Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portugaliae Electrochimica Acta
Print version ISSN 0872-1904
Port. Electrochim. Acta vol.34 no.2 Coimbra Mar. 2016
https://doi.org/10.4152/pea.201602119
Inhibitive effect by Psidium guajava leaf extract on the corrosion of double thermally-aged Al-Si-Mg (SSM-HPDC) alloy in simulated seawater environment
L. M. Sania,* , M. Abdulwahaba , S. A. Yaroa,b and F. O. Kolawolec
a Department of Metallurgical and Materials Engineering, Ahmadu Bello University, Zaria, Nigeria.
b Shell chair professorial occupant, Dept of Mechanical Engineering, Ahmadu Bello Univ., Zaria, Nigeria.
c Department of Materials and Metallurgical Engineering, Federal University Oye-Ekiti, Nigeria.
Abstract
The assessment of Psidium guajava leaf extract as corrosion inhibitor for double thermally-aged Al-Si-Mg (SSM-HPDC) alloy in 3.5%wt NaCl solution using the gravimetric and potentiodynamic polarization techniques was investigated. The gravimetric test was carried out at different inhibitor concentration, time and temperature ranges of 0.1-0.5%v/v, 1-5 hrs and 30-70oC. The results revealed that Psidium guajava leaf extract in 3.5%wt NaCl solution-aluminium environment decreased the corrosion rate at various concentrations considered. Inhibition efficiency (IE) of 72.1% at 0.5% v/v Psidium guajava leaf extract addition using the gravimetric method was demonstrated in 3.5%wt NaCl solution. The IE from the potentiodynamic polarization method was significantly enhanced as high as 98.89%/0.5%v/v. The additions of Psidium guajava leaf extract as corrosion inhibitor in the solution indicate higher potential value, IE and polarization resistance with decrease in current density. The methods for assessment of the alloy were in agreement and mixed-type corrosion exist which obeyed the Langmuir adsorption isotherms. The optical microscopy (OPM) revealed that the microstructure of the double thermally-aged sample has finer grains and enhanced grain boundaries compared to the untreated sample. The scanning electron microscope (SEM) surface morphology of as-corroded uninhibited condition showed severe damage and pit formation than as-corroded inhibited.
Keywords: Aluminium, Psidium guajava, Corrosion, Thermal ageing, SEM, OPM, Thin film.
Introduction
Aluminum is the second widely used metal due to its desirable chemical, physical and mechanical properties. It is alloyed with elements like Si, Mg, Cu, Mn, Fe etc. Addition of silicon to aluminum can improve its fluidity as well as castability and mechanical properties [2]. Addition of Magnesium to Al-Si alloy improves its strength to weight ratio and yield stress by combining with silicon to form the age hardening phase (Mg2Si) which precipitates from a super saturated solid solution during heat treatment [4].
Aluminium and its alloys are widely used for different applications in industries and marine environment because of their excellent properties [12]. However, the alloys still exhibit insufficient and poor performance in service condition that requires high hardness, wear and aggressive corrosion environment [1]. Due to different industrial applications and economic importance of aluminium and its alloys, their protection against corrosion by inhibiting the working environment is worth studying [3, 10]. The safety and environmental issues of corrosion inhibitors arising in industries has always been a global concern [13]. The use of inhibitors for corrosion control of materials, which are in contact with aggressive environment, is an accepted practice [11]. The corrosion resistance of aluminium and its alloys using inhibitors have been investigated in many environments [5, 7, 8, 10. 14, 15] Although synthetic inhibitors when used for corrosion control, have been reported to indicate an excellent performance. But majority of these inhibitors are not eco-friendly and are expensive [12]. Therefore effort towards identifying any potential eco-friendly and less expensive corrosion inhibitors remain relevant and important. The growing interest among researchers for green inhibitors remained a top research focus. In this direction, plant extracts and oil have gained acceptance as corrosion inhibitors that are considered safe, eco friendly, available and less expensive for most metals and alloys [12].
Accordingly, the present study involved the use of guava (Psidium guajava) leaf extracts as corrosion inhibitors for double thermally-aged Al-Si-Mg (SSMHPDC) alloy.
Experimental Procedure
Materials and sample preparation
Aluminium alloy specimen with chemical composition as shown in Table 1 was sectioned into coupons of dimension 12×12×2 mm for the corrosion study in Psidium guajava-3.5%wt NaCl solution.

Initially, the coupons were mechanically polished with emery papers down to 800 grit size. The coupons were degreased in ethanol, dried, weighed and stored in desiccators. The initial weight of each sample was taken and recorded.
Heat treatment
The coupons were solution treated at a temperature of 540oC for 1hr in a heat treatment electric resistance furnace and then rapidly quenched in warm water (65 °C). The quenched samples were pre-aged at 105 °C for 2hrs, and then finally aged at 180 °C for 2 hrs before cooling in air. Gravimetric measurement The corrosion rate by gravimetric analysis was carried out on the previous weighed coupons with and without inhibitor at 303K, 323K and 343K. The volume of the solution was 150ml with and without the addition of Psidium guajava inhibitor. The Psidium guajava inhibitor concentration was varied from 0.1, 0.2, 0.3, 0.5 %v/v in 150ml of 3.5 %wt NaCl solution. For each sample, using gravimetric method, the samples were washed, dried and weighed at interval of 1, 2, 3, 4 and 5 hrs of exposure time. The corrosion rate and inhibition efficiency were determined for each inhibitor concentration at 30, 50 and 70 °C.
Potentiodynamic polarization technique
The potentiodynamic polarization and linear polarization resistance was used to characterize the corrosion rate of double thermally-aged aluminium alloy in Psidium guajava-simulated seawater medium. In the electrochemical test, a glass corrosion cell kit with a platinum counter electrode, a saturated Ag/Ag reference electrode and aluminium sample as working electrode were used. The working electrode samples were positioned at the glass corrosion cell kit, leaving 1.14cm2 surfaces in contact with the solution. Polarization test was carried out in simulated seawater solution at room temperature using a potentiostat. The polarization curves were determined by stepping the potential at a scan rate of 0.003V/sec. The polarization curves were plotted using Autolab data acquisition system and both the corrosion rate and potential were estimated by the Tafel extrapolation method.
Microstructure and surface morphology
The samples for the microscopic examination were ground with emery papers of progressively fine grade 80, 220, 600, 800 grits sizes using water as coolant. The ground samples were then polished using one-micro size alumina powder suspended in distilled water, followed by etching in Kellerâs reagent. The optical microscope (OPM) with an inbuilt camera was used for the microstructure observation of the as-received and age-hardened samples while Scanning electron microscope (SEM) was used to assess the surface morphology of the inhibited and uninhibited samples.
Results and Discussion
Hardness measurement and double thermal ageing treatment
Table 2 presents the average hardness value for Al-Si-Mg (SSM-HPDC) alloy, the result of the age-hardened sample showed an increase in hardness value (82.8 HRF) compared to the as-received sample (59.1 HRF) indicating an increase of 28.62%.
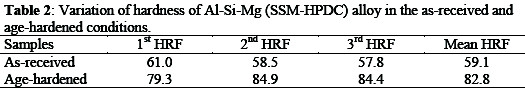
The age-hardening mechanisms responsible for strengthening are based on formation of intermetallic compounds during decomposition of metastable supersaturated solid solution obtained by solution treatment and quenching (Precipitation hardening)[9].
Gravimetric-based mass loss measurement and corrosion rate
From result, the corrosion rate (Figures 1-3) showed the variation of corrosion rate of the age-hardened alloy in simulated seawater environment decrease with addition of inhibitor.
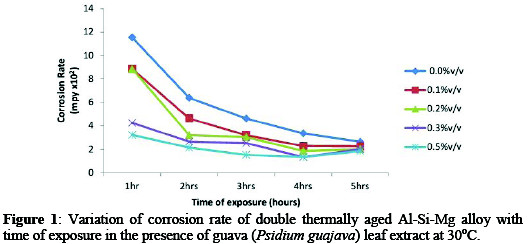
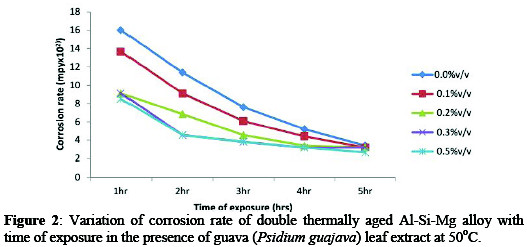
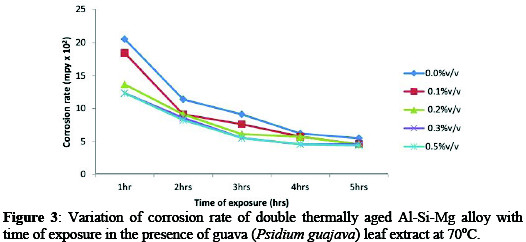
In Figure 1, the sample in NaCl/0.5%v/v Psidium guajava leaf extract exhibit an excellent corrosion resistance. Similar trend was observed in Figure 2 and 3. For example, corrosion rate decreased from 11.54×102 mpy (control) to 3.32×102 mpy (0.5%v/v) at 30 °C for 1hr immersion time (Figure 1).
While at 50 °C/70 °C (0.5%v/v) for 1hr immersion time, corrosion rate decreased from 16.01×102 / 20.58×102 mpy (control) to 8.51×102 / 12.35×102 mpy. The significant decrease in corrosion rate at 0.5%v/v inhibitor concentration can be attributed to the adsorption of inhibitor molecules on alloy surface which act as physical barrier to restrict the diffusion of ions to and from the alloy surface and prevent the alloy atoms (ions) from participating in further anodic or cathodic reactions, hence resulting in decrease in corrosion rate [3].
Effect of inhibitor concentration and time on inhibitor efficiency
Figures 4-6 showed the variations of inhibitor efficiency with time for guava leaf extract with maximum inhibitor efficiencies of 72.10, 66.61, 67.10, 60.30, 29.92% for alloy with time of 1-5hrs respectively were obtained at concentration of 0.5%v/v at 30 °C.
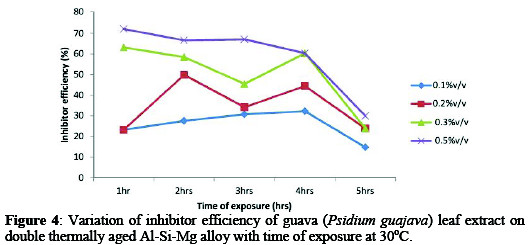
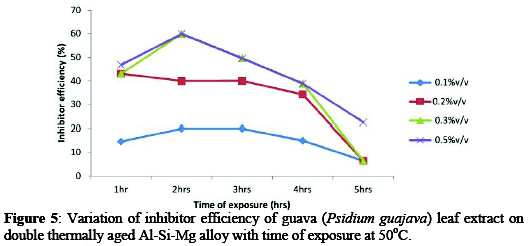
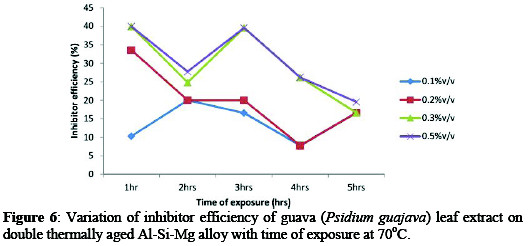
This may be attributed to the fact that, as the extract addition increases, the surface area covered by the inhibitor increased hence higher IE was achieved [12].
Effect of temperature on inhibitor efficiency
It is evident from figure 7 that inhibition efficiency decreases with increasing temperature.
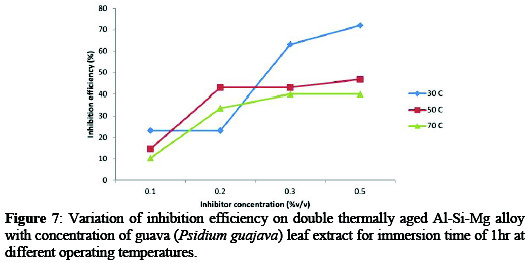
The decrease in inhibitor efficiency with increase in temperature can be attributed to the fact that at a lower temperature the inhibitor molecules adsorbed onto the alloy surface, while at higher temperature increased rate of dissolution process takes place along with partial desorption of inhibitor molecules from the alloy surface as a result of dissociation of constituents of inhibiting substance [2, 11].
Potentiodynamic polarization
In the potentiodynamic polarization for Al-Si-Mg (SSM-HPDC) alloy in 3.5%wt NaCl/ Psidium guajava environment (Table 3), different measurement consisting of potentiodynamic polarization-corrosion rate (PP-CR), potentiodynamic polarization-corrosion density (PP-Jcorr), and linear polarization resistance (LPR) were used as a criteria for evaluation of corrosion resistance of Al-Si-Mg alloy in the environment.
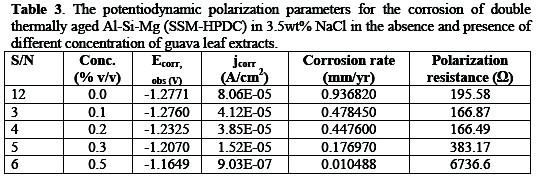
Figure 8 indicate the polarization curves for age- hardened Al-Si-Mg (SSM-HPDC) alloy in 3.5%wt NaCl/ Psidium guajava at 298K.
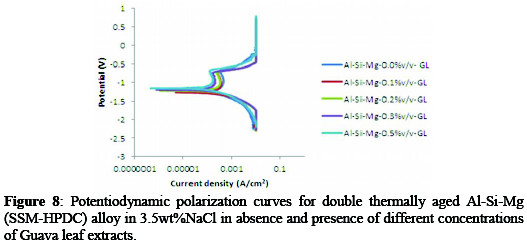
Generally, the studied environment demonstrated a decreased corrosion rate and corrosion current density with addition of Psidium guajava inhibitor. While the corrosion potential (Ecorr) and polarization resistance (Rp) increases with inhibitor concentrations. The corrosion potential of the alloy in blank solution was -1.2771 V but at various concentration of guava leaf extract, the corrosion potential values shifted towards the anodic side (-1.2760 V,-1.2325 V,1.2070 V,-1.1649 V) indicating that the extract affects the anodic reaction predominantly. This is in agreement with previous reports [1, 12]. The inhibited age-hardened Al-Si-Mg (SSM-HPDC) alloy in 3.5%wt NaCl showed that corrosion rate decreased from 0.936820 mm/yr to 0.478450, 0.447600, 0.176970 and 0.010488 mm/yr at 0.1, 0.2, 0.3, and 0.5%v/v Psidium guajava. The linear polarization values showed that addition of guava leaf extract resulted in an increase in polarization resistance value (Rp) for the alloy from 195.58Ω (uninhibited) to 6736.6Ω (inhibited) condition with an IE of 97.1%. The increase in Rp and corresponding decrease in jcorr generally suggested an improvement in corrosion resistance of the age-hardened alloy in the presence of inhibitor. This shows that the alloy in both the as-received and age-hardened condition was protected within the immersion time considered [12].
Inhibitor efficiency and adsorption behavior
The computed inhibitor efficiency (% IE) of age-hardened Al-Si-Mg (SSMHPDC) alloy-Psidium guajava in 3.5%wt NaCl solution was done using the equation reported [1,12]. The variation in % IE using the gravimetric (G.M), potentiodynamic polarization-corrosion rate (PP-CR), potentiodynamic polarization-corrosion density (PP-Jcorr), and linear polarization resistance (LPR) are presented in Figure 9 for 3.5 wt% NaCl/ Psidium guajava.
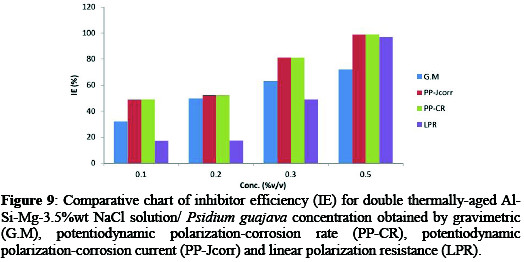
The results (see Table 4)show that addition of Psidium guajava increased the IE with an increase in the inhibitor concentrations.
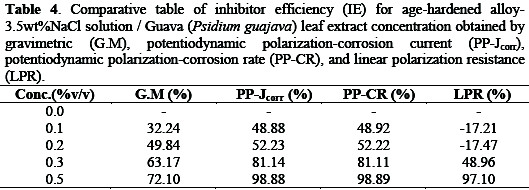
This may be attributed to the fact that, as the Psidium guajava addition increases, the surface covered by this inhibitor increased hence higher IE was achieved. Psidium guajava extracts can be said to exhibit mixed-type corrosion because of the simultaneous change in the anodic and cathodic region during the electrochemical evaluation of the corrosion condition.
The adsorption mechanism of the extracts in showed that the relationship between C/θ and C is linear for both extracts in simulated seawater environment (Figure 10).
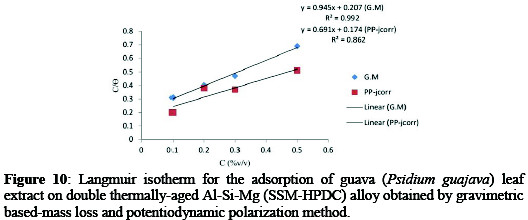
Since the linear regression coefficient/or correction factors (R2) close to unity: G.M (0.992), PP (0.862) the adsorption behavior is assumed to have obeyed Langmuir adsorption isotherms. In this case, the adsorption of inhibitor molecules belongs to a monolayer adsorption, assuming that lateral interactions between the inhibitor molecules are ignored.
Microstructure examination and surface morphology
From microstructure analysis, it can be seen that the microstructure of the age- hardened alloy (Plate II) has finer grains and enhanced grain boundaries than the as-received alloy (Plate I).
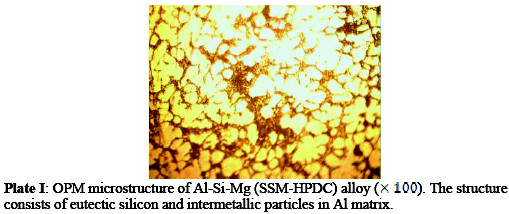
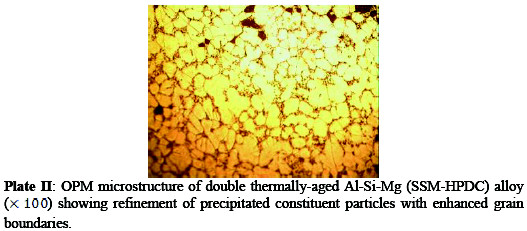
This is based on the fact that there was an increase in volume of the precipitated intermetallic compounds during ageing process and also refinement of the precipitated constituent particles [6].
The SEM micrographs of uninhibited Al-Si-Mg sample in 3.5%NaCl solution (Plate III) indicate severe surface degradation and pit formation compared to the inhibited sample (Plate IV).

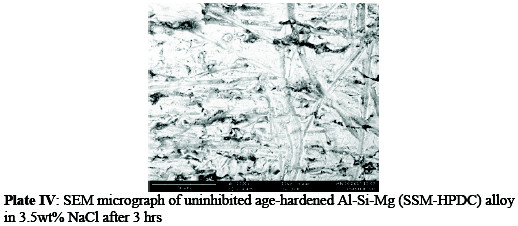
This indicates that the guava leaf extract was able to exhibit some degrees of inhibition which retarded the corrosion rate of the Al-Si Mg alloy. This occurrence has been attributed to formation of thin oxides on the alloy surface which interfere with the reaction sites, thus impede the formation of pits and their growths.
Conclusions
The hardness of the Al-Si-Mg (SSM-HPDC) alloy was improved by 28.62% employing the double thermal ageing treatment. Psidium guajava have been demonstrated to be an effective corrosion inhibitor for Al-Si-Mg (SSM-HPDC) alloy in 3.5wt% NaCl. The corrosion resistance and inhibitor efficiency of age- hardened Al-Si-Mg (SSM-HPDC) alloy increased with addition of Psidium guajava as inhibitor. IE as high as 72.1% and 98.89% in 3.5wt% NaCl-Psidium guajava condition was obtained for the gravimetric and potentiodynamic polarization techniques respectively. The adsorption behavior of Psidium guajava leaf extract in 3.5wt% NaCl for age-hardened Al-Si-Mg (SSM-HPDC) alloy can be said to obey the Langmuir adsorption isotherms. Potentiodynamic polarization measurements show that Psidium guajava is a mixed type inhibitor predominant to the anodic branches. The SEM examination showed that there was improvement in surface morphology of the as-corroded inhibited alloy compared to uninhibited sample.
References
1. Abdulwahab M, Popoola A P I, Fayomi O S I. Int J Electrochem Sci. 2012;7:11706. [ Links ]
2. Asuke F, Yaro S A, Oloche O B, et al . J Met Mat Engineering. 2009;4:4854. [ Links ]
3. Ayeni F A, Madugu I A, Sukop P, et al . J Min Mat Charact Eng. 2012;11:667. [ Links ]
4. Birol Y. J Alloys Compounds. 2009;484:164. [ Links ]
5. Hongwei S, Han E H, Lamaka S V, et al. Prog Inorg Coating. 2014;77:765. [ Links ]
6. Hossain A, Kurny A S. Int J Chemical, Nuclear, Met Mat Eng. 2014;8:288. [ Links ]
7. Howida A F, Tarek M A, Mohamed S E. Int J Electrochem Sci. 2014;9:1565. [ Links ]
8. Loto C A, Loto R T, Popoola A P I. Int J Phys Sci. 2011;6:2249. [ Links ]
9. Mohammed R A, Abdulwahab M, Madugu I A, et al. J Mater Environ Sci. 2013;4:93. [ Links ]
10. Nnanna L A, Obasi V O, Nwadiuku O C, et al. Arch Appl Sci Res. 2012;4:207. [ Links ]
11. Olusegun S J, Adeiza B A, Ikeke K I, et al. J Emerging Trends Eng Appl Sci (JETEAS). 2013;4:138. [ Links ]
12. Popoola A P I, Fayomi O S I, Abdulwahab M. Int J Electrochem Sci. 2012;7:5817. [ Links ]
13. Stephen G Y, Sylvester O A, Tersoo G T, et al. Int J Adv Research Chem Sci (IJARCS). 2014;1:38. [ Links ]
14. wan Nik W B, Sulaiman O, Ayob A F, et al. Int J Eng Res Appl. 2012;1:723. [ Links ]
*Corresponding author. E-mail address: sanilawal66@yahoo.com
Received 15 April 2015; accepted 04 March 2016














