Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.34 no.1 Coimbra jan. 2016
https://doi.org/10.4152/pea.201601053
Corrosion Inhibition of Aluminium in 2 M Phosphoric Acid Using the Essential Oil of Mentha Pulegium Leaves
M. S. Uwinezaa,* , M. Essahlib and A. Lamiric
a University Hassan I, Laboratory of Applied Chemistry and Environment, Faculty of Sciences and Technology, BP 577 Settat, Morocco
b University Hassan I, Department of Applied Chemistry and Environment, Faculty of Sciences and Technology, BP 577 Settat, Morocco
b University Hassan I, Director of the Superior School of Technology of Berrechid, B.P 218 Berrechid, Morocco
Abstract
The corrosion inhibition characteristics of essential oil of mentha pulegium leaves have been studied as a green inhibitor of corrosion of aluminum in 2 M phosphoric acid using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS). The effect of inhibitor concentration shows that efficiency increases with increase of concentration with maximum of 79% at 1800 ppm. Polarization curves reveal that the essential oil of mentha pulegium leaves acts as a cathodic inhibitor. The effect of temperature on aluminum corrosion behavior was also studied and thermodynamic data of activation was determined.
Keywords: corrosion, phosphoric acid, aluminum, inhibitor, mentha pulegium.
Introduction
Aluminum and its' alloys are a very important class of materials for their high technological value and a wide range of industrial applications particularly in aerospace and food industry, due to their low weight, low cost and favorable mechanical properties [1, 2]. Aluminum and its' alloys are resistant to corrosion due to the surface forming a thin oxide layer that protects the metal against the oxidizing environments and a possible chemical attack [1, 3]. However, this corrosion resistance can be influenced by the way in which metals are used [3]. Efforts to stop or delay the maximum attack of metals in various corrosive media are made [4]. The use of green eco-friendly natural polymeric structures, extracted from leaves or seeds as corrosion inhibitors, is gaining large preference and interest due to its safe effect to the environment, practical use, low cost and renewable sources of materials [4-6]. The extent of inhibition by those compounds depends on the effective organic groups and increases in the order oxygen < nitrogen < sulfur < phosphorus [7, 8].
Mentha pulegium, commonly known as pennyroyal, is one of mentha species and it is a native species of Europe, North Africa, and Asia. It has been traditionally used as an antiseptic for treatment of cold, sinusitis, cholera, tuberculosis [9]. Its' essential oil is known to have biological activities such as antibacterial [10], antioxidant and antimicrobial properties [11, 12].
In this work, essential oil from mentha pulegium leaves has been studied for its' inhibition of activity on aluminum corrosion in 2 M phosphoric acid by electrochemical methods.
Experimental
Preparation of the solution
The 2 M phosphoric acid solution was prepared by dilution of the phosphoric acid of 85% analytical grade with distilled water. Test solutions were freshly prepared before each experiment by adding the oil directly to the corrosive media. Experiments were conducted in triplicate to ensure the reproducibility.
Preparation of material
The aluminum disc was abraded with sandpaper to different particle sizes of 400, 600, 800, 1000, and 1200, degreased with acetone, rinsed with distilled water and dried before each test.
Electrochemical measures
The electrochemical experiments were performed using an electrochemical work station. The electrochemical cell used was a conventional three-electrode compartment with a pyrex cell: aluminum as the working electrode in the form of discs cut with a geometric area of 1 cm2; platinum as a counter electrode; and a saturated calomel electrode (SCE) as the reference. The measurements are performed with an assembly comprising a potentiostat-galvanostat PGZ100 radiometer type associated with "voltamaster4" software.
Tafel polarization measures
A freshly polished disc was exposed to corrosion solution of 2 M phosphoric acid. After a 30 minutes open-circuit potential steady-taste, the current-potential curves are obtained by potentiodynamic method; the potential applied to the disc varies continuously from -1200 mV to -500 mV with a scanning rate of 30 mV / min.
Electrochemical impedance spectroscopy measures
The electrochemical impedance spectroscopy (EIS) measures were performed with the same electrochemical system. The frequencies from 100 kHz to 40 mHz were superposed on the corrosion potential. The diagrams given in the impedances are Nyquist representation.
Results
Analysis of the essential oil of mentha pulegium
Table 1 gives the principal chemical component of the essential oil of mentha pulegium, which is mostly composed of 84,75 % of pulegone (5-methyl-2propan- 2-ylidene cyclohexan-1-one) and 14,72 % of geraniol (2,6-dimethyltrans- 2,6-octadien-8-ol).
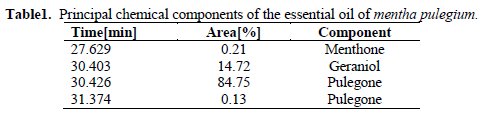
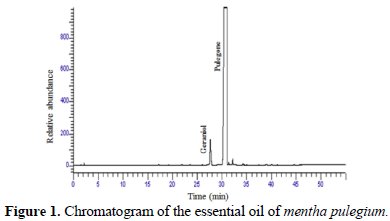
The corresponding chromatogram is in Fig. 1.
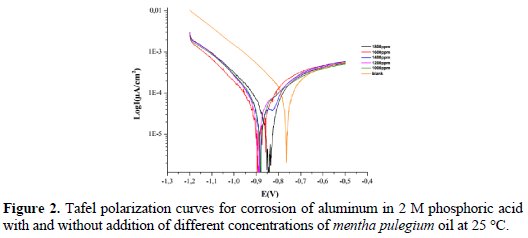
Polarization curves
The polarization behavior of aluminum in 2 M phosphoric acid with and without addition of this inhibitor is presented in Fig. 2.
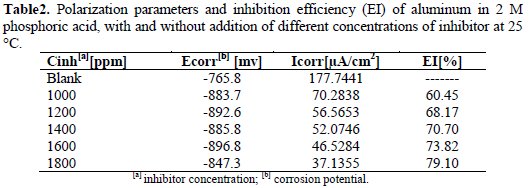
Electrochemical parameters, values of corrosion current (Icorr), the corrosion potential (Ecorr), and inhibitor efficiency (IE) are given in Table 2.
The inhibitor efficiency, in Equation 1, is defined by the following relationship:
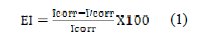
where I'corr and Icorr, respectively, represent corrosion current densities determined by extrapolation of the straight Tafel corrosion potential, with and without addition of inhibitor.
Electrochemical impedance spectroscopy
Electrochemical impedance (EIS) is a technique with a small perturbative signal, and the surface damage of the sample is very little [13]. Besides, the corrosion mechanism can be estimated by analyzing the measured electrochemical impedance spectrum.
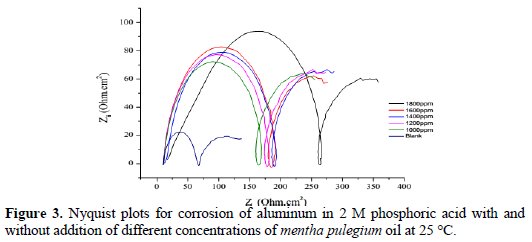
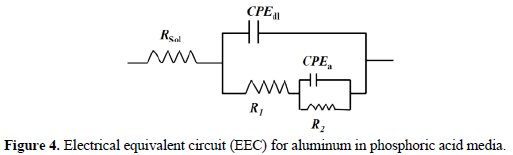
The Nyquist plots for this compound are shown in Fig. 3 and the corresponding circuit is shown in Fig. 4.
Nyquist plots
Every spectrum shows two capacitive loops. A first large well-trained high frequency loop and a small low frequency loop.
The inhibition efficiency was calculated using the Equation 2:
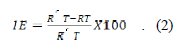
Where, R'T and RT are respectively the charge transfer resistance of aluminum in 2 M phosphoric acid in the presence and absence of inhibitor. The impedance parameters are given in Table 3.
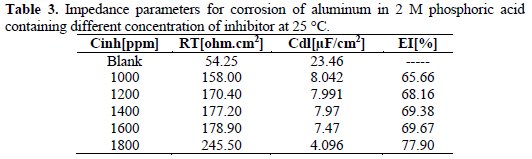
The relationship between the double layer capacity (Cdl) and the maximum frequency (fmax) of the imaginary component of the impedance is shown in Equation 3:
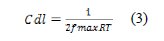
Electrical equivalent circuit
In the circuit, in Fig. 4, Rsol represents the solution resistance of the system, R1 is the charge transfer resistance for the corrosion reaction and the double layer resistance, and CPEdl is the capacitance of the electrical double layer at the metal-solution interface, corresponding to the high frequency capacitive loop. R2 and CPEa represent the pseudo resistance and pseudo capacitance of the blasters layer.
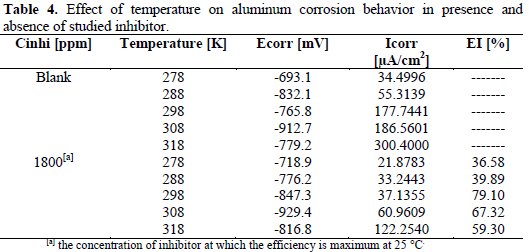
Effect of temperature
The polarization experiments were also used to investigate the effect of temperature on the corrosion behavior of aluminum in 2 M phosphoric acid with and without inhibitor at a concentration of 1800 ppm, in the temperature range of 278 °K-318 °K. Tafel curves are represented in Fig. 5 and Fig. 6.
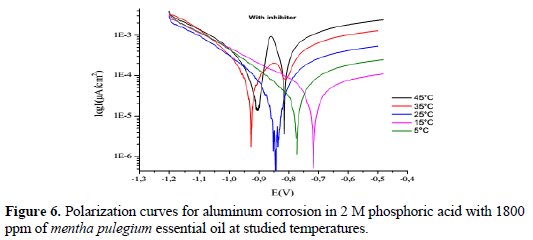
Results obtained are reported in Table 4.
Discussion
Polarization curves
From Fig. 2 and Table 2, it can be observed that, with the addition of this oil, the corrosion rate of aluminum decreases with an increase in the concentration of the inhibitor. Therefore, the inhibitor efficiency increases with an increase in the concentration of the inhibitor.
The changes observed in the polarization curves after the addition of the inhibitor are usually used as the criteria to classify inhibitors as cathodic, anodic or mixed [14]. According to literature report, when the corrosion potential is more than ± 85 mV, with respect to the corrosion potential of the blank, the inhibitor can be considered distinctively as either cathodic or anodic type [14, 15]. However, the minimum displacement in this study is more than ±85 mV.
This suggests that the essential oil of mentha pulegium oil functions as a cathodic-type inhibitor.
Observing Figure 3, the diameter of the capacitive loop increases with the increase in the inhibitor concentration. This indicates that the impedance of the inhibited substrate has increased with the inhibitor concentration. The fact that impedance plots are semicircles both in the absence and in the presence of the inhibitor indicates that the corrosion process is charge transfer controlled [16]. From Table 3, the Cdl (double layer capacitance) values decrease with an increase in inhibitor concentrations, which could be due to the formation of a protective layer by inhibitor molecules [17].
Effect of temperature
On Table 4, it is observed that the corrosion rate (Icorr) increases with an increase of temperature in the presence and absence of the inhibitor. At low temperatures, the inhibitor efficiency EI increases with an increase of temperature. This can be due to the adsorption of inhibitor molecules on aluminum surface [18]. At elevated temperatures, the efficiency of the inhibitor decreases with an increase of temperature. This decrease in inhibition performance of the inhibitor is due to desorption of the adsorbed inhibitor molecules from aluminum surface [18].
Thermodynamic parameters
The corrosion inhibition of aluminum in M phosphoric acid can be more explained in terms of molecular adsorption of inhibitor on its' surface [18]. The value of apparent activation energy (Ea), in both presence and absence of inhibitor, was calculated from Arrhenius equation:
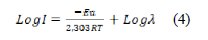
where, Ea is the apparent activation energy (kJ mol-1), R is the molar gas constant (8.314 JK-1 mol-1), T is the absolute temperature (K), and λ is the Arrhenius pre- exponential factor.
The Arrhenius plots give a straight line between LnI vs. 1000/T, as shown in Fig. 7.
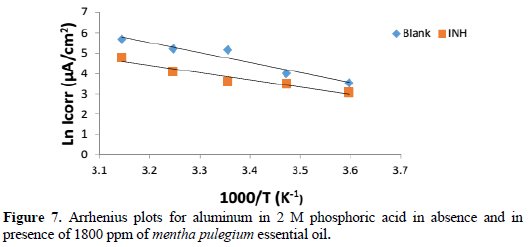
The Ea values were calculated by multiplying the slope of Arrhenius plots (LnI vs. 1000/ T) by R, represented in Table 5, from which it is noticed that the activation energy is greater (40.98 kJmol-1) in absence of the inhibitor than in presence of 1800 ppm of the inhibitor (29.48 kJmol-1).
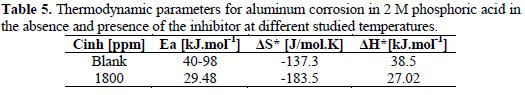
This shows the chemical adsorption of the inhibitor and the effective formation of a surface film [8, 19].
The corrosion current intensity dependency on temperature can also be represented by the transition state equation:
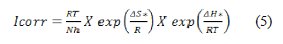
where, ΔH* is the apparent enthalpy of activation, ΔS* the apparent entropy of activation, h is the Planck's constant and N is the Avogadro number. The transition state plots, in Figure 8, give a straight line between Ln (I/T) vs. 1000/T.
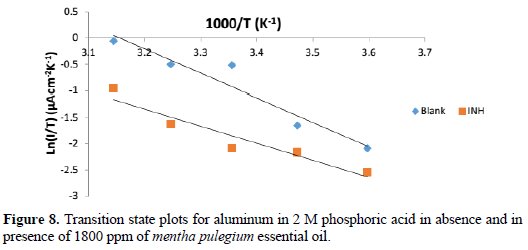
From the slope of (-ΔH*/R) and intercept of [Ln(R/Nh) + (ΔS*/R)], values of ΔS* and ΔH* were calculated and listed in Table 5.
From Table 5, ΔH*, in both absence and presence of the inhibitor, are positive. This reflects the endothermic process of adsorption of this inhibitor on aluminum surface, suggesting that higher temperature favors the corrosion process [20]. The negative values of ΔS* may reflect the association mechanism of corrosion, i.e., the decrease in disorder takes place on going from reactants to the activated states. It has also been noticed that the addition of the inhibitor to the test solution gives lesser negative values of ΔS* than in the absence of the inhibitor, i.e., it decreases the corrosion rates. This result may be considered as an indirect evidence to support the cited proposed mechanism [21].
Conclusions
- Mentha pulegium leaves essential oil is a good aluminum corrosion inhibitor in 2 M phosphoric acid solution. Its effectiveness increases with an increase of concentration, reaching the maximum efficiency of 79 % at 1800 ppm by potentiodynamic polarization studies. The effectiveness decreases with an increase in temperature.
- Polarization results indicate the cathodic type of the used inhibitor.
- Impedance plots shape indicates that the corrosion process is charge transfer controlled.
- According to thermodynamic parameters and due to the addition of 1800 ppm of the inhibitor, the apparent activation energy (Ea*) shows the chemical nature of the adsorption of the studied oil on aluminum. The apparent enthalpy of activation (ΔH*) proves that the same adsorption is an endothermic process and the apparent entropy of activation (ΔS*) confirms the decrease of corrosion rate.
References
1. Constantin F. PhD Thesis. Lyon, France: INSA; 2011. [ Links ]
2. Halambek J, Zutinic A, Berkovic K. Int J Electrochem Sci. 2013;8:11201. [ Links ]
3. Musa A Y. Recen Reseac Corr Evalu Protec. 2012. [ Links ]
4. Bensabah F, Houbairi S, Essahli M, et al. Port Electochim Acta. 2013;31:195. [ Links ]
5. Fares M M, Maayta A K, A-Qudah M M. Corros Sci. 2012;60:112. [ Links ]
6. Deng S, Li X. Corros Sci. 2012;64:253. [ Links ]
7. Haleem S M A E, Wanees S A E, Aal E E A E. Corros Sci. 2013;68:1. [ Links ]
8. Houbairi S, Lamiri A, Essahli M, Chem Sci Rev Lett. 2014;3:353. [ Links ]
9. Darwich E, Benziane Z, Taouil R. Chem Bull âPOLITEHNICAâ Univ (Timisoara). 2010;55:103. [ Links ]
10. Boukhebti H, Chaker AN, Belhadj H, et al. D Pharma Lett. 2011;3:267. [ Links ]
11. Erhan M K, Bolukbasi SC, Urusan H. Liv Stoc Sci. 2012;146:189. [ Links ]
12. Teixeira B, Marques A, Ramos C, et al. Indust Crop Prod. 2012;36:81. [ Links ]
13. Prabhu D, Rao P. J Environ Chem Eng. 2013;1:676. [ Links ]
14. Zarrouk A, Zarrok H, Salghi R, et al. J Mater Environ Sci. 2013;4:177. [ Links ]
15. Ameer M A, Ghoneim A A, Fekry AM. Int. J. Electrochem.Sci. 2012;7:4418. [ Links ]
16. Hmamou D B, Salghi R, Zarrouk A, et al. D Pharma Lett. 2013;5:135. [ Links ]
17. Victoria S N, Prasad R, Manivannan R. Int J Electrochem Sci. 2015;10:2220. [ Links ]
18. Korbe R, Verma CB, Ebenso EE, et al. Int J Electrochem Sci. 2015;10:1081. [ Links ]
19. Bhola S M, Singh G, Mishra B. Int J Electrochem Sci. 2013;8:5635. [ Links ]
20. Vashi R T, Naik D. E-J Chem. 2010;7(S1):S1. [ Links ]
21. Hassan R M, Zaafarany I A. Materials. 2013;6:2436. [ Links ]
*Corresponding author. E-mail address: m.uwineza@uhp.ac.ma
Received 15 February 2016; accepted 23 February 2016














