Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.33 no.6 Coimbra nov. 2015
https://doi.org/10.4152/pea.201506323
Synthesis Characterization and Anticancer Activity of Co(II)-Flutamide Complex
Birjesh Singha,* , Jyotsna Mishraa and Alka Pradhanb
a Department of Chemistry, Institute of Science and Technology, AISECT University, Bhopal, India
b Department of Chemistry, MVM College, Bhopal (M.P), India
Abstract
Cobalt complex with flutamide was synthesized and physico-chemically characterized by amperometry, polarography, elemental analysis and IR spectrometry. After synthesizing the metal complex, it was evaluated for antibacterial and antifungal activities against various pathogenic microorganisms such as Streptococcus aureus, Prosteus mirabilis, Klebsiella pneumonia and Aspergillus niger, Nigrosporan sp Fungi. B16-F10 melanoma cell and C-57BL/6 mice have been used for anticancer screening of the metal complex for in vitro and in vivo study. The result of pharmacological studies with M:L revealed that the complex is more potent as compared to the pure drug as regards to its anticancer activity.
Keywords: Anticancer drug, Cobalt Complex, Polarography, Antibacterial activity, Pharmacology.
Introduction
Flutamide 2-methyl-N-[4-nitro-3-(trifluoromethyl) phenyl] propanamide is used as antineoplastic and antiandrogen drug [1]. It is a powerful nonsteroidal androgen antagonist which has treated prostate cancer and is believed to block androgen receptor sites. This drug and its primary hydroxyl metabolite decrease metabolism of C-19 steroid by the cytochrome P-450 system at the target cells in the secondary sex organ [2]. Prostate cancer is the most common cancer in men in western countries and it is the second leading cause of cancer death [3]. Cobalt ion is present in cobamide, which is a derivative of vitamin B12 and one of the most extraordinary in all biologically active substances. Cobamide is important in the in-vivo synthesis of amino acids used to make proteins. Vitamin B12 is the first metallo complex in living systems being studied in great depth [45]. Cobalt is a life essential element; it must be supplied in diet in its physiologically activity from vitamin B12. The complexation behavior of Co (II) for medicinal purposes has been widely published [6].
The study on cobalt complex of anticancer flutamide drug has been carried out physico-chemically, microbially and pharmacologically. The metal ligand complexation equilibrium has been studied using amperometric titration polarographic studies and elemental analysis. IR spectral analysis has been worked out, giving the probable formula for the complex as being 1:1. Various pathogenic bacteria like Streptococcus aureus, Prosteus mirabilis, Klebsiella pneumonia, Aspergillus niger, and Nigrosporan sp, have been used to microbial study using the disc diffusion method.
B16-F10 melanoma cell and C-57BL/6 mice were used for the in vitro and in vivo anticancer study of the complex, respectively. The results of the physicochemical method, and of microbial and pharmacological studies with Co(II)-flutamide complex may be suggested to the therapeutic experts for their possible use as a more potent anticancer drug.
Experimental
The entire chemicals used were of analytical ''grade''; the flutamide drug was purchased from Sigma Chemical Company®, USA. Standard solutions of Co(II) 1 mM, flutamide 1 mM, and potassium chloride 0.1 M, were 5% of 95% ethyl alcohol and prepared according to our choices.
Polarographic / voltammetric measurement was carried out using a Metrohm® instrument, Model 797A Computrace, which stands three electrodes containing a DME dropping mercury electrode as a working electrode, a coiled platinum wire as an auxillary electrode, and a saturated calomel electrode (SCE) as a reference electrode.
Electrochemical studies of Co(II)-flutamide complex
For the study of the metal : ligand (M:L) complexation equilibrium, experiment sets were prepared by keeping overall Co(II) and potassium chloride (supporting electrolyte) concentrations fixed at 1 mM and 0.1M, respectively. The ligand concentration was varied until 15 mM. The pH of the test solution was adjusted to 7.0 ±0.1 using HCl/NaOH solution. The test solutions were deaerated by bubbling nitrogen gas for 15 min before recording the polarogram. The amperometric titrations were performed on a manually operated set up equipped with a polyflex galvanometer (sensitivity 8.1 × 10-9 amp per div) and an Agco Vernier potentiometer. The capillary characteristics of DME had m2/3 t1/6 value of 2.5 mg2/3 S-1/2 at 50 cm effective height of mercury column. A systronics digital pH meter-335 was used for the pH measurements. Experimental sets each having different but known amount of Co(II) were prepared in appropriate quantity of the supporting electrolyte (KCl) and the pH was adjusted to 7.0 ±0.2 and titrated separately against the standard solution of the titled flutamide whose pH was also adjusted to that of the titrate ( 7.0 ±0.1 using NaOH / HCl) at -1.20 V versus SCE (the plateau potential of Co(II)). The current offered by each addition of the titrant was read, and a curve was plotted between current against volume of the titrant added.
Synthesis procedure of the solid complex
Cobalt chloride and flutamide were prepared separately in water and were mixed in 1:1 molar ''ratio''. The mixture was then refluxed in a round bottom flask for 2 h. The complex was marked by precipitation; after reducing (complex) it was filtered and washed thoroughly to remove any unreacted material; then it was dried at low temperature and stored over P4O10.
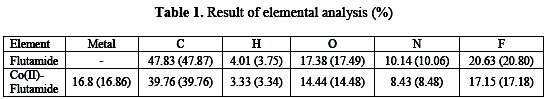
The results of elemental (C, H, N) and F analysis on the drug and Co(II)flutamide complex were furnished by CDRI Lucknow, India. The gravimetric method was used for the estimation of cobalt in the complex [7].
Antimicrobial screening
The microorganisms used in this study were Klebsiella pneumonia, Proteus mirabilis, Stretococus aureus, fungi Asperginus niger and Nigrosporas SP. All strains were obtained from the microbiological department Govt. Motial Vigyan mahavidyalal Bhopal (M.P.) India. Each microorganism was maintained on Mueller-Hinton (MH) agar medium at 4 °C.
Kirby-Baller et al. disc diffusion was followed for the antimicrobial activity screening of the complex against various microorganisms: Klebsiella pneumonia, Proteus mirabilis, Streptococcus aureus, fungi Asperginus niger and Nigrosporas S.P. [8]. The number of replicates in each case was three; the percentage of inhibition was calculated using the following formula [9]:

where a represents the diameter of the zone of inhibition for flutamide control, and b represents the zone of inhibition for the complex (Co(II)-flutamide).
Pharmacological studies
In-vitro and in vivo studies of anticancer activity of the prepared metal drug complex have been done using the following procedure [10-12]. In-vitro B16-F10 melanoma cell line strains were obtained from Jawaharlal Nehru Cancer Hospital and Research Centre, Idgah Hills Bhopal M.P, India, as a monolayer culture in Roux battles (Corning® plastics U.S.A.).
Cell Culture
The cells B16-F10 (13) melanoma obtained were in culture in 5 mL 24 well culture plates (corning® plastics, USA). The cells were seeded in 2×105 cell per well, grown in 1.0 mL dulbecco's modified Eagles medium (DMEM Gibco®), supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acid, 1mM sodium pyruvate, 100 μg/mL penicillin, 100 μg/ mL streptomycin, and 5% v/v heat inactivated fetal calf serum. The B16F10 cell line was growth at 37 °C. The cells were kept in an incubator at 37 °C for 8 h in 5% CO2 atmosphere and 95% humidity. The cell counter was made on Neubaur's Chamber (Fine optic®, Germany).
Two dilutions viz 1 μm, 10 μm of pure drug and its complex were made and then the cells were treated as follows:
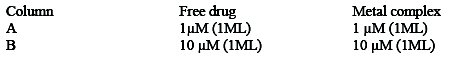
After addition of the respective solutions, the culture plate was incubated at 37 °C for 8 hours. Finally, the cell counts were made where appropriate to be shown. These are compared with the cell cultured in DMEM without treatment.
Cells vialibility counts
Cell vialibility counts were made by trypan blue dye exclusion test. Two drops of trypan blue were added to each cell culture well and kept for 15 minutes. Now a drop of culture was added to hemocytometer (Neubaurs chambers) and the number of stained, unstained, and the total numbers of cells were counted; then the % inhibition was calculated using the equation
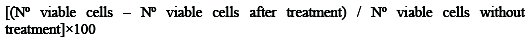
The experiment of each concentration of the drug and the complex was repeated thrice and statistical conclusions were drawn.
In vivo
The comparative efficiency of pure and complex forms of flutamide drug was evaluated from the difference in response after treatment with two forms of the drug.

Cells growing in nutrient medium (DMEM) were obtained from NCCS Pune. They were brought into a single cell suspension by trypsinization (0.2% trypsin). The cell suspension was centrifuged to obtain a concentrated suspension (2×105 cell/mL); approximately 105 cells of tumor were injected on the dorsal skin of an adult C57BL/6 mice and allowed the tumor grow; palpable size was reached by 6-8 days.
The time required to double the tumor volume (volume doubling time (VDT)) from 100 to 200 mm3 was taken as a criterion to assess the antitumor efficiency of pure and complex drug in B16F10 tumor bearing mice. The treatment was started after tumor size reached 100 ±10 mm3. Indicated doses (0.2 mg) of free drug and drug complex were injected intravenously and the tumor growth was monitored. The tumor size was calculated by the formula V=(π/6) D1 D2 D3, where D1 D2 D3 = diameters in three perpendicular planes, measured using a vernier caliper, and V=volume of tumor [14].
Results and discussion
Polarographic behaviour of flutamide with Co(II)
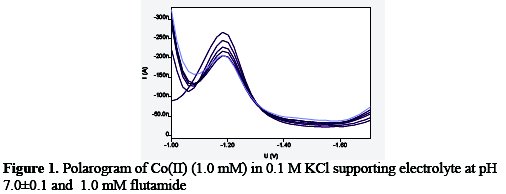
In 0.1 M KCl at pH 7.0±0.1 the Co(II) and its complex with the ligand under study were found to be reversible, and the diffusion controlled polarographic wave, which was revealed by the log plot slop id versus √h (effective height of mercury column) respectively on gradual addition of ligand the E1/2 of metal shift more electronegative value indicating the formation of complex (Fig. 1). Lingane's treatment [15] of the observed polarographic data revealed 1:1 [M:L] complex formation in solution with log β1=5.2.
Amperometric determination of flutamide with Co(II)
Co (II) with flutamide gives a well defined polarographic waves peak in 0.1 M KCl; at 7.0±0.1 pH the diffusion current was found to be proportional to the concentration of Co(II). The plateau potential for the polarographic wave of Co (II) (-1.25V) vs. Hg Pool was applied for carrying out amperometeric titration. The current goes on decreasing to minimum and then attends a constant value. The plot of id versus volume (V+vV) of the titrant added revealed L shaped curve (Fig. 2).
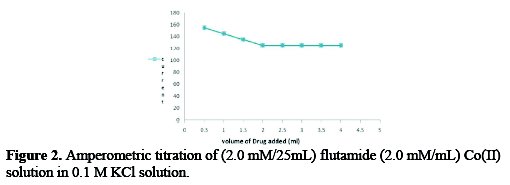
The end point was indicated by the intersection of the two lines, which confirmed 1:1 [M: L] complex formation.
Elemental analysis
Percentage of C, H, O, N, F and Co found and calculated in the complex and flutamide drug are summarized in Table 1.
Spectroscopic measurement
A comparison of IR data for the flutamide drug and its complexes reveals that the bond at 3356 cm-1, 1717 cm-1, 1513 cm-1 and 1243 cm-1 in the drug are shifted to 3350 cm-1, 1656 cm-1, 1540 cm-1 and 1280 cm-1 respectively in the spectrum of the drug complex display nitrogen and (C=O) vibration in the spectrum of the free ligand suggesting ketoform of ligand and nitrogen indicating the coordination.
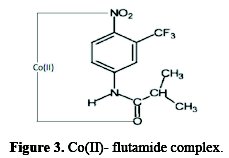
On the basis of the above discussion, a tentative of structure to the flutamide and its complexes may be assigned as shown in Fig. 3.
Antimicrobial activity
Antimicrobial behavior of Co(II)-flutamide complex against various pathogenic bacteria and fungi is reported in Table 2.
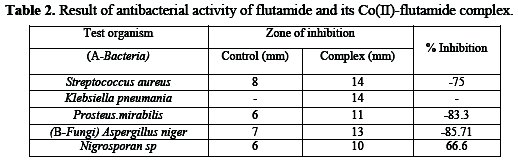
A perusal of data in the table reveals that the complex shows increased antimicrobial activity against all the pathogenic bacteria under study, as compared to the parent drug flutamide.
Pharmacological studies
In vitro
The result of in-vitro experiments of the pure drug and its complex is shown in Table 3.
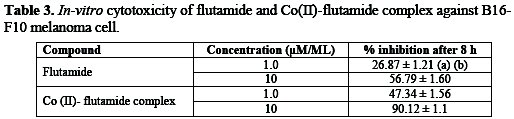
Perusals of results show that cobalt flutamide complex was found to be more effective than the pure drug. The complex under study showed an increased inhibition against the melanoma cell line B16F10 at all the test concentrations, i.e., 1, 10, μM/ML. The increased inhibition activity of the complex was 47.34±1.56%, 90.12±1.10% as against 26.87±1.21%, 56.79±1.60% shown by the drug, respectively. The statistical treatment of the observed inhibition data, i.e., standard deviation and coefficient of variance, which never exceeded 0.9 and 18 %, respectively, speaks the reliability of the observed inhibition data.
In vivo
The results of the average of mice tumor against flutamide drug and cobalt complex under study are shown in Fig. 4.
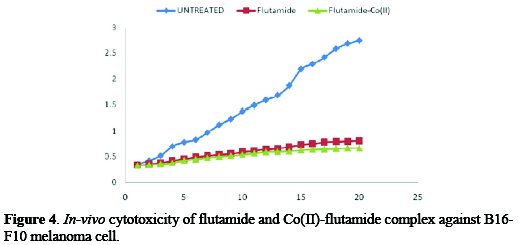
The results indicated that the tumor volume was 0.2 cm3 on the mice tumor cells injected without administering drug or complex; 20 days after administering the flutamide drug in the mice tumor injected, the tumor size was reduced 0.81± 0.63 cm3. The inhibition rate was found to be 70.54%. However, in the case of Co(II) flutamide, the administrated mice (tumor cell injected) show significant decrease in the tumor volume of 0.67 ± 0.73 cm3, and the inhibition rate was 75.63%, thus indicating the increasing in vivo of the tumor inhibition power of the complex.
Conclusions
To investigate the structure and behavior of complex of flutamide with life essential metal ion Co(II) some physicochemical method i.e. IR spectral analysis, elemental analysis, amperometry and polarography have been successfully used. The obtained results suggest that the complexes are more stable as compared to the pure drug. On the basis of the observed results we can conclude that Co(II) with flutamide complex is more effective and non toxic in nature as compared to the parent drug. Thus, polarographic and amperometric methods may be recommended to study more potent drugs in lieu of the drug taken for present study, having excellent potential for clinical application.
References
1. Budavari S O, Neil M, Smith A, et al. Merck Index. 11th ed. Rathway: Merck index and Co Inc;1989. [ Links ]
2. Snycerski A. J Pharm Biomed Anal. 1989;7:1513. [ Links ]
3. Ferlay J, Bray F, Pisani P, et al. Globocan 2000 cancer incidence. 2001. [ Links ]
4. Underwood E J. Trace element in human and nutrition. 4th ed. New York: Acad Press; 1997. [ Links ]
5. William D R. The metal of life. London: Van Nostrand Reinhold; 1971. [ Links ]
6. Brualand K W, Chester K. Chemical oceqnography. 8th ed. London: Acad Press; 1983. [ Links ]
7. Bassett J, Denny R C, Jeffery G H, et al, editors. Vogels Text Book of quantitative inorganic Analysis. 6th ed. London: ELBS Longman; 1996. [ Links ]
8. Bauer A W, Kirby W M M, Sherris J C, et al. Am J Clin Pathol. 1966;36:493. [ Links ]
9. Kumar R, Giri G, Nizamuddin. J Indian Chem Soc. 1988;64:571.
10. Tiwari S B, Udupa N, Rao B S S, et al. Ind J Pharmacology. 2000;32:214. [ Links ]
11. Ghosh M N. Fundamentals of experimental pharmacology. 2nd ed. Scientific Book Agency; 1984. [ Links ]
12. Devi P U, Sharoda A C, Soloman F E, et al. Indian J Expl Biol. 1993;607. [ Links ]
13. Fidler I J. Cancer Res. 1975;35:218. [ Links ]
14. Rajanaresh R A, Udupa N, Uma D P. Indian J Expt Biol. 1996;34:764. [ Links ]
15. Lingane J. J Chem Rev. 1941;1:29. [ Links ]
*Corresponding author. E-mail address: vision_bsin@yahoo.com
Received 20 January 2015; accepted 13 October 2015













