Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portugaliae Electrochimica Acta
Print version ISSN 0872-1904
Port. Electrochim. Acta vol.33 no.4 Coimbra July 2015
https://doi.org/10.4152/pea.201504231
Anticorrosive Properties of Chitosan for the Acid Corrosion of Aluminium
B. A. Abd-El-Nabeya,* , Y. M. Goherb , H. A. Fetouha,* and M. S. Karama
a Department of Chemistry, Faculty of Science, Alexandria University, Ibrahimia, P.O. Box 426, Alexandria 21321, Egypt
b Department of Botany, Faculty of Science, Alexandria University, Ibrahimia, P.O. Box 426, Alexandria 21321, Egypt
Abstract
Potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques were used to measure the corrosion rate of aluminium in 0.1 M HCl in the absence and presence of different concentrations of chitosan. Inhibition efficiency up to 90% in the presence of 0.028 g/L chitosan was achieved. Increasing the concentration of chitosan shifted the breakdown potential, Eb, of aluminium to more noble values and inhbitied the pitting corrosion of aluminium. Measurements of the break potential, Eb, and the electrical double layer capacity (Qdl) indicated that chitosan is adsorbed at the aluminium/solution interface.
Keywords: aluminium, polarization curves, pitting corrosion, Electrochemical Impedance Spectroscopy (EIS), charge transfer, adsorption.
Introduction
Aluminium was reported to be widely applied in aluminium-air technology, food industry and desalination plants. These industrial applications are due to the low density, favorable mechanical properties, good finishing, benign effect on the environment and the human health. The high corrosion resistance of aluminium is attributed to the natural surface protective oxide film. Aluminium suffers from pitting corrosion by chloride ion and many organic inhibitors are reported for the corrosion of aluminium in hydrochloric acid solutions [1-3].
Chitosan was reported to be used in the smart self-healing coating and as based polymer with 2-mercaptobenzothiazole anticorrosive coating for the aluminium alloy against the atmospheric corrosion [4, 5]. Chitosan molecules are rich in hydroxyl and amino groups (Fig. 1), so it is a good potential inhibitor.
El-Haddad [6] has investigated the inhibition characteristics of chitosan for the corrosion of copper in 0.5 M HCl using the potentiodynamic polarization and the electrochemical impedance spectroscopy (EIS) techniques. The polarization results indicated that chitosan acted as cathodic inhibitor and shifted the corrosion potential of copper from (Ecorr.= -94 mV) into (Ecorr.= -174 mV) for 0.5 M HCl solution in the absence and prescence of 8×10-6 M chitosan respectively. The results also indicated that the inhibition efficiency of chitosan increased with increasing its concentration reaching the maximium value of 92% at the concentration of 8×10-6 M chitosan at 25 °C. This work aims to study the electrochemical behavior and the inhibition effect of chitosan for corrosion of aluminium in 0.1 M HCl at 30 °C.
Experimental
Solution preparation
Chitosan, that is a natural biopoymer, Fig. (1), was prepared in laboratory following the method reported elsewhere [7]. The structure of chitosan was elucidated by Infra red (IR) and UV-visible spectroscopy. Further characterization of chitosan was carried out by the determination of average number molecular weight and the degree of acetylation as reported previously[8]. Hydrochloric acid was purchased from Aldrich Chemicals Company. A stock solution of 1.0 M HCl solution was prepared using double distilled water. A stock solution of 0.1 g/L chitosan was prepared in 10% acetic acid. The test solutions of 0.1 M HCl containing different concentrations of chitosan were prepared by appropriate dilutions.
Electrochemical techniques
The impedance measurements were achieved by applying an alternating potential (AC) signal of 10 mV amplitude around the rest potential (Erest) to the electrode surface at the frequency range (0.1 Hz-1.0×104 Hz). The data indicate that 88 reading points were recorded per decade.
The polarization curves were recorded by polarizing the aluminium electrode surface at a scan rate of 0.5 mV/sec of direct current (DC) starting from -250 mV below the rest potential, Erest to +250 mV above Erest.
The working electrode was the aluminium sample of the following chemical composition: 0.37 C, 0.212 Si, 0.002 Mn, 0.007 Cu, 0.001 Mg, 0.008 Zn, 0.007 Ti, 0.089 Fe, 0.004 Pb, 0.003 B, 0.001 Zr, 0.0004 V, 0.0002 Cd, 0.002 Gu , 0.0003 Ag , Al (98.64). The aluminium sample was fixed in a Teflon rod in such a way that only one surface area (1.227 cm2) was exposed to the test solution. This area was mechanically polished with emery papers of 320, 600 and 1000 grades, washed thoroughly with double distilled water then with absolute ethanol. The working electrode was introduced in the electochemical cell containing the reference saturated calomel electrode, the counter platinium electrode and 25 mL of the tested solution. The cell was thermostated at 30 °C for 20 min before starting the experiment, then connected to Gill AC-potentiostat that forced alternating or direct potential to the working electrode, then recording the current signal. The open circuit or the rest potential of the working electrode was followed as a function of time until steady state potential (equilibrium potential at which the variation of potential is 1.0 mV/ min.) was established to ensure reliable measurements in polarization and impedance measurements in aerated unstirred solutions [9].
Results and discussion
Characterization of the structure of chitosan
The chemical structure of the natural polymer that is shown in Fig. 1 was confirmed by various tools. Infrared spectroscopy, that is a measurement of intensity of the absorption of infrared (IR) radiation by a sample of chitosan at different wave numbers (cm-1). The IR spectrum gave a characteristic band at 3450 cm-1 due to the stretching vibrations of NH2 and OH groups and another band at 2925 cm-1 that is a characteristic of CH2(s) symmetric vibrations. Chitosan showed a broad absorption peak at the wavelength (λ) of 425 nm in the UV-visible range of electromagnetic radiations. This peak corresponds to the electronic transition (n-σ*) of the free electrons in chitosan molecules [4]. The number of average molecular weight, Mn of chitosan molecules was determined by the colligative properties methods and was found to be nearly 24 kilo Dalton. Another evidence of the structure of chitosan is the degree of deacetylation of chitin (the precurser of chitosan). The deacetylation process of chitin was carried out by adding 50% NaOH and then boiled at 100 °C for 2 hours on a hot plate. The samples are then placed under the hood and cooled for 30 min at the room temperature. Then the samples are washed continuously with 50% NaOH and filtered in order to retain the solid chitosan. The samples were then left uncovered and oven dried at 110 °C for 6 hours [8]. The chitosan obtained will be in a creamy-white form. The degree of acetylation was achieved to be 85%.
Potentiodynamic polarization results
Fig. 2 showed the potentiodynamic polarization curves for aluminium in 0.1 M HCl in the absence and presence of different concentrations of chitosan.
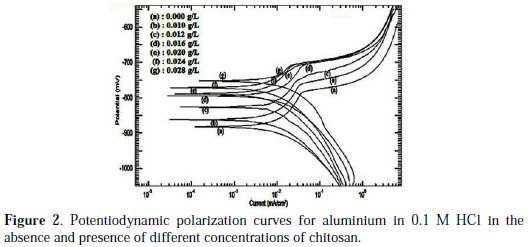
Increasing the concentration of chitosan shifted the corrosion potential, Ecorr. to more noble values and retarded the anodic and the cathodic reactions indicating that chitosan is a mixed-type inhibitor [9]. At highly cathodic overvoltages, the current density continuously increases. The highly anodic overvoltage causes breakdown of the oxide film by chloride ion that replaced the adsorbed oxygen gas in some defect points at the breakdown potential, Eb and the current density increases quickly.
The anodic polarization curves showed an inflection (breakdown potential, Eb) corresponding to the pitting corrosion of aluminium. The breakdown potential was shifted to more noble values when chitosan is added, indicating retardation of pitting corrosion [10]. Applying Tafel extrapolation method on the cathodic and anodic Tafel lines gives the values of polarization parameters, including the corrosion potential, Ecorr, the corrosion current density, icorr, and the anodic and cathodic Tafel slopes, ba, bc respectively, Table 1.
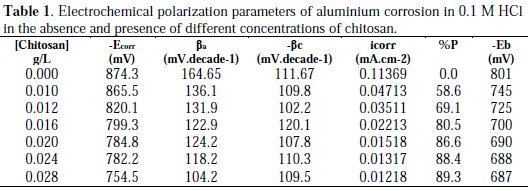
The slope of the cathodic Tafel line (βc) remains nearly constant upon the addition of chitosan, indicating that the cathodic reaction which is the reduction of the hydrogen ion is not affected by the presence of chitosan and this step is charge transfer controlled. On the other hand, the slope of the anodic Tafel line (βa) decreases by increasing the concentration of chitosan and largely decreases in the presence of high concentration of chitosan. This behavior indicated that the oxidation of aluiminium in the absence and presence of small concentrations of chitosan is charge transfer controlled and Tafel equation is applied. While, in the presence of higher concentrations of chitosan, the oxidation of aluminium is controlled by pitting corrosion and Tafel equation is not applicable [11].
Electrochemical Impedance Spectroscopy (EIS) results
The impedance plots of aluminium as Nyquist plots and the equivalent circuit model fit well the impedance spectrum with a small error and are represented in Fig. 3 (a, b).
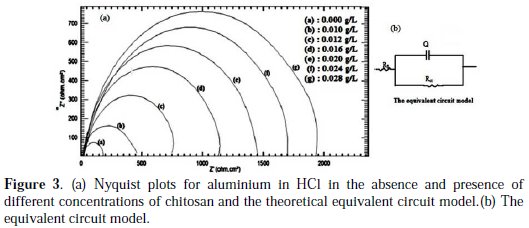
The Nyquist plots showed one capacitive semi-circle at high frequency region for all the examined solutions. These capacitive semi-circles indicate that the aluminium dissolution is mainly controlled by charge transfer process across the aluminium/solution interface. This capacitive loop is related to the dielectric properties of the oxide film [12, 13].
The Bode magnitude plots for aluminium in 0.1 M HCl at 30 °C in the absence and presence of different concentrations of chitosan are shown in Fig. 4.
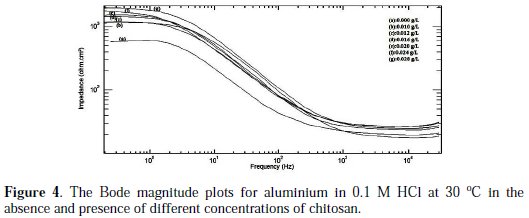
The Bode plots explain the impedance at the high frequency region. The simulated spectra tof these plots indicated that the impedance increased with increasing the concentration of chitosan. The straight line obtained in Bode plots has a slope less than -0.5 indicating that the corrosion reaction is controlled by charge transfer, as predicted from Nyquist plots [14].
The diameter of the semicircle in the Nyquist plots increased with increasing the concentration of chitosan. The impedance parameters including the solution resistance (Rs), the charge transfer resistance, Rct, and the capacitance of the double layer (Qdl), are collected in Table (2).
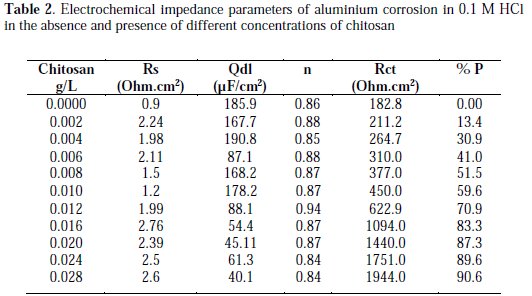
The surface coverage (Θ) of chitosan was calculated using the relations [9]:
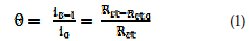
where io and Rcto, and I and Rct, are the current density and the charge transfer resistance in the absence and presence of different concentrations of chitosan, respectively.
The protection efficiency % P = Θ × 100 in Tables 1 and 2 indicated that there is a fairly agreement between the results of polarization and the impedance measurements.
The dependence of the inhibition efficiency on the concentration of chitosan is shown in Fig. 5.
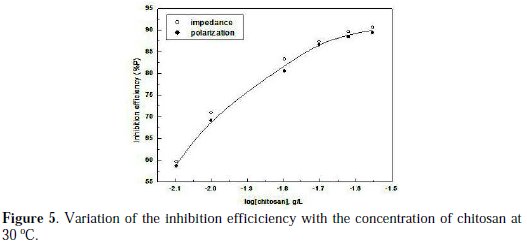
The rapid linear increase of (%P) followed by steadily rising part indicates the formation of a monomolecular film of chitosan on aluminium surface [15].
Statistical linear regression analysis of the results
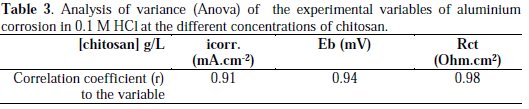
The statistical analysis of variance (Anova) method was used to analyze the significance of the influence of chitosan concentration on the thermodynamic (break down potential, Eb), and on the kinetic factors (corrosion current density icorr. and charge transfer resistance, Rct). High correlation coefficients (r) have been obtained and indicated a strong relationship between the concentration of chitosan and the experimental variables icorr., Rct and Eb, as shown in Table 3.
Anticorrosive action of chitosan
The pitting corrosion of aluminium occured near copper and iron containing intermetallic particles. Both Cu and Fe are more cathodic than aluminium matrix resulting in galvanic interaction [16]. Fig. (6) presents the variation of each of the breakdown potential (Eb) and the double layer capacitance (Qdl) of aluminium in 0.1 M HCl with the concentration of chitosan.
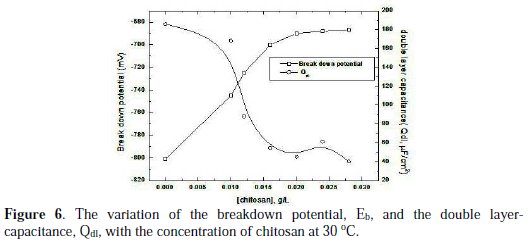
It is clear that the increase of the concentration of chitosan leads to the shift of the breakdown potential towards more anodic values, while the capacitance of the double layer decreases. A maximum shift in the breakdown potential is attained at 0.02 g/L chitosan, the same concentration at which the double layer capacity has the minimum value.
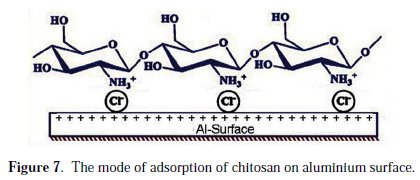
This behavior leads to the suggestion that 0.02 g/L chitosan is the concentration at which the adsorbed monolayer is completely formed at the aluminium surface and the repesentative mode of adsorption is shown in Fig. (7).
Conclusion
1) Increasing the concentration of chitosan shifted both the corrosion potential (Ecorr.) and the breakdown potential (Eb) of the aluminium in 0.1 M HCl to more noble values and retarded both the anodic and the cathodic reactions, indicating that chitosan is a mixed-type inhibitor.
2) Oxidation of aluminium in 0.1 M HCl in the absence and presence of small concentrations of chitosan is charge transfer controlled and Tafel equation is applied, while in the presence of higher concentrations of chitosan, oxidation of aluminium is controlled by pitting corrosion and Tafel equation is not applicable.
3) The increase of the concentration of chitosan leads to the decrease of the electrical double layer capacity at the aluminium/solution interface in 0.1 M HCl solution and shifted the breakdown potential towards more noble values. A maximum shift in the breakdown potential is attained at 0.02 g/L chitosan, and at the same concentration the double layer capacity has the minimum value. This concentration is required to form an adsorbed monolayer of chitosan at the aluminium/solution interface.
4) Chitosan inhibits the pitting corrosion of aluminium in 0.1 M HCl with efficiency up to 90% at 0.028 g/L and acts by adsorption at the aluminium/solution interface.
References
1. Safak S, Duran B, Yurt A, et al. Corrosion Sci. 2012;54:251. [ Links ]
2. Fares M M, Maayta A K, Al-Qudah M M. Corrosion Sci. 2012;60:112. [ Links ]
3. Abd El Aal E E, Abd El Wanees S, Farouk A, et al. Corrosion Sci. 2013;68:14. [ Links ]
4. Yasakau K A, Tedim J, Zheludkevich M L, et al. Handbook of Smart Coatings for Materials Protection. 2014. P. 451. [ Links ]
5. Carneiro J, Tedim J, Fernandes SCM, et al. Surf Coat Technol. 2013;226:51. [ Links ]
6. El-Haddad M N. Int J Biolog Macromol. 2013;55:142. [ Links ]
7. Islama M M, Masumb S M, Rahmana M M, et al. Int J Basic Appl Sci. 2011;11:77. [ Links ]
8. Hussain M R, Iman M, Maji T K. Int J Adv Eng Appl. 2013;6:4. [ Links ]
9. Abdel-Gaber A M, Abd-El-Nabey B A, Saadawy M. Corrosion Sci. 2009;51:1038. [ Links ]
10. Williams G, Coleman A J, McMurray H N. Electrochim Acta. 2010;55:5947. [ Links ]
11. Kelly G R, John R S, David W S, et al. Electrochemical Techniques in Corrosion Science and Engineering. 2002. [ Links ]
12. Zhang Q B, Hua Y X. Mater Chem Phys. 2010;119:57. [ Links ]
13. Fetouh H A, Abdel-Fattah T M, El-Tantawy M S. Int J Electrochemical Sci. 2014;9:1565. [ Links ]
14. Lenderink H J W, Linden M V D, de Wit J H W. Electrochim Acta. 1993;38:1989. [ Links ]
15. Abd-El-Nabey B A, Abdel-Gaber A M, Elewady G Y, et al. Int J Electrochem Sci. 2012;7:11718. [ Links ]
16. Meng Q, Frenkel G S. J Electrochem Soc. 2004;151:61. [ Links ]
*Corresponding author. E-mail address: howida_fetouh@yahoo.com
Received 11 June 2015; accepted 30 August 2015














