Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.32 no.5 Coimbra set. 2014
https://doi.org/10.4152/pea.201405355
Evaluation of Corrosion Inhibition of Mild Steel: Chemically Polymerized PpAP/ Al2O3 Composite in the Presence of Anionic Surfactants
G. Thenmozhia , P. Arockiasamya , G. Mohanrajb and Jaya Santhi Ra,*
a P G & Research Department of Chemistry, Auxilium College, Vellore-632006, Tamil Nadu, India
b Department of Physics, Martin Luther University of Halle-wittenberg, Halle(saale)-06120, Germany
Abstract
Poly para aminophenol (PpAP)/Al2O3 composite was prepared by in situ chemical polymerization of para aminophenol using ammonium persulphate as an oxidizing agent in an aqueous medium containing anionic surfactants like DBSA (dodecyl benzene sulphonic acid) and SDS (sodium dodecyl sulphate) at 0 °C. The synthesized composites were characterized by UV-VIS-NIR spectroscopy, FTIR, XRD and TGA. The inhibiting effect of synthesized composites on the corrosion of mild steel in 1 M HCl solution has been investigated by different techniques like potentiodynamic polarization and electrochemical impedance spectroscopy methods for four different concentrations ranging from 50 to 300 mg/L. The results indicated that the corrosion inhibition efficiency increased on increasing composites concentration till 200 mg/L and decreased on further increasing concentration. It has been found that PpAP/Al2O3DBSA has got higher corrosion protection efficiency than that of PpAP/ Al2O3-SDS. Polarization studies reveal that composites act as a mixed type corrosion inhibitor and adsorption follows Langmuir adsorption isotherm.
Keywords: aminophenol, oxidative polymerization, surfactant, corrosion protection, mild steel.
Introduction
The damage by corrosion generates not only high cost for inspection, repairing, and replacement, but in addition it constitutes a public risk, thus the necessity of developing novel substances that behave like corrosion inhibitors especially in acid media [1]. The use of inhibitors is one of the most effective and often successful methods of protection of corrosion of metals in acidic media. It is a well-known fact that acids are used in many operations such as pickling, cleaning, descaling, etc. Because of their aggressiveness, inhibitors are used to reduce the rate of dissolution of metals [2]. Among the acid solutions, hydrochloric acid is one of the most widely used in the pickling processes of metals. The use of organic molecules as corrosion inhibitors is one of the most practical methods for protecting against corrosion, and it is becoming increasingly popular. It has been shown that organic compounds containing heteroatoms with high electron density, such as phosphorus, nitrogen, sulfur, and oxygen, as well as those containing multiple bonds which are considered as adsorption centers, are effective as corrosion inhibitors [3-6].
A derivative of polyaniline, aminophenols are interesting electrochemical materials since, unlike aniline and other substituted anilines, they have two groups (-NH2 and -OH) which can be oxidized [7]. Recently, poly aminophenol has attracted considerable attention for the preparation of its composites with inorganic particles in the presence of surfactants to improve their process ability. Surfactant inhibitors have many advantages, for example, high inhibition efficiency, low price, low toxicity, and easy production. Moreover, the investigation of surfactants adsorbed on metal surfaces is extremely important in electrochemical studies such as corrosion inhibition, adhesion, lubrication, and detergency [8]. The most important action of inhibition is the adsorption of the surfactant functional group onto the metal surface, as adsorption is critical to corrosion inhibition. The ability of a surfactant molecule to adsorb is generally related to its ability to aggregate to form micelles. Zhao and Mu [9] observed the effect of anionic surfactants such as dodecyl sulphonic acid sodium salt (DSASS), dodecyl benzene sulfonic acid sodium salt (DBSASS) and SDS on aluminium surfaces.
In the present work, PpAP/Al2O3 composite was prepared in the presence of two anionic surfactants like DBSA and SDS by chemical oxidation method and characterized by different spectroscopic techniques. An attempt has been made to investigate the corrosion protection behaviour of chemically synthesized PpAP/Al2O3-DBSA and PpAP/Al2O3-SDS composites over mild steel (MS) in acidic environment using potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) measurements.
Experimental method
Synthesis of PpAP/Al2O3 composites
The equimolar volumes of 0.1 M solution of para aminophenol and HCl were prepared in double distilled water, mixed and kept in the freezing mixture. 2 g of Al2O3 were added to the above solution and kept for vigorous stirring to keep the Al2O3 suspended in the solution. 0.1 M ammonium per sulphate and 0.025 M DBSA were taken in separate beakers. The oxidant and surfactant were added slowly to the mixture containing para aminophenol and Al2O3 and the stirring was continued for six hours at 0 °C. The product was filtered, washed with excess amount of water, methanol and acetone to remove excess of HCl, APS and DBSA. The resulting composite was then dried at 50 °C for 24 h. The same procedure was adopted for the synthesis of PpAP-Al2O3 /SDS composite.
Characterization
UV-VIS-NIR spectra of composites dissolved in DMSO solvent were obtained using a Varian, Cary-5000 spectrophotometer in the range of 200-2500 nm. The FT-IR spectra were recorded by a Thermo Nicolet, Avatar 370 spectrophotometer. The spectrum of the dry polymer powder in KBr pellet was recorded from 500 cm-1 to 4000 cm-1. X-ray diffraction (XRD) scan was done with a Bruker AXS D8 Advance diffractometer at room temperature using Cu Kα (λ=1.5406 Å). Thermo gravimetric analysis (TGA) was carried out in nitrogen atmosphere at a heating rate 10 °C/min up to 750 °C temperature by a Perkin Elmer, Diamond TG/DTA analyzer.
Preparation of specimens
Mild steel specimens having nominal composition of 0.023% P; 0.04% Si; 0.017% Ni; 0.37% Mn; 0.078% C; 0.02% S; 0.002% Mo and Fe balance were used. Specimen of dimension 1 × 1× 0.1 cm was used for electrochemical studies. The specimens were embedded in epoxy resin leaving a working area of 1 cm2. The surface preparation of the mechanically abraded specimens was carried out using different grades of silicon carbide emery paper (up to 1200 grit) and subsequent cleaning with acetone and rinsing with double-distilled water were done before each experiment.
Electrolyte
An electrolyte of 1 M HCl solution was prepared by diluting 37% HCl (Merck) using double-distilled water, being used as a corrosive solution. The four concentrations of PpAP/Al2O3 composites varying from 50 to 300 mg/L were used for the present investigations.
Electrochemical measurements
Electrochemical measurements, including potentiodynamic polarization curves and EIS were performed in a conventional three electrode cell using a computer- controlled potentiostat/galvanostat (Autolab PGSTAT 302N potentiosat from Eco-chemie, Netherlands). A platinum electrode was used as the counter electrode, Ag/AgCl, 3 M KCl as the reference electrode, and the mild steel specimen was used as a working electrode. Before each potentiodynamic polarization (Tafel) and EIS studies, the electrode was allowed to corrode freely and its open circuit potential (OCP) was recorded as a function of time up to 20 min, which was sufficient to attain a stable state. After this, a steady-state of OCP corresponding to the corrosion potential (Ecorr) of the working electrode, was obtained. The potentiodynamic measurements were started from cathodic to the anodic direction, E = Ecorr ± 250 mV, at a scan rate of 10 mVs-1. The linear Tafel segments of the anodic and cathodic curves were extrapolated to obtain Ecorr and the corrosion current density (Icorr). The inhibition efficiency was evaluated from the measured Icorr with and without inhibitor using the relationship,
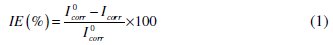
where I0corr is the corrosion current density without inhibitor, and Icorr is the corrosion current density with inhibitor. The corrosion rates of MS with polymers were calculated from polarization curves using the following equation [10],
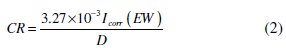
where CR is the corrosion rate (mmpy), Icorr is the corrosion current density (μA cm-2), EW is the equivalent weight of the specimen, and D is the density (g cm-3) of the specimen.
EIS measurements were carried out using AC signals of 10 mV for the frequency spectrum from 100 kHz to 10 mHz at the stable OCP. The potentiodynamic polarization and EIS data were analyzed and fitted using graphing and analyzing impedance software, Nova 1.4. Fresh solution and fresh steel samples were used after each sweep. The charge transfer resistance (Rct) was obtained from the diameter of the semicircle of the Nyquist plot. The inhibition efficiency of the inhibitor has been found out from the charge transfer resistance values using the following equation

where R0ct and Rct are the charge transfer resistance in the absence and in the presence of the inhibitor, respectively.
Results and discussion
UV-VIS-NIR spectroscopy
The UV/VIS/NIR absorption spectra of PpAP and PpAP/Al2O3 composites dissolved in DMSO are shown in Fig. 1, where we clearly observe two characteristic absorption peaks of PpAP: the first peak at 252-265 nm is assigned to the π-π* transition of the phenyl rings which is related to the extent of conjugation between the phenyl rings in the polymer chain; the second absorption peak at 425-440 nm shows n-π* transitions within the quinoid structure [11].
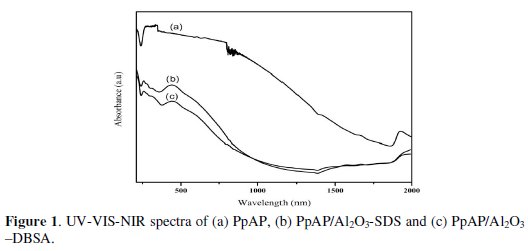
In addition, from comparison of UV-VIS-NIR spectra PpAP/ Al2O3-DBSA and PpAP/Al2O3-SDS can be seen that the shapes of peaks were very similar, but there are some shifts due to the interaction between PpAP/ Al2O3 and surfactants. It is well known that in composites systems with PpAP, strong guest-host interactions, such as hydrogen bonding, occur in the form of NH⋅⋅⋅O-Al in Al2O3 [12].
FT-IR analysis
Fig. 2 shows the FTIR spectra of PpAP and PpAP/Al2O3 composites synthesized in two different surfactants.
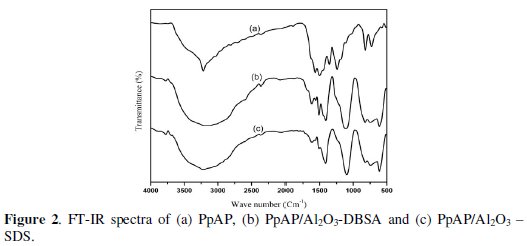
The main characteristic peaks of PpAP are assigned as follows: the broad intense peak around 3219 cm-1 is assigned to O-H stretching vibrations; a shoulder peak at 1504 cm-1 is due to stretching vibrations of N-H in the secondary amine, indicating that the polymerizing chains grow through the amino groups; the two main peaks around 1600 and 1560 cm-1 correspond, respectively, to the ring-stretching vibrations of the quinoid and benzenoid rings [13]; the peak at 1364 cm-1 is due to C -N stretching vibration of a secondary aromatic amine; the peak at 1121 cm-1 is ascribed to the stretching vibration of C-O-C linkages and further support that the Paminophenol changed into PpAP [14]. From the FTIR spectra of PpAP/Al2O3 composites, it is shown that all the characteristic peaks of PpAP are present and these peaks, when compared to that of PpAP/Al2O3, are found to be shifted slightly. It can be concluded that there is an interaction between PpAP macromolecule and Al2O3 particles. The interaction may be associated with the interaction of aluminium and nitrogen atoms in PpAP macromolecule. Moreover, the action of hydrogen bonding between Al2O3 particles and PpAP molecule is also contributory to the shift of peaks.
XRD analysis
Fig. 3 shows the XRD pattern of TiO2, PpAP and PpAP/Al2O3 composites.
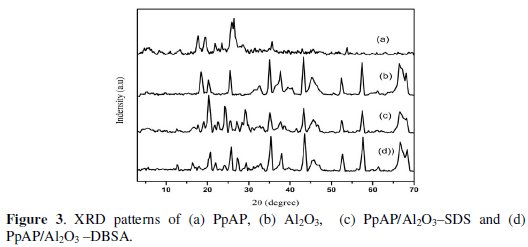
The crystalline regions in PpAP are shown by the presence of relatively sharp peaks. The amorphous regions are visible by the broad low intense peaks. PpAP exhibits broad Bragg diffraction peaks at 2θ angles of 17.6°, 19.4°, 24.2° and 25.5°; 2θ = 25.5° is characteristic of the van der Waals distances between stacks of phenylene rings (poly aminophenol rings). These strongest peaks indicate crystalline domains in the amorphous structure of the polymers [15]. It can be seen that the XRD patterns of PpAP/Al2O3-DBSA and PpAP-Al2O3/SDS are very much similar to that of Al2O3 and the broad diffractive peak of PpAP has become weak. The result suggests that the addition of Al2O3 hampers the crystallization of the PpAP molecular chain. This is because when the deposited PpAP is absorbed on the surface of the Al2O3 particle, the molecular chain of absorbed PpAP is tethered, and the degree of crystallinity decreases. It also confirms that the PpAP deposited on the surface of Al2O3 has no effect on the crystalline structure of Al2O3.
Thermo Gravimetric analysis
The most significant and consistent study of heat stable polymer and composite is the estimation of thermal stability. Thermal properties and interactions between polymer molecule and composite can also be studied from the decomposition through TGA. TGA thermogram (Fig. 4) of PpAP and PpAP/Al2O3 composites undergoes three weight loss steps.
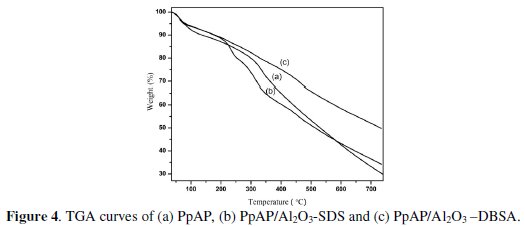
The first weight loss occurring around 65 °C is due to the loss of water vapor from both the oxide and polymer surface. The second weight loss stage which occurs in the range 230 to 310 °C corresponds to the removal of HCl, sublimation and removal of low molecular weight polymer/oligomer from the polymer matrix. The third weight loss, around 330 to 455 °C, is due to decomposition of the polymer backbone in PpAP, removal of DBSA from PpAP/Al2O3-DBSA and SDS dopant from PpAP/Al2O3-SDS in composites. The decomposition of PpAP is continuous up to 700 oC and even after 700 oC the complete decomposition had not take place. The decomposition of PpAP and PpAP/Al2O3 composites leaves some char content.
From the TGA curve it can be seen that the degradation of PpAP/Al2O3 composites is somewhat similar to that of PpAP. The noticeable difference is that the thermal decomposition of the composites starts at much lower temperature than that in pure PpAP, which may be due to the strong interaction between PpAP and Al2O3 [16]. This is also clear from the XRD where sharp peak of PpAP disappeared in the composites probably due to the interaction of PpAP and Al2O3. Several reports show that the lowered thermal stability of polymer composites with the incorporation of inorganic materials is due to the weakened interfacial interaction between the two components [17-18].
Potentiodynamic polarization
The potentiodynamic anodic and cathodic polarization plots for MS specimens in 1 M HCl solution with the synthesized PpAP/Al2O3 composites at four different concentrations like 50, 100, 200 and 300 mg/L are given in Fig. 5 and their respective electrochemical parameters, such as corrosion current density (Icorr), corrosion potential (Ecorr), cathodic Tafel slope (bc), anodic Tafel slope (ba), inhibition efficiency (IE), degree of surface coverage (θ) and corrosion rate (CR) are given in Table 1.
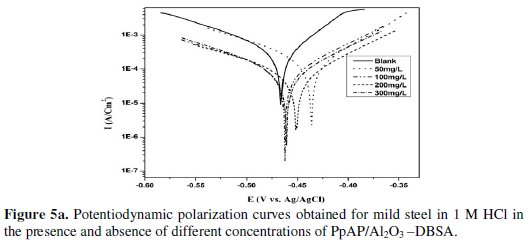
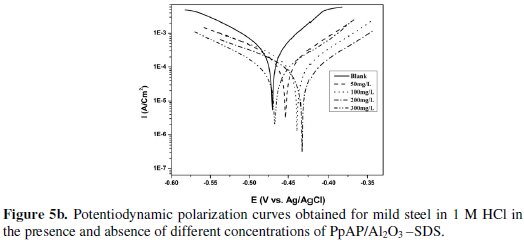
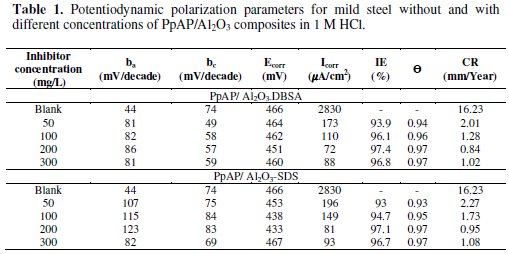
From the values given in this table it is evident that as the inhibitor concentration increased, the corrosion current densities of MS decreased till 200 mg/L of PpAP/Al2O3 composites and this behaviour is due to the adsorption of the inhibitor on MS/solution interfaces. The Icorr values decrease from 2830 μAcm-2 to 72 and 81 μAcm-2 with the addition of 200 mg/L of PpAP/Al2O3-DBSA and PpAP/Al2O3-SDS which resulted in 97.4 and 97.1% of IE. It is clear that the Icorr values decrease with the presence of composites, which indicated that composites adsorbed on the metal surface and hence inhibition occurs. Both the composites reached optimum inhibition concentration at 200 mg/L and further increase in concentration the IE value decreases. The Ecorr, ba and bc values do not change appreciably with the addition of the inhibitors, indicating that the inhibitors are not interfering with the anodic dissolution or cathodic hydrogen evolution reactions independently, but they act as a mixed type of inhibitor [19]. These results confirm that the composites on MS act as a highly protective layer, which is mainly attributed to the presence of π electrons in aromatic ring coexisting with quaternary nitrogen atom and large molecular size [7]. The surface coverage is approximately 0.960 (PpAP/Al2O3-DBSA) and 0.955 (PpAP/Al2O3-SDS) which is good to act as corrosive inhibitors.
Electrochemical impedance spectroscopy
Nyquist plots of MS in 1 M HCl solution in the absence and presence of PpAP /Al2O3 composites at different concentrations as inhibitor during an immersion time of 20 min at room temperature are given in Fig. 6.
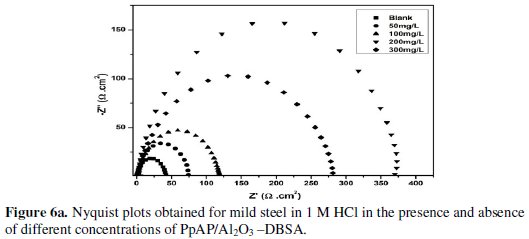
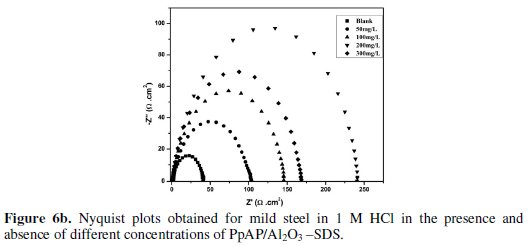
The depression in the figure is the characteristic of the inhomogeneities of the metal surface during corrosion [20]. The diameter of the capacitive loop stands for the resistance of the corrosion and it can be seen that the resistance decreases significantly with the decrease in diameter. Considering the impedance diagrams, the size of the capacitive loop increased by increasing the concentration of the inhibitor. The Nyquist impedance plots were analyzed by fitting the experimental data to a simple equivalent circuit model given in Fig. 7.
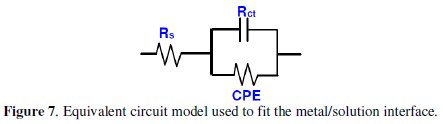
In this equivalent circuit (Fig. 7), Rs is the solution resistance, Rct is the charge transfer resistance, and CPE is a constant phase element, which is placed in parallel to charge transfer resistance element. Thus, in these situations, pure double layer capacitors are better described by a transfer function with constant phase elements (CPE) to give a more accurate fit [21] and its impedance is given by

where A is the proportionality coefficient, ω is the angular frequency, i is an imaginary number, and n is an exponent related to the phase shift and can be used as a measure of the surface irregularity. For ideal electrodes, when n = 1, CPE can be considered as a real capacitor. The Rct values are calculated based on the difference in impedance at lower and higher frequencies. According to Helmholtz, the decrease in CPE can be attributed to increase in the thickness of the electrical double layer [22]. The electrochemical parameters derived from Nyquist plots are calculated and listed in Table 2, indicating that the values of both Rct and IE are found to increase with increasing the inhibitor concentration, while the values of double layer capacitance (Cdl) are found to decrease.
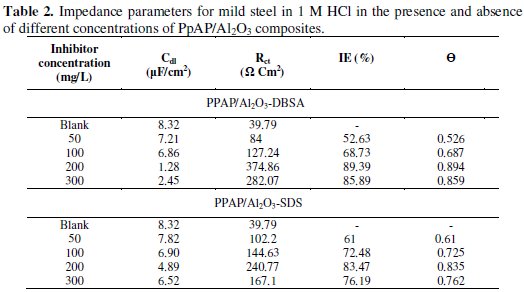
The Rct value is increased from 39.79 Ω cm2 for blank solution to 374.86 and 240.77 Ω cm2 upon addition of 200 mg/L of PpAP/Al2O3-DBSA and PpAP/Al2O3-SDS, resulting in 89.39 and 83.47% IE, respectively. The increase in Rct value is attributed to the formation of an insulating protective film at the metal/solution interface [23]. The Cdl decreases from 8.32 μF cm-2 to 1.28 and 4.89 μF cm-2 in the presence of 200 mg/L of PpAP/Al2O3-DBSA and PpAP/Al2O3-SDS, respectively. The effect is reversed with further increase in the concentration of the composites as seen in the result. As the concentration of the composites increased above 200 mg/L, the corrosion resistance decreased. In fact, the highest IE was reached at 200 mg/L of both the composites, which is considered as being an optimum inhibitor concentration. The initial decrease in Cdl value from blank solution to that of the inhibitor containing electrolyte is due to a decrease in the local dielectric constant, while further decrease in Cdl with increasing concentrations of the inhibitor is due to increase in the thickness of the electrical double layer. The θ is approximately 0.742 (PpAP/Al2O3-DBSA) and 0.733 (PpAP/Al2O3-SDS) which is found to be good to act as an anticorrosive agent. The results obtained from EIS method are in good agreement with the linear polarization measurement.
The enhanced corrosion protection of MS by PpAP/Al2O3 composites can be explained on the basis of the molecular adsorption. The composites inhibit corrosion by controlling both the anodic and cathodic reactions. In acidic solution, the composite molecules exist as protonated species [24]. These protonated species adsorb on the cathodic sites of MS and decrease the evolution of hydrogen. The adsorption on anodic sites occurs through long π-electrons of aromatic rings (benzenoid and quinoid) and lone pair of electrons of nitrogen atoms, which decrease the anodic dissolution of MS [25]. It is well known that the species having high molecular weight and bulky structure may cover more area on the active electrode surfaces. The slightly higher performance of the PpAP/ Al2O3-DBSA than PpAP/ Al2O3-SDS is due to the higher molecular size and high electron density on the adsorption centers.
Adsorption isotherm
The interaction between an inhibitor and MS surface can be described by the adsorption isotherm. We have obtained the adsorption isotherm through the degree of surface coverage which is defined as θ = IE%/100. It was determined at different inhibitor concentrations using Langmuir adsorption isotherm and can be expressed as

where K is the equilibrium constant of adsorption and Cinh is the inhibition concentration. Many researchers have explained the Langmuir adsorption isotherm with the interaction of adsorbed species on the metallic surfaces [26]. The Langmuir adsorption isotherm was drawn by plotting Cinh/θ vs. Cinh, considering the θ values from potentiodynamic polarization and EIS measurements at 25 °C for MS in 1 M HCl at different concentrations of composites; the graph is shown in Fig. 8 and 9.
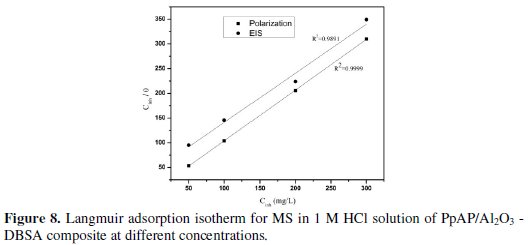
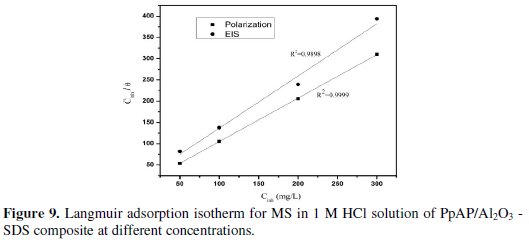
The straight line obtained in the graphs clearly shows that the chosen inhibitor obeys the Langmuir adsorption isotherm. From this, it can be concluded that the PpAP /Al2O3 composites can act as a good inhibitor.
Conclusion
PpAP/Al2O3 composites were successfully synthesized by in situ chemical oxidative polymerization method using ammonium per sulphate as an oxidant and two anionic surfactants DBSA and SDS at 0 °C. From different spectroscopic techniques it can be concluded that there are strong interactions between PpAP/Al2O3 and the surfactants. The percentage inhibition efficiency of these polymers obtained from potentiodynamic polarization and EIS measurements are in good agreement, and the corrosion inhibition efficiencies are in the order PpAP/Al2O3-DBSA > PpAP/Al2O3-SDS. Polarization curves demonstrated that the examined composites behave as a mixed type of inhibitors. In these composites, the uniform increasing inhibition efficiency as a function of the concentration deals with the adsorption phenomenon; the adsorption of inhibitors on the surface of MS is indicated by decrease in the double layer capacitance. The inhibition is due to the adsorption of the inhibitors on the steel surface and results in blocking the active sites. Adsorption follows Langmuir adsorption isotherm.
References
1. Trabanelli G. Corrosion 1991;47:410. [ Links ]
2. Jeyaprabha C, Sathiyanarayanan S, Venkatachari G. J Appl Polym Sci. 2006;101:2144. [ Links ]
3. Abdallah M. Corros Sci. 2002;44:717. [ Links ]
4. Singh A K, Quraishi M A. Corros Sci.2010;52:1529. [ Links ]
5. Singh A K, Quraishi M A. Corros Sci. 2010;52:1373. [ Links ]
6. Hosseini M, Mertens S F L, Ghorbani M, et al. Mater Chem Phys. 2003;78:800. [ Links ]
7. Hur E, Bereket G, Duran B, et al. Prog Org Coat. 2007;60:153. [ Links ]
8. El Azhar M, Mernari B, Traisnel M, et al. Corros Sci. 2001;43:2217. [ Links ]
9. Zhao T, Mu G. Corros Sci. 1999;41:1937. [ Links ]
10. Srikanth A P, Sunitha T G, Raman V, et al. Mater Chem Phys. 2007;103:241. [ Links ]
11. Yavuz A G, Gok A. Synth Met. 2007;157:235. [ Links ]
12. Xu J C, Liu W M, Li H L. Mater Sci Eng C. 2005;25:444. [ Links ]
13. Thenmozhi G, Santhi R J. Int J Sci Resear. 2014;3:378. [ Links ]
14. Ehsani A, Mahjani M G, Jafarian M. Synth Met. 2012;162:199. [ Links ]
15. Thenmozhi G, Mohanraj G, Madhusudhana G, et al. J Polym. 2014;ID827043. [ Links ]
16. Zheng J, Li G, Ma X, et al. Sens Actuators B: Chem. 2008;133:374. [ Links ]
17. Bian C, Yu Y, Xue G. J Appl Polym Sci. 2007;104:21. [ Links ]
18. Li J, Zhu L, Wu Y, et al. Polymer. 2006;47:7361. [ Links ]
19. Thenmozhi G, Arockiasamy P, Santhi R J. Int J Electrochem. 2014; ID961617. [ Links ]
20. Saliyan V R, Adhikari V. Corros Sci. 2008;50:55. [ Links ]
21. Arockiasamy P, Sheela X Q R, Thenmozhi G, et al. Int J Corros. 2014; ID679192. [ Links ]
22. Tang Y, Yang X, Yang W, et al. Corros Sci. 2010;52:1801. [ Links ]
23. Bentiss F, Traisnel M, Lagrenee M. Corros Sci. 2000;42:127. [ Links ]
24. Aridoss G, Kim MS, Son SM, et al. Adv Polym Tech. 2010;21:881. [ Links ]
25. Quraishi MA, Rawat J, Ajmal M. J Appl Electrochem. 2000;30:745. [ Links ]
26. Bayol E, Kayakirilmaz K, Erbil M. Mater Chem Phys. 2007;104:74. [ Links ]
*Corresponding author. E-mail address: shanthijaya02@gmail.com
Received 15 October 2014; accepted 25 October 2014














