/mole: 9-Hexadecenoic acid) and C22H42O2 (338 g/mole: Erucic acid, 13-Docosenoic acid). The extract contains organic compounds with polar atoms capable of being adsorbed onto the metal surface through the polar atoms [15].
Results from the thermometric method
Effects of the inhibitor's concentration on the reaction number (RN) and the inhibition efficiency (IE) are shown in Table 1.
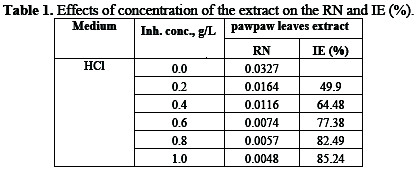
Increase in concentration lowers the reaction number, but increases the inhibition efficiency of the inhibitor. It agrees with previous observations [2, 5].
Results from the Gravimetric Method
In Table 2, corrosion rate decreases with an increase in the extract concentration.
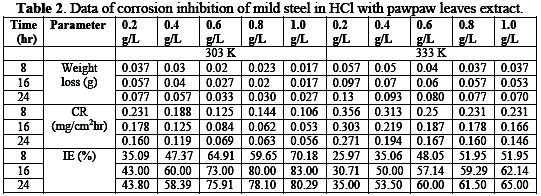
Highest inhibition efficiency of 83.00% was obtained. It showed that pawpaw leaves extract can be used as an additive for pickling, cleaning and descaling operations. The extract is efficient for corrosion control of mild steel in acid medium.
Energy activation and adsorption heat process
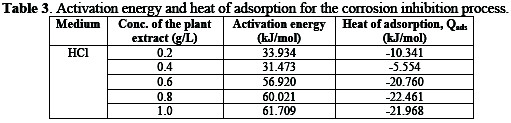
In Table 3, the values of the activation energy and heat of adsorption indicate that the adsorption of the pawpaw extract on the surfaces of mild steel has been conformed with the mechanism of physical adsorption and exothermic reaction.
Adsorption parameters
The parameters of Langmuir, Frumkin, Temkin and Flory-Huggins isotherms are presented in Table 4.
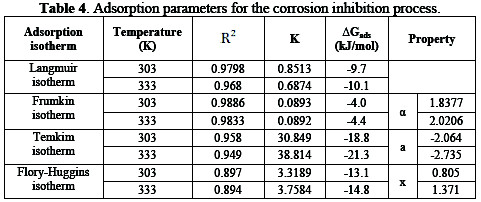
Considering the fitted data to Langmuir isotherm, R2 values are very close to unity, indicating strong adherence to Langmuir adsorption isotherm [10, 16]. The application of Langmuir isotherm to the adsorption of the extract indicates that there is no interaction between adsorbate and adsorbent. The lateral interaction term (α) gave positive values, suggesting attractive behaviour of the inhibitor on the mild steel surface. The attractive parameter values (a) were negative, indicating that repulsion in the adsorption layer exists [10]. The values of the size parameter (x) were positive. They show that the adsorbed species of the extract were bulky [9]. The values of ΔGads were negative and lower than the threshold value of -40 kJ/mol required for chemical adsorption. It showed that adsorption of the extract on the metal surface followed the mechanism of physical adsorption [9, 17].
Results as analyzed by RSM
Table 5 showed the dependency of weight loss, corrosion rate and inhibition efficiency on concentration, temperature and time.
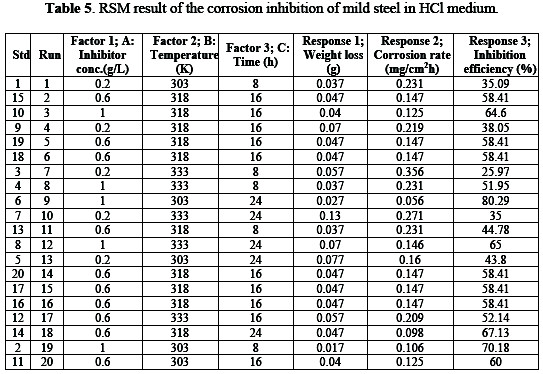
Optimum inhibition efficiency of 80.29% was obtained.
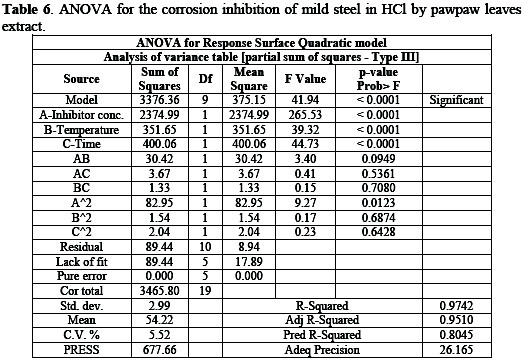
The statistical analysis of the data is shown in Table 6.
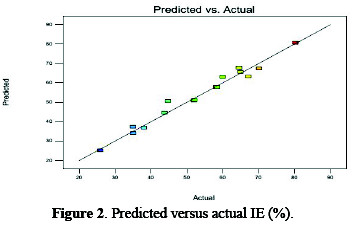
In graphical form, predicted versus actual values of the inhibition efficiency were highly correlated (Fig. 2).
The general model with combined significant and insignificant terms is presented in Equation (14).
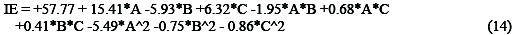
The quadratic model relates the inhibition efficiency (IE) with concentration (A), temperature (B) and time (C). Considering only the significant terms, the model is reduced to Equation (15).
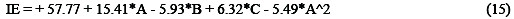
From the statistical analysis, there was only a 0.01% chance that large F-value could occur due to noise. The source of noise may be attributed to non-controllable factors of oxygen content and flow rates of the inhibitors' functional groups. The models are significant and can be used to navigate the design space.
Conclusion
A quadratic model was generated, with high inhibition efficiency of 80.29% obtained. The extract is effective for surface treatment of mild steel in acid medium. It is therefore recommended that pawpaw leaves extract should be employed as corrosion inhibitor in oil well acidizing and other related surface treatment of mild steel.
References
1. Dubey AK, Singh G. Port Electrochim Acta. 2007;25:221. [ Links ]
2. Omotioma M, Onukwuli OD. Der Pharma Chem. 2015;7:373. [ Links ]
3. El Ouariachi E, Paolini J, Bouklah M, et al. Acta Metal Sin. 2010;23:13. [ Links ]
4. Rosaline-Vimela J, Leema RA, Raja S. Der Chemica Sinica. 2012;3:582. [ Links ]
5. Mabrouk EM, Shokry H, Al-Naja KMA. Chem Met Alloys. 2011;4:98. [ Links ]
6. Eddy NO, Ita BI, Dodo SN, et al. Green Chem Lett Rev. 2012;5:43. [ Links ]
7. Nagm AN, Kandile NG, Badr EA, et al. Corrosion Sci. 2012;65:94. [ Links ]
8. Octave L. Chemical reaction engineering. 3rd ed. New York: John Wiley & Sons; 2003. [ Links ]
9. Nwabanne JT, Okafor VN. J Emerging Trends Eng Appl Sci (JETEAS). 2011;2:619. [ Links ]
10. Nnanna LA, Owate IO, Nwadiuko OC, et al. Int J Mater Chem. 2013;3:10. [ Links ]
11. Orubite-Okorosaye K, Oforka NC. J Appl Sci Environ Mgt. 2004;8:57. [ Links ]
12. Alinno IJ, Ejikeme PM. Am Chem Sci J. 2012;2:122. [ Links ]
13. Li X, Deng S. Corrosion Sci. 2012;65:299. [ Links ]
14. Patel NS, Jauhariand S, Mehta GN, et al. Int J Electrochem Sci. 2013;8:2635. [ Links ]
15. Rani BEA, Basu BBJ. Int J Corrosion, 2012;15. [ Links ]
16. Vasudha VG, Shanmuga PK. Res J Chem Sci. 2013;3:21. [ Links ]
17. Ndibe OM, Menkiti MC, Ijomah MNC, et al. EJEAFChe. 2011;10:2847. [ Links ]
Acknowledgements
For the GC-MS characterization of the pawpaw extract, the authors are grateful to National Research Institute for Chemical Technology (NARICT), Zaria, Nigeria.
*Corresponding author. E-mail address: omorchem@yahoo.com
Received June 18, 2016; accepted 26 August, 2016














