Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.33 no.1 Coimbra jan. 2015
https://doi.org/10.4152/pea.201501049
Effect of Process Parameters on Corrosion Resistance of Ni-P-Al2O3 Composite Coatings Using Electrochemical Impedance Spectroscopy
Prasanna Gadhari and Prasanta Sahoo*
Department of Mechanical Engineering, Jadavpur University, Kolkata, 700032, India
Abstract
Electroless nickel composite coatings are developed by incorporating soft/hard particles into Ni-P coatings, to improve mechanical as well as tribological properties. The objective of the present work is to investigate the effect of various coating process parameters on the corrosion behavior of Ni-P-Al2O3 composite coating deposited on mild steel substrate. The electrochemical impedance spectroscopy test is used to evaluate the corrosion behavior of the heat treated composite coatings at various annealing temperatures (300 °C, 400 °C, and 500 °C). Corrosion properties, charge transfer resistance (Rct) and double layer capacitance (Cdl), are optimized using Taguchi based grey relational analysis to improve the corrosion resistance of the coating. Concentration of nickel source, concentration of reducing agent, concentration of alumina particles and annealing temperature, are considered as a main design factor for optimization of electrochemical properties. Analysis of variance (ANOVA) is used to find out the optimum combination of coating process parameters. From ANOVA result, it is found that the concentration of Al2O3 particles and annealing temperature have significant influence on the corrosion resistance of the composite coatings. Concentration of reducing agent has moderate influence on the corrosion resistance. Surface morphology of the coated surface is studied using SEM (scanning electron microscopy) and chemical composition of the coating is studied using EDX (energy dispersive X-ray analysis). The XRD (X-ray diffraction analysis) is used to understand the phase transformation behavior of the composite coatings.
Keywords: Ni-P-Al2O3 composite coating, corrosion, electrochemical impedance spectroscopy, optimization.
Introduction
Electroless nickel coatings are not only used for environmental protection of base metal but also to protect it from corrosion and wear. Most machine parts, tools and equipments in the industries are affected due to environmental changes, corrosion, erosion, wear and friction. It is essential to protect the machine tools, equipments and materials from corrosion, wear and erosion. It may be achieved by applying specific coatings on the base material to protect them from corrosion and environmental changes and also to increase hardness, wear and friction resistance. Ni-P electroless plating is one of the most important surface- engineering technology, which is applied in industrial fields. Composite coatings are more popular in various industries like as mechanical, chemical, automobile, textile, aerospace, etc., due to excellent mechanical and tribological properties [1]. Electroless nickel plating is a metal deposition process involving chemical reaction. It is an auto-catalytic chemical technique used to deposit layers of nickel and phosphorus on ferrous or non-ferrous solid substrates. This process relies on the presence of a reducing agent, which reacts with metallic ions to deposit metal. When the substrate is dipped in the electroless bath, it develops potential. Due to this, negative and positive ions are attracted towards the surface of the substrate and release the energy by charge transfer process. Electroless bath consists of source of metallic ions, source of reducing agent, complexing agent, wetting agent, stabilizer, and pH controlling agent. The electroless Ni-P coatings containing more than 7% phosphorus have an excellent corrosion resistance. Therefore, these coatings are used in industries which have corrosive environments such as in mining, chemical, textile, oil and gas, etc. In general, electroless coating is divided into four group viz., pure nickel coatings, alloy and poly alloy coatings, composite coatings, and nano coatings [2]. These coatings have uniform thickness across all the surfaces irrespective of complex geometry and sharp edges.
Incorporation of fine inert (soft/hard) particles into electroless nickel coatings yields electroless nickel composite coatings. In composite coatings, second phase particles are deposited on the surface of the substrate and are well embedded in the Ni-P matrix during the deposition process. To improve corrosion resistance, lubricity and to reduce friction, soft particles like as PTFE. MoS2, HBN, and graphite are introduced in the electroless Ni-P coatings. On the other hand, hard particles like as SiC, WC, Al2O3, Si3N4, CeO2, TiO2, ZrO2, and diamond, etc., are incorporated in the electroless Ni-P coatings to increase hardness, wear resistance, corrosion resistance, and frictional resistance [3]. To get excellent properties, the second phase particles must be uniformly distributed during the deposition process otherwise, due to non-uniform distribution of particles, numerous defects are formed because of segregation and agglomeration of composite or nano particles with high surface energy and activity in the electroless bath [4]. The tribological and mechanical properties of composite coatings are significantly improved with appropriate heat treatment at and above 400 °C for one hour [5]. Various hard particles like, SiC, TiC, TiB2, B4C particles are normally used as reinforcement phase. Out of these particles Al2O3 is widely used because of its high elastic modulus, strength retention at high temperature, and high wear resistance [6]. Usually, the presence or availability of composite particles in the electroless Ni-P composite coatings depends on incorporation of particles on the coating surface and holding time of the particle on the coating surface [7]. Deposition rate, incorporation rate of the composite particles, roughness and hardness of the composite coating depend on the concentration of alumina particles in the elecroless bath [8]. Corrosion resistance of electroless Ni-P coatings is higher than chromium alloy and pure nickel. In a particular environment higher corrosion resistance is due to amorphous nature and the passivity of the Ni-P coating [9]. Various factors such as phosphorus content, porosity of the coating and heat treatment of the coating significantly affect the corrosion resistance of the composite coating. The porosity of the composite coating is increased with increase in the surface roughness. Roughness of the coating depends on the mechanical preparation of the surface of the substrate and deposition of the second phase particles on the coated surface of the sample. The porous composite coatings have lower corrosion resistance as compared to non-porous composite coatings. Hence, to avoid the porosity of the coating, the second phase particles must be uniformly distributed over the coated surface of the sample. Wetting agents play important role in the deposition of the composite coating. In the presence of wetting agent, the second phase particles get uniformly distributed over the coated surface and it also improves the corrosion resistance of the composite coating. Recently, much attention is being paid on composite coatings due to their excellent performance, instead of Ni-P and Ni-B coatings [4].
Abdel Salam Hamdy et al. [10] have observed the improvement in corrosion resistance (100 times) of the coatings, in the presence of alumina particles as compared to steel substrate. The surface resistance of Ni-P coating is 0.78 × 104 Ω cm2 and for composite coatings it is 7.00 × 104 Ωcm2. Bigdeli and Allahkaram [11] have found increase in corrosion resistance (Rc) with decrease in the constant phase elements (CPE) of the Ni-P and Ni-P-SiC composite coatings. The Ni-P-SiC composite coating has higher corrosion resistance as compared to Ni-P coating, due to reduction in effective metallic area for corrosion. Zarebidaki et al. [12] have confirmed that the corrosion resistance of Ni-P-SiC coating depends on the dispersion of nano particles throughout the coating. At higher concentration, SiC particles are agglomerated on the coated surface, which provokes the porosity of the composite coating. On the basis of Nyquist plot, it is confirmed that Ni-P coatings have better corrosion resistance as compared to Ni-P/nano-SiC composite coatings. The porosity of the composite coatings is decreased with increase in incorporation of alumina particles in the composite coatings, which improves the corrosion resistance of the coating. Whereas Stankiewicz et al. [13] have observed that the addition of zirconium oxide in Ni-P and Ni-P-W coatings adversely affect corrosion resistance after 1 hour immersion in 3.5% NaCl medium, Allahkaram et al. [14] have found that addition of nano-particles in Ni-P coating reduces the effective metal area, which is prone to corrosion. The charge transfer resistance (Rct) of a nano composite coating is higher than that of Ni-P coating, meaning that the composite coating has higher corrosion resistance than the electroless nickel coating. The corrosion resistance of EN silicon carbide composite coatings depends on the spreading of nano particles throughout the coatings. Parveen et al. [15] have found the improvement in corrosion resistance of zinc coating in the presence of carbon nanotube particles.
The corrosion resistance of a coating depends on various factors such as phosphorus content, type of corrosion solution and incorporation of second phase particles in Ni-P coatings. Zarebidaki et al. [16] have observed that the proper heat treatment of the coating significantly improves the coating density and structure. The experimental results show that the Ni-P-CNT composite coating has better corrosion resistance as compared to Ni-P coating. Corrosion resistance of the Ni-P coating is reduced due to increase in effective metallic area. Lee [17] has performed immersion tests in 3.5 wt.% NaCl solution for different test durations (one hour to 720 hours). The experimental result shows that the Ni-P- CNT composite coating has higher corrosion shield compared to Ni-P/nano composite coating. It might be due to denser and uniform distribution of composite particles with higher phosphorus content. Novakovic and Vassiliou [18] have found that, after vacuum heat treatment, a composite coating has less corrosion resistance as compared to electroless Ni-P coating and observed the same trend for as-deposited Ni-P coating with higher corrosion resistance as compared to annealed Ni-P coating.
To identify the basic characteristics of the corrosion behavior of the coated surface in different corrosive environments, the measurement of electrochemical corrosion is very essential. From literature review on Ni-P coating, it is confirmed that the dissolution of nickel ions occurs at open circuit potential, leading to the enrichment of phosphorus on the surface layer. During chemical reaction the phosphorus available on the coated surface reacts with water from the electrolyte to form a layer of adsorbed hypophosphite anions, which blocks the supply of water to the electrode surface and forms a passive nickel film. Electroless Ni-P coatings are known as barrier coating, which protects the surface of the specimen by sealing it off from corrosive environments. Corrosion resistance of the coating also depends on the amorphous structure and passivity of the electroless coating. Amorphous alloys offer better corrosion resistance as compared to crystalline or polycrystalline materials.
Different electrochemical tests, namely, potentiodynamic polarization test and electrochemical impedance spectroscopy tests are used to study the corrosion behavior of electroless nickel coatings. The resistance of the coatings towards corrosion is evaluated on the basis of different corrosion parameters obtained from such tests such as corrosion potential (Ecorr), corrosion current density (Icorr), charge transfer resistance (Rct), double-layer capacitance (Cdl), and corrosion rate (Rc), etc. The present study deals with the assessment of corrosion behavior of Ni-P-Al2O3 composite coating using electrochemical impedance spectroscopy (EIS) technique. EIS is a non-destructive technique often used to understand the corrosion behavior in electrochemical systems and is best suitable for the electrochemical characterization of the coating deposited on the metal surface. It provides the exhaustive data on the usefulness of coating over a relatively small area. It also indicates the occurrence of the rate of corrosion, and moisture content in the coating. To maximize the corrosion resistance (Rct and Cdl) of the composite coating, the process parameters are optimized in order to identify the optimum combination of parameters using Taguchi method with grey relational grade. Analysis of variance (ANOVA) is used to observe the level of significance of the process parameters and their interactions. The surface morphology and composition of Ni-P-Al2O3 coatings are studied with the help of scanning electron microscopy (SEM), energy dispersed X-ray analysis (EDX) and X-ray diffraction analysis (XRD).
Experimental details
Parameters selection and coating deposition
Effective deposition of the coating on the surface of the substrate depends on the preparation of the substrate. Hence, it is important to prepare the substrate surface very carefully and properly. In the present work AISI 1040 (mild steel) of size 20 mm × 20 mm × 2 mm is used as substrate material for Al2O3 composite coatings. Different machining processes such as shaping, parting, milling and grinding processes are used accordingly for the preparation of the substrate. The substrate is mechanically cleaned to remove foreign matters and corrosion products, and after that cleaned with de-ionized water. Sequentially a pickling treatment is given to the substrate with dilute hydrochloric acid (50 % HCl and 50% de-ionized water) for short duration of time to remove any surface layer formed like rust followed by rinsing with de-ionized water and methanol cleaning. To start the coating deposition quickly, the specimen is activated using a warm palladium chloride solution, maintained at 55 °C. The activated substrate is then dipped into the electroless bath maintained at 85 °C and the coating is carried out for three hours duration. For every substrate the constant deposition time is maintained to get uniform thickness of the coating. The coating thickness is found in the range of 28-32 microns. After the completion of the coating deposition process, the samples are cleaned with distilled water and wrapped with soft tissue paper.
Fig. 1 shows the schematic experimental setup for electroless Ni-P-Al2O3 composite coating.
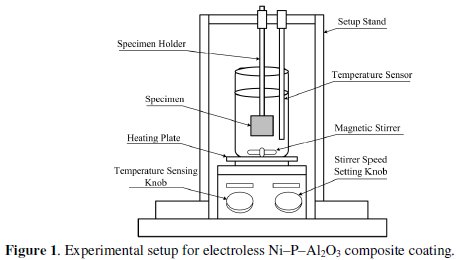
It consists of a heater with a magnetic stirrer (IKA®r; RCT basic). A fixed rigid stand is provided to grip and support the substrate and a glass coated temperature sensor. Chemical solution for electroless bath (200 mL) is poured into the glass beaker (250 mL size) placed on the heating plate. With the help of a temperature sensing knob, the temperature is set at 85 °C and a stirrer speed is set with the help of a speed setting knob at 150 rpm. The function of PTFE coated magnetic stirrer is to maintain the composite particles in suspension without agglomeration at the bottom of the glass beaker. The stirrer speed is fixed after a large number of iterations to avoid the decomposition of the electroless bath due to the agglomeration of particles.
The operating conditions and the ranges of chemicals used in electroless bath for electroless Ni-P-Al2O3 composite coatings are selected after several trials. The three important coating parameters are varied and the others are kept constant for coating deposition. The electroless bath composition and operating conditions used for the deposition of the electroless Ni-P-Al2O3 composite coatings are shown in Table 1.
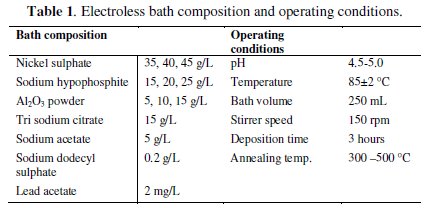
To have better suspension of the second phase alumina particles and to avoid agglomeration of particles, a specified amount of surfactant SDS (Sodium Dodecyl Sulphate) is added to the electroless nickel poly alloy bath. About 50 mL of electroless nickel solution containing the required amount of alumina powder are thoroughly mixed using a Magnetic stirrer (Remi make 2MLH) to get a uniform suspension of particles in the solution. At first a Ni-P layer is deposited in the first hour to prevent the porosity of the coating and then the solution containing Al2O3 particles and surfactant is introduced into the same bath for subsequent 2 hours for Ni-P-Al2O3 co-deposition.
During a chemical reaction within the electroless bath, nickel sulphate, which is used as a source of metallic ions, supplies the nickel ions in the solution. In the same bath sodium hypophosphite (used as a reducing agent) reduces the nickel ions from their positive valence state to zero valence state. At the given temperature the chemical reaction between nickel sulphate and sodium hypophosphite is quite fast and tremendous, which results in instant decomposition of the electroless bath. Consequently, complexing agents (tri sodium citrate and sodium acetate) are used to slow down the chemical reaction into a feasible form. Complexing agent forms metastable complexes with nickel ions and releases them slowly for the reaction, which helps to maintain the stability of the electroless bath. Even though the complexing agents have performed their duty in the electroless bath, there remains a possibility of decomposition of the chemical solution. In such situations the stabilizer (Lead acetate) plays an important role in the stabilization of the electroless bath for total duration of coating. To increase the wettability and surface charge of alumina particles, surfactant Sodium Dodecyl Sulphide is used in the electroless bath. The imperative functions of the surfactant are to reduce the surface tension of the liquid, easy dispersion of the particles and to reduce the interfacial tension between the solid and liquid surfaces. Surfactant reduces the agglomeration of the particles and electrostatic adsorption of suspended particles on the substrate [19]. To understand the effect of heat treatment on the corrosion resistance of the composite coatings, the coated samples are annealed in a box furnace for 1 hour at different temperatures (300 °C, 400 °C and 500 °C) according to the Orthogonal Array (OA). After annealing, the samples are cooled to room temperature without application of any artificial cooling.
Parameters optimization process and design of experiments
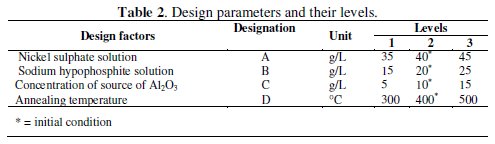
During coating deposition several factors like nickel source concentration, reducing agent concentration, pH of the solution, bath temperature, stabilizer and surfactant concentration, concentration of second phase particles and substrate material affect the coating characteristics. To obtain an optimum combination for maximum corrosion resistance, various coating parameters are varied within the specific range. From literature review, it is seen that three factors viz. nickel suphate (A), sodium hypophosphite (B) and concentration of second phase particles, i.e., Al2O3 particles (C), are the most commonly used by the researchers to control the properties of the composite coatings [19-21]. Besides, heat treatment (annealing) is found to have great effect on the corrosion resistance of the coating. Therefore, annealing temperature (D) is consider as the fourth parameter in the experimental design to study its effect on the corrosion resistance of the coating. The different design factors and their levels are shown in Table 2.
The present work considers the optimization of the corrosion behavior of electroless Ni-P-Al2O3 composite coating using a grey based Taguchi method [22] that uses loss function to measure the quality characteristics. The value of the loss function is transformed into a statistical measure, called as signal to noise ratio (S/N ratio). It is the ratio of the desirable value or signal to standard deviation or noise. S/N ratio can effectively consider the variation encountered in a set of trials. On the basis of the objective of the experimental work, the S/N ratios are classified into three basic criteria: lower-the-better (LB), higher-thebetter (HB) and nominal-the-best (NB). A larger S/N ratio represents minimization of noise factors. The combination of parameter levels which gives a maximum S/N ratio is known as the optimum combination of parameter levels. The present work is related to assessment of corrosion characteristics by measuring the charge transfer resistance (Rct) and double layer capacitance (Cdl). Because of using two different corrosion characteristics, it becomes a complex multivariate problem, which cannot be solved by Taguchi method. It may be possible that higher S/N ratio of one response variable corresponds to the lower S/N ratio of the other. Grey relational analysis [23] is an efficient tool which is used for the overall evaluation of the S/N ratio to optimize the multiple response characteristics. The optimization of the process is performed in a range of steps. In the first step the results of the experiments are normalized in the range of one and zero by performing the grey relational generation. In the next step grey relational coefficients are calculated with the help of normalized data. The normalized data represent the correlation between actual experimental data and desired experimental data. In the third step grey the relational grade is calculated by averaging the grey relational coefficients. The grey relational grade is treated as the overall response of the process instead of multiple responses of charge transfer resistance and double layer capacitance.
A statistical technique, analysis of variance (ANOVA), is performed to find the significant parameters of the experiment. The optimum combination of the coating process parameters is predicted with the help of ANOVA and grey relational analysis. Finally, a confirmation test is conducted to verify the optimum process parameters obtained from the analysis. In the present work, corrosion behavior of electroless Ni-P-Al2O3 composite coating is studied with the help of electrochemical impedance spectroscopy. Charge transfer resistance (Rct) and double layer capacitance (Cdl) are obtained from the Nyquist plot. Rct and Cdl are taken as the response variables for the current study. A higher value of Rct and lower value of Cdl indicate better corrosion resistance of the composite coating.
Design of Experiment (DOE) is a systematic and rigorous approach that provides the maximum amount of conclusive information from limited experimental runs. The DOE of the present study includes an orthogonal array (OA) based on the Taguchi method to reduce the number of experiments for the optimization of the coating process parameters.
For successful completion of the experiments it is very essential to choose the proper orthogonal array. It allows to compute the total degree of freedom (DOF) of main and interaction effects via a minimum number of experimental trials. In the present experiment, four process parameters are used with three levels, therefore the total DOF considering the individual factors and their interaction is 20. Hence the L27 OA is chosen which has 27 rows corresponding to the number of experiments and 26 DOF with 13 columns. The L27 OA is shown in Table 3.
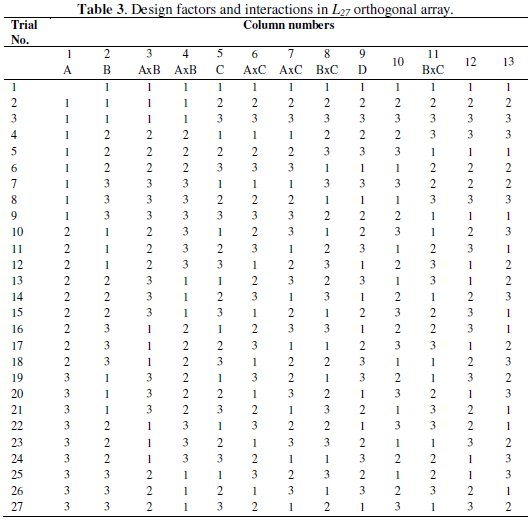
Each row in the table represents the specific combination of the experimental run and each column represents a specific factor or interactions. The cell value indicates the level of the corresponding factor or interaction assigned to that column. The experimental run is controlled by the setting of the design factors and not by the interactions.
Electrochemical study
The electrochemical impedance spectroscopy (EIS) test of heat-treated electroless Ni-P-Al2O3 composite coated samples is carried out using a potentiostat (Gill AC) of ACM instrument, UK. The test is conducted using 3.5% NaCl solution as electrolyte at ambient temperature of 30 °C. The electrochemical cell consists of three types of electrodes. A saturated calomel electrode (SCE) is used as reference electrode, which provides a stable 'reference' against which the applied potential may be accurately measured. A platinum electrode works as counter electrode or auxiliary electrode, which provides the path for the applied current into the solution. The coated specimen is used as working electrode. The design of the cell is such that only an area of 1 cm2 of the coated surface is exposed to the electrolyte.
Fig. 2 shows an equivalent electrical circuit model, which is used to arouse and fit the corrosion property parameters.
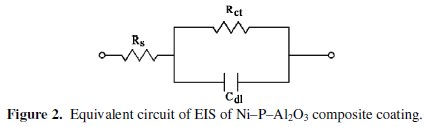
In the circuit the charge transfer resistance (Rct) is represented by the resistance of electron transfer during the electrochemical reaction course. The double layer capacitance (Cdl) is correlated to delamination of the coating. Solution resistance (Rs) is refered to the resistance between the work electrode (WE) and the reference electrode. The values of charge transfer resistance and double layer capacitance are calculated from the Nyquist plot by fitting a semicircle using the accompanying software.
The experimental setup of the electrochemical impedance spectroscopy test is shown in Fig. 3.
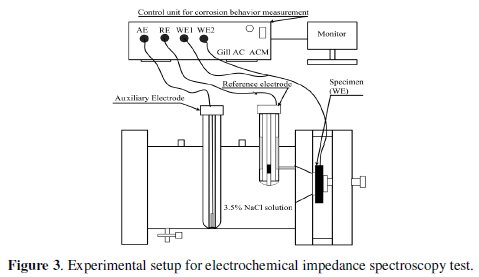
A settling time of 15 minutes is assigned before every test in order to stabilize the open circuit potential (OCP). The potentiostat is controlled with the help of a sophisticated desktop computer, which also collects and stores the data of EIS test. During the test the applied frequency of corrosion setup is varied from 10 KHz to 0.01 Hz. The Nyquist plot obtained from the EIS test appears in a single semicircle. The semicircle in the high frequency region signifies the charge control reaction and is quite consistent [14].
Microstructure and characterization study
Energy dispersive X-ray analysis (EDAX Corporation) is used to find out and verify the weight percentage of nickel, phosphorus, aluminum oxide, and oxygen in the composite coating. Scanning electron microscopy (JEOL, JSM-6360) is used to observe the surface morphology of the composite coating before and after heat treatment. SEM is done in order to investigate the effect of heat treatment on the electroless Ni-P-Al2O3 composite coatings. The phase compositions of composite coatings before and after heat treatment are detected using an X-ray diffraction analyzer (Rigaku, Ultima III).
Results and discussion
Microstructure study and characterization of the Ni-P-Al2O3 composite coating
SEM micrographs of as-deposited and heat treated Ni-P-Al2O3 composite coatings are shown in Fig. 4.
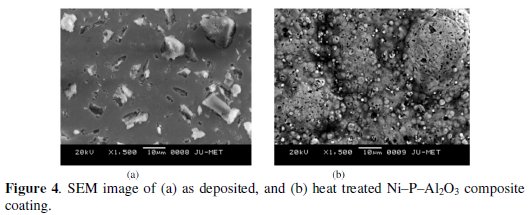
From the figure it is confirmed that the surface of the as-deposited composite coating has a smooth surface with uniform distribution of alumina particles with no porosity. The alumina particles are properly embedded on the surface of the alloy coating. The uniform distribution of the composite particles along with the smooth surface of the coating is due to the use of surfactant (SDS) in the electroless bath. After heat treatment of the composite coating at 400 °C, the globules of nickel and phosphorus are seen with embedded alumina particles. The globules become more compact due to heat treatment, which further reduces the porosity of the coating. This may be the reason for increase in corrosion resistance of the composite coating.
Fig. 5 shows the EDAX analysis of as deposited and heat treated Ni-P-Al2O3 composite coating.
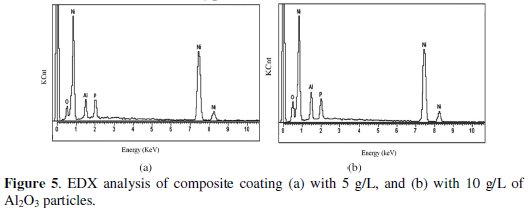
The compositional analysis of the coatings deposited at different concentrations of nickel sulpahte, sodium hypophosphite, and Al2O3 particles is carried out using energy dispersive X-ray analysis. From analysis, it is confirmed that the composite coating deposited using 5 g/L of Al2O3 particles in the bath has 78.07 wt% of nickel, 6.67 wt% of phosphorus, 8.74 wt% of oxygen, and 6.52 wt% of alumina particles. Similarly, the composite coating deposited using 10 g/L of Al2O3 particles in the bath has 72.46 wt% of nickel, 6.92 wt% of phosphorus, 11.06 wt% of oxygen, and 9.56 wt% of alumina particles. It means that the amount of alumina particles in the composite coating increases with increase in Al2O3 particles in the electroless bath.
X-ray diffraction (XRD) analyzer (Rigaku, Ultima III) is used for identification of different compounds in the electroless Ni-P-Al2O3 composite coating. Fig. 6 shows XRD plots of as deposited and heat treated composite coatings.
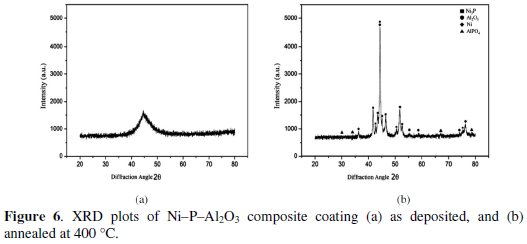
From the plots it is confirmed that in as-deposited condition the phase is mostly amorphous in nature, as a single broad peak is available at diffraction angle of 44.468. After heat treatment at 400 °C for one hour, the amorphous phase of composite coating is converted into crystalline phase. Different peaks are seen at different diffraction angles. The highest peak of Ni3P with Al2O3 is observed at the diffraction angle of 44.320. Ni3P peaks are also observed at the diffraction angles of 43.1, 43.5, 43.74, 44.26, 45.04 and peaks of Al2O3 are observed at diffraction angles of 37.06, 53.46, 55.7, and 77.28. Similarly, peaks of Ni are observed at diffraction angles of 52.18, and 77.38.
Coating process parameter optimization and confirmation test
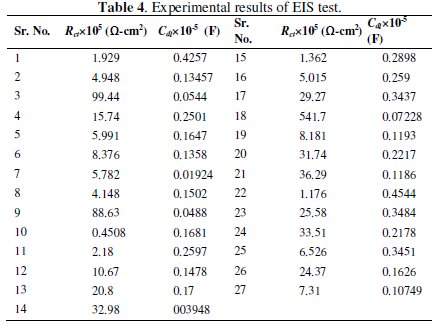
The present work deals with two responses, charge transfer resistance and double layer capacitance, for optimization of the corrosion resistance of Ni-P-Al2O3 composite coatings. Grey analysis is a technique which converts a multi response (variable) problem into a single response problem. The experimental data for charge transfer resistance (Rct) and double layer capacitance (Cdl) of the electrochemical impedance test are given in Table 4.
The particular sets of analysis are performed to convert the given multiple responses into a single performance index, called as grey relational grade.
Experimental results of the linear normalization, i.e., charge transfer resistance and double layer capacitance in the range of zero and one are vital for generating the grey relational coefficient. A material will have a lower tendency to corrode if Rct value tends to be a higher and Cdl to be a lower value. From Table 4 it is observed that the values of charge transfer resistance for all experiments are positive, hence higher the better criterion is used for normalization. Similarly, for all values of double layer capacitance lower the better criterion is used. The expressions for higher the better and lower the better criterion are given below.
Equation for higher the better:
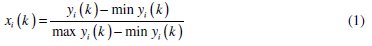
Equation for lower the better:
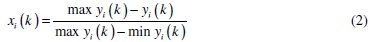
where xi(k) is the value after grey relational generation, while min yi(k) and max yi(k) are the smallest and largest values of yi(k) for the kth response. The data after grey relational grade are shown in Table 5.
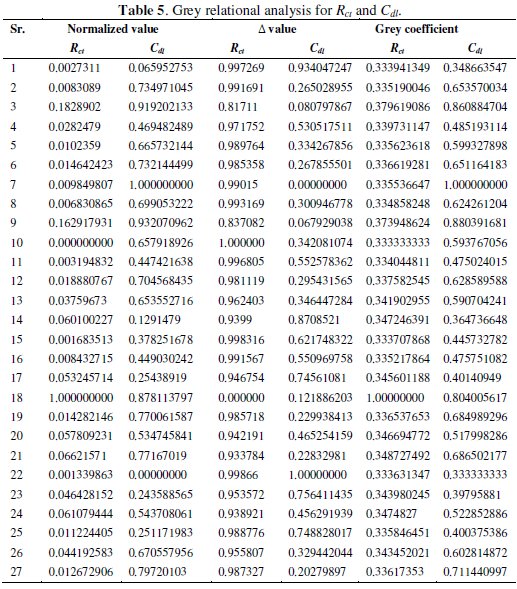
The grey relational coefficient is calculated from the normalized value and the equation for the grey relational coefficient is as follows.

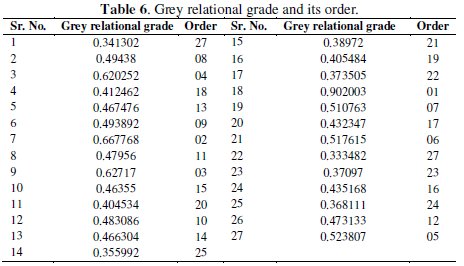
where Δ0i = ∥ x0(k) - xi(k) ∥ is the difference of the absolute value between x0(k) and xi(k). Δmin and Δmax are the minimum and maximum values of the absolute differences (Δ0i) of all comparing sequences. The overall multiple response characteristics evaluation is based on the grey relational grade and is calculated as follows:

where n is the number of process responses. The grey relational grades are considered in the optimization of multi-response parameter design problem. The values of grey relational grade are shown in Table 6.
As the grey relational grade is to maximized, the S/N ratio is calculated using higher the better criterion which is given by:
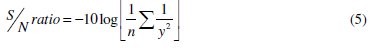
where y is the observed data and n is the number of observations. As the experimental design is orthogonal, it is possible to separate out the effect of each coating parameter at different levels. The mean grey relational grade for three levels of the four factors is summarized in Table 7.
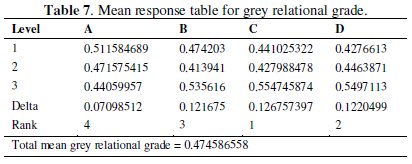
All the calculations are performed with the help of Minitab software [24]. The response table shows the average of the selected characteristic for each level of the factors. The ranks shown in the table are based on Delta statistics and it compares the relative magnitude of effects. The Delta statistics is the difference of highest average and lowest average of each factor. Ranks are assigned on the basis of Delta values; rank 1 is assigned to the highest Delta value, rank 2 is assigned to next highest value, and so on. Fig. 7 shows the main effects plot for mean S/N ratio and Fig. 8 shows the interaction plots between the process parameters.
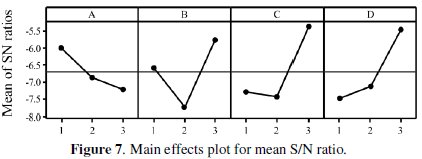
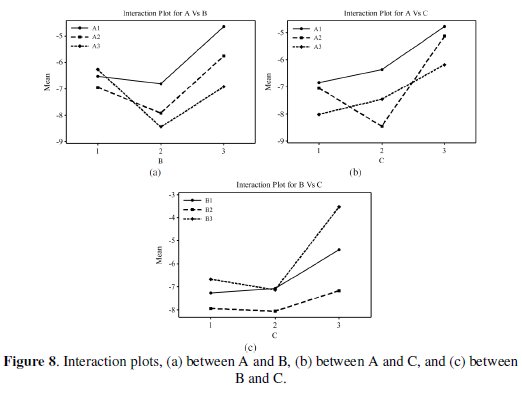
If the line for a particular parameter in the main effect plot is horizontal, it means that the parameter has no significant effect. On the other hand, if the line has maximum inclination to horizontal line, it means that the parameter has the most significant effect. From Fig. 7 it is clear that annealing temperature (parameter D) has a significant effect, the concentration of Al2O3 particles (parameter C) has the most significant effect and the reducing agent (parameter B) has a moderate significant effect. Parameter A is less significant due to less inclination to the horizontal line. Increase in the corrosion resistance of the composite coating in the presence of Al2O3 particles has also been observed earlier. In Ni-P-(Al2O3- TiC) composite coating, the corrosion resistance is increased in presence of Al2O3 particles upto a certain level of particle concentration in the bath and after that it gets decreased [6]. In a further case [3], the researchers have observed that the corrosion resistance is increased with increase in Al2O3 particles. Interactions between parameters A, B and C are shown in Fig. 8. From the plots it is observed that almost all lines are intersecting to each other, i.e., all factors have some amount of interaction between each other. From the figure it is seen a considerable interaction between parameters B and C and between parameters A and C. Thus, from the present analysis, it is confirmed that annealing temperature and concentration of Al2O3 particles are the significant parameters for the corrosion characteristics of Ni-P-Al2O3 composite coatings. In Taguchi method, optimum level of combinations is selected for those levels, which are having higher S/N ratios. In the present study the optimal combination is found to be A1B3C3D3.
ANOVA is useful to investigate the effect of the process parameters and of their significance level. This technique gives various important conclusions based on analysis of the experimental data. ANOVA separates the total variability of the response into contribution of each of the factors and the error. A sophisticated software Minitab is used to obtain the results through ANOVA with the S/N ratio. ANOVA results for the corrosion behavior of the composite coatings are shown in Table 8.
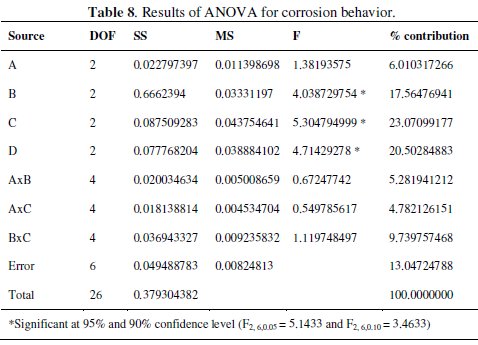
ANOVA calculations are based on the F-ratio or variance ratio. It is used to measure the significance of the parameters under investigation with respect to variations of all the terms included in the error term at the desired significance level. If the calculated value is higher than the tabulated one, the factor is significant at the desired level. The ANOVA table shows the percentage contribution of each parameter. From the table it is observed that parameter C (concentration of Al2O3 particles) has the most significant effect on the corrosion behavior at the confidence level of 95% within the specific test range. The annealing temperature (D) and concentration of reducing agent (B) are significant at 90% confidence level. Among the interactions, the interaction of parameters between B and C has a significant contribution. The percentage contribution of the factors and interactions are calculated to know the influence of the process parameters. From ANOVA table it is clear that parameter C has the largest contribution (23.071%) followed by parameter C (20.50%). Among interactions, B×C interaction has the highest contribution (9.74%). In the final step, confirmation results of optimum parameters are calculated by Taguchi method. The confirmation test is performed by conducting the experiment with optimal settings of the factors and levels previously calculated.
The predicted values of the S/N ratio at the optimum level his calculated as
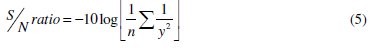
where ηm is the total mean grey relational grade, hiis the mean grey relational grade at optimal level, and 'o' is the number of main design parameters that significantly affect the corrosion resistance of Ni-P-Al2O3 composite coating. The results of the validation test are shown in Table 9.
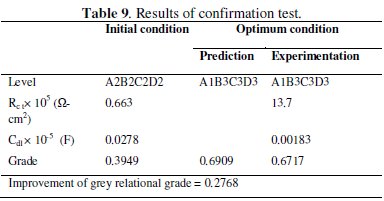
The significant improvement in grey relational grade from initial to optimum condition is 0.2768, which is about 41.21% of the mean relational grade. Nyquist plots for the Ni-P-Al2O3 composite coatings developed at initial and optimal condition are shown in Fig. 9.
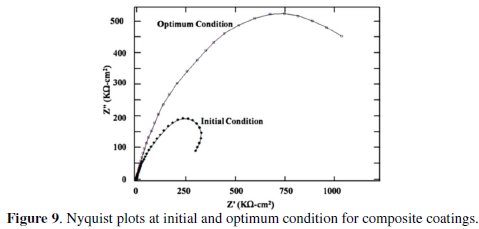
From the plots it is confirmed that the semicircle for optimum condition is larger than that of initial condition. Therefore the corrosion resistance at optimum condition is higher than that of initial condition.
Conclusion
Taguchi method in combination with grey relational analysis is used in order to optimize the electrochemical parameters of electroless Ni-P-Al2O3 composite coating using electrochemical impedance spectroscopy test in 3.5% NaCl solution. The optimum parameter combination is found to be A1B3C3D3. At optimum combination, the nickel concentration is 35 g/L, the reducing agent concentration is 25 g/L, the concentration of Al2O3 particles is 15 g/L, and the annealing temperature is 500 °C. From ANOVA results it is confirmed that the concentration of Al2O3 particles and the annealing temperature have the most significant influence on corrosion resistance of the composite coating. The interaction between the reducing agent and the concentration of Al2O3 particles has good significant effect among the interactions. The corrosion resistance of the composite coating improves with increase in alumina content and annealing temperature at 500 °C. The improvement in the grey relational grade from initial condition to optimal condition is found to be 41.21%. The coating composition is studied using EDX analysis. The micro structural analysis and crystallization behavior of the coating is studied with the help of SEM and XRD analysis. From SEM micrograph, it is confirmed that the alumina particles are uniformly distributed over the smooth coated surface without porosity. From EDX analysis, it is confirmed that the coating is deposited with the alumina particles, nickel, phosphorus and oxygen. From XRD plots it is confirmed that the as deposited composite coating has amorphous structure and it converts into a crystalline structure after heat treatment at 400 °C with Ni3P as a major compound.
References
1. Balaraju J N, Sankara Narayanan T S N, Seshadri S K. J Appl Electrochem. 2003;33:807. [ Links ]
2. Sudagar J, Lian J, Sha W. J Alloys Comp. 2013;571:183. [ Links ]
3. Sharma A, Singh AK. J Mater Eng Perform. 2013;22:176. [ Links ]
4. Sahoo P, Das SK. Mater Design. 2011;32:1760. [ Links ]
5. Apachitei I, Tichelaar F D, Duszczyk J, et al. Surf Coat Techn. 2001;148:284. [ Links ]
6. Abdel Aal A, Zaki Z I, Hamid Z A. Mater Sci Eng A. 2007;447:87. [ Links ]
7. Balaraju J N, Sankara Narayanan T S N, et al. Mater Res Bull. 2006;41:847. [ Links ]
8. Alirezaei Sh, Monirvaghefi S M, Salehi M, et al. Surf Coat Technol. 2004;184:170. [ Links ]
9. Riedel W. Electroless nickel plating. UK: Finishing Publications Ltd; 1991. [ Links ]
10. Hamdy A S, Shoieb M A, Hady H, et al. Surf Coat Technol. 2007;202:162. [ Links ]
11. Bigdeli F, Allahkaram S R. Mater Design. 2009;30:4450. [ Links ]
12. Zarebidaki A, Allahkaram SR. Micro Nano Lett. 2011;6:937. [ Links ]
13. Stankiewicz A, Masalski J, Szczygiel B. Mater Corros. 2013;64:908. [ Links ]
14. Allahkaram S R, Zoughi M, Rabizadeh T. Micro Nano Lett. 2010;5:262. [ Links ]
15. Praveen B M, Venkatesha T V, Naik Y A, et al. Surf Coat Technol. 2007;201:5836. [ Links ]
16. Zarebidaki A, Allahkaram S R. Micro Nano Lett. 2012;7:90. [ Links ]
17. Lee CK. Int J Electrochem Sci. 2012;7:12941. [ Links ]
18. Novakovic J, Vassiliou P. Electrochim Acta. 2009;54:2499. [ Links ]
19. Liu D, Yan Y, Lee K, et al. Mater Corros. 2009;60:690. [ Links ]
20. Sahoo P. J Phys D: Appl Phys. 2008;41:95305. [ Links ]
21. Das SK, Sahoo P. Adv Mechanical Eng. 2012;703168:1. [ Links ]
22. Roy RK. A Primer on the Taguchi Method. Mich, USA: Dearborn Society of Manufacturing Engineers; 1990. [ Links ]
23. Deng J. J Grey System. 1989;1:1. [ Links ]
24. Minitab user manual (release 13.2). Making data analysis easier, MINITAB Incorporation, State College, PA, USA. 2001. [ Links ]
*Corresponding author. E-mail address: psjume@gmail.com
Received 9 February 2015; accepted 25 February 2015














