Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.32 no.5 Coimbra set. 2014
https://doi.org/10.4152/pea.201405325
New Approach for Measuring Antioxidant Activity Via a Graphite Sensor
A. M. El-Kosasy, L. A. Hussien and M. H. Abdel-Rahman*
Pharmaceutical Analytical Chemistry Department, Faculty of Pharmacy, Ain Shams University, Abbassia, 11566 Cairo, Egypt
Abstract
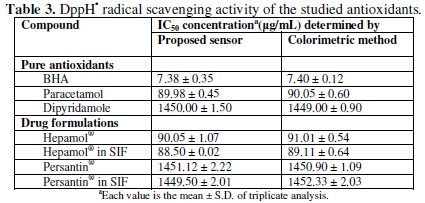
An ion selective membrane sensor from dioctyl phthalate as a plasticizer in a polymeric matrix of polyvinyl chloride (PVC) and β-cyclodextrin as an ionophore was constructed and evaluated according to IUPAC recommendations. Linear Nernstian response of DPPH• within the concentration ranges of 10-6 to 10-2 mol L-1 was obtained with average recovery 99.87 ± 0.617. Nernstian slope of 58.5 mV/decade with excellent selectivity over the pH range of 3-8 was observed. The suggested method was standardized using butylated hydroxyl anisole (BHA). The 50% radical scavenging activity (IC50) determined by the proposed sensor correlated well with that of the common spectrophotometric method based on scavenging of 2,2-diphenyl-1picrylhydrazyl (DPPH•). An algorithm implemented in Microsoft Visual Basic® 6.0 was used for calculating (IC50) values which are 7.38 μg/mL ± 0.35, 89.98 μg/ mL±0.45 and 1.45 mg/ mL ± 1.50 for BHA, Paracetamol and Dipyridamole, respectively. The proposed sensor represents a simple and reproducible tool for measuring DPPH• scavenging activity of Paracetamol and Dipyridamole in bulk powder, pharmaceutical formulations and simulated intestinal fluid (SIF) without sophisticated separation techniques.
Keywords: Sensor, antioxidant, Paracetamol, Dipyridamole.
Introduction
The chemistry of free radicals and antioxidants is of great importance, particularly in the areas of clinical medicine and nutritional science [1]. Antioxidants act as free radical scavengers and can prevent the damage caused by oxidative reactions, including cancer, Alzheimer's and Parkinson's diseases [2, 3-6]. Hence, the evaluation of the antioxidative activity of medical, cosmetic and food samples provides useful clinical information [7].
One of the most commonly used methods for in vitro evaluation of antioxidant capacity is DPPH• scavenging method [8-13]. Recently, a number of DPPH• -based tests for the assessment of antioxidant activity have been developed. The evaluation of antioxidant capacity based on the amperometric reduction of DPPH• at the glassy carbon electrode was reported [14, 15], TLC [16], HPLC techniques [17-20] and flow injection based methods for determination of scavenging capacity against DPPH• were also reported [21-25]. A DPPH• based optical sensor for screening of antioxidant activity was introduced by Steinberg et al. [26].
In the present work, we have studied the feasibility of using a graphite ion- selective membrane sensor for evaluation of DPPH• scavenging activity of certain antioxidants and using BHA, a standard antioxidant, to study its response. The proposed sensor can be considered superior to the common colorimetric method since it can be used for evaluation of antioxidant activity of colored or turbid solutions and it applies neither sophisticated instruments nor any separation step; also, the proposed method is more simple, faster and cheaper than the amperometric and chromatographic methods.
Experimental
Apparatus
Mettler Toledo compact titrator model G20 with Labx software version 3.1 accompanied with Ag/AgCl double junction reference electrode was used for potential measurements. A Jenway pH meter 3310 pH / mV / °C meter with a Jenway pH glass electrode (UK) was used for pH adjustments. Thermostatic multiple water bath, model BT-15 (Spain). Thermometer. Double beam Shimadzu (Japan) 1601Pc UV-VIS spectrophotometer connected to a computer fitted with UVPC personal spectroscopy software version 3.7.
Chemicals and reagents
2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), 99.98%, was obtained from Sigma Aldrich, Cairo, Egypt. Hepamol® tablets (500 mg paracetamol/tablet, Hikma Pharmaceutical Company, Egypt) and Persantin tablets (75 mg dipyridamole/ tablet, Sideco Pharmaceutical Company, Egypt).
All reagents and chemicals used throughout this work were of analytical grade and the water used was bi-distilled. Poly (vinyl chloride) carboxylate (PVC carboxylate) and β-cyclodextrin (β-CD) were obtained from Fluka (Chemie Gmbh, Germany), dioctyl phthalate (DOP) was obtained from Aldrich (Germany). Tetrahydrofuran (THF) was obtained from Merck (Darmstadt, Germany). Sodium hydroxide, hydrochloric acid, potassium chloride, citric acid, sodium bicarbonate, nickel chloride hexahydrate and magnesium chloride were obtained from Prolabo (Pennsylvania, USA). Simulated intestinal fluid (SIF) was prepared from 6.8 g monobasic potassium phosphate, 0.2 M sodium hydroxide (NaOH) (to adjust pH to 6.8) and water to 1000 mL and the temperature was adjusted to 37 ± 0.2 °C.
Procedures
Fabrication of the membrane sensor
0.04 g of (β-cd) was mixed with 0.19 g PVC carboxylate and dissolved in 0.4 mL (DOP) and then mixed thoroughly with 5 mL (THF) till complete homogeneity in a Petri dish (5 cm diameter), the solvent was slowly evaporated at room temperature until an oily concentrated mixture was obtained. The coated graphite electrode was constructed using a graphite bar 3 cm length, 3 mm diameter). One end of the bar was used for connection, while the other, about 1 cm length, was dipped in the electro-active membrane mixture. The process was repeated several times until a layer of proper thickness was formed covering the terminal of the graphite bar. The electrode was left standing at room temperature to dry. The uncoated end of the graphite rod was sealed in a poly tetra ethylene tube; the tube was filled with metallic mercury into which a copper wire was dipped.
Sensor calibration
The sensor was conditioned by soaking in 10-2 mol L-1 DPPH• solution for only 2 hours before measurement, storage was in distilled water when not in use, the conditioned sensor was calibrated by separately transferring 50 mL aliquots of solutions covering the concentration range of (10-7 to 10-2 mol L-1) DPPH•, into a series of 100 mL beakers. The electrode system was immersed in each solution with constant stirring at speed 20% in conjunction with a Mettler Toledo reference electrode. The sensor was washed in distilled water between measurements. The sensor potential was plotted versus each negative logarithmic concentration of DPPH•, the calibration plot obtained was used for subsequent measurements of unknown samples.
Effect of pH and temperature
The influence of pH on the response of the membrane sensor was checked at various pH values over a pH range of (1-9). 1×10-3 and 1×10-4 mol L-1 DPPH• solutions were prepared. Effect of temperature also was checked by applying the same procedures at all concentrations at different temperatures using a water bath and a thermometer to adjust the temperature of each concentration.
Sensor selectivity
The potentiometric selectivity coefficients (KpotA.B) of the proposed sensor towards different substances were determined by a separate solution method using the following equation [27]:
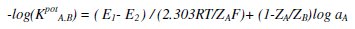
where KpotA.B is the potentiometric selectivity coefficient, E1 is the potential measured in 10-3 mol L-1 DPPH• solution, E2 is the potential measured in 10-3 mol L-1 of the interfering solution, ZA and ZB are the charges of DPPH• and interfering ion, respectively, aA is the activity of DPPH• and 2.303RT/AF represents the slope of the investigated sensor (mV/concentration decade).
Potentiometric determination of % DPPH• scavenging of pure paracetamol and dipyridamole
0.5 mL of BHA, paracetamol and dipyridamole standard solutions (1.01-15.15 mg mL-1) were taken separately to obtain concentrations (10-150 μg/mL), 50 mL DPPH• working standard solution (1×10-4 mol L-1) were added, BHA prepared in methanol but paracetamol and dipyridamole prepared in water: methanol (1:1) then incubated at room temperature for 15 min after good shaking. Control solutions were prepared, in which no drug was added and the same procedure was carried out. The reagent solutions were prepared daily. Percentages scavenging and IC50 values were calculated using an algorithm [28] implemented in Microsoft Visual Basic® 6.0 (Microsoft Corporation, Redmond, WA, USA).The software can be freely downloaded at http://www.pharm.unipmn.it/rinaldi/software/blesq/BLeSq.html. Results were compared with those obtained from the colorimetric method.
Potentiometric determination of % DPPH• scavenging of paracetamol and dipyridamole in dosage forms
The suggested procedure was applied for the evaluation of DPPH• scavenging activities of dipyridamole in Persantin® tablets and paracetamol in hepamol® tablets in which ten tablets were weighed and their mean weight was determined. The tablets were finely powdered and accurately weighed portions of powders equivalent to (101.00-1515.00 mg) were transferred to 100-mL volumetric flasks, then the volume was completed with water: methanol (1:1) then filtered. The same previous procedure was then repeated. Percentages scavenging of paracetamol and dipyridamole in dosage forms, separately, were calculated.
Potentiometric determination of % DPPH• scavenging of paracetamol and dipyridamole in dosage forms in (SIF)
The same procedure was applied but the volume was completed with simulated intestinal fluid (SIF): methanol (1:1) then filtered. Percentages scavenging of paracetamol and dipyridamole in tablet dosage forms, separately, were calculated.
Colorimetric analysis
The DPPH• scavenging activity was measured by following the methodology described by Sharififar [29] where 50 μL of BHA, paracetamol and dipyridamole standard solutions (1.01-15.15 mg mL-1) were taken separately to obtain concentrations (10-150 μg/mL), 5 mL DPPH• working standard solution (1×10-4 mol L-1) were added, BHA prepared in methanol but paracetamol and dipyridamole prepared in water: methanol (1:1), then incubated at room temperature for 15 min after good shaking. Absorbances were measured at 517 nm. Control solutions were prepared, in which no sample was added and the same procedure was carried out. The assays were carried out in triplicate. The percent radical scavenging activity is determined from the difference in absorbance of DPPH• between the control and samples by the following equation:
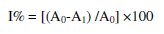
where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the antioxidant [30]. The same procedure was applied on pharmaceutical formulations and in SIF.
Results and discussion
Sensor fabrication
The central cavity of the cyclodextrin molecule is lined with skeletal carbons and ethereal oxygens of the glucose residues (Fig. 1a).
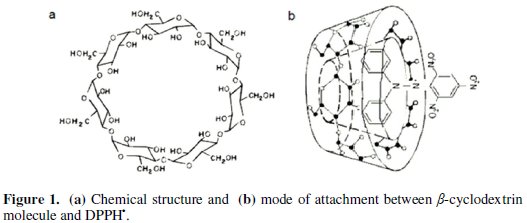
It has therefore lipophilic cavity into which suitably sized drug molecules are included. No covalent bonds are formed or broken during drug-cyclodextrin complex formation [31]. We proposed that the two benzene rings of DPPH• are located well inside the cavity with the tri nitro benzene group protruding from β-cd cavity with the formation of hydrogen bonds between hydroxyl groups of β-cd and nitro groups of DPPH• (Fig. 1b).
It has been reported that PVC matrix is a regular support and reproducible trap for ion association complex in ion selective electrodes [32]. Nevertheless, its use creates a need for plasticization and places a constraint on the choice of the mediator [33].
In the present study, DOP was used in the sensor fabrication, which plasticized the membrane and adjusted permittivity of the final organic membrane [34]. The electrochemical cell of the suggested membrane electrode for the determination of DPPH• can be illustrated diagrammatically as follows:
Double junction Ag/AgCl reference electrode ∣ ∣ Test solution (DPPH•) ∣ Membrane (PVC,β-CD, DOP) ∣ Graphite rod ∣∣ Metallic mercury.
Choice of solvent
The reaction mechanism between DPPH• and antioxidant molecule is based on an electron transfer reaction [35]; as a result, the scavenging capacity against DPPH• radical is strongly influenced by the solvent and the pH of the reaction [36]. It was concluded that 50% (v/v) aqueous/methanol solutions are a suitable choice for lipophilic and hydrophilic antioxidants and the reaction rate between DPPH• and the antioxidant may increase considerably with increasing water ratios. However, at water content over 60% (v/v) the measured antioxidant capacity decreased, since a part of the DPPH• coagulates and it is not easily accessible to the reaction with the antioxidant [37].
Sensor calibration and response time
The electrochemical performance characteristics of the investigated DPPH•selective membrane sensor were evaluated according to IUPAC recommendation data [27] and summarized in Table 1.
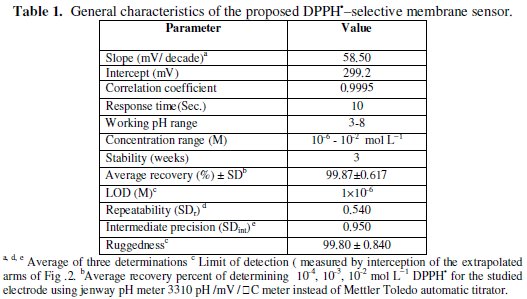
The potential displayed by the proposed electrode for the measurements of the standard drug solution in the same day and linearity range from day-to-day does not vary by more than ± 1 mV. Calibration slopes didn't change by more than ± 1 mV/decade concentration over a period of 3 weeks. The required time for the sensor to reach values within ± 1 mV of the final equilibrium potential after increasing the drug concentration 10-folds was found to be 10 seconds. Typical calibration plot is shown in Fig. 2.
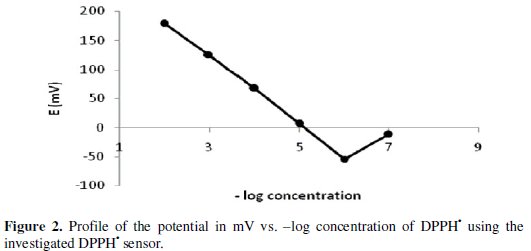
The slope of the calibration plot was 58.5 mV/concentration decades. The slight deviation from the ideal Nernstian slope (60 mV) stems from the fact that the electrode responds to the activities of drug anion and cation rather than to their concentration. Nernstian relation of the sensor is:
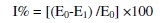
where [C] is the molar concentration. The detection limit of the proposed sensor was estimated according to the IUPAC definition [27]. LOD value was found to be 10-6 mol L-1.
The effect of pH and temperature
The influence of pH on the potential response of the sensor was studied at different concentrations, 10-3 and 10-4 mol L-1 over the pH range 1-9; the potential pH profile (Fig. 3) indicated that the sensor potential is fairly constant over the pH range of 3-8, therefore, this range can be chosen as the working pH range for the sensor assembly; above pH 8, the hydroxide anion reacts with DPPH•, in two ways, the first one in which the anion acts as a nucleophile and makes a complex , which decomposes after that by losing a hydride anion or a nitrite anion, leading to different compounds, or the DPPH• is strong enough to abstract one electron from the anion and to oxidize it to the short-lived radical X, which reacts with DPPH• , yielding also finally the nitro derivative of DPPH• [38], as shown in Fig. 4.
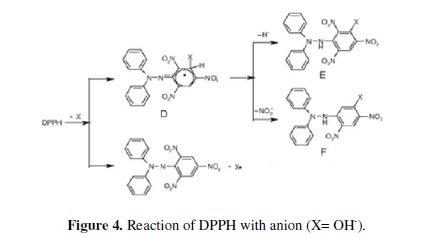
Upon studying the effect of temperature, the proposed sensor exhibits slight increase in its potentials as the temperature rises in the range of 25-40 °C; however, the calibration graphs obtained at different temperatures were parallel, as shown in Fig. 5.
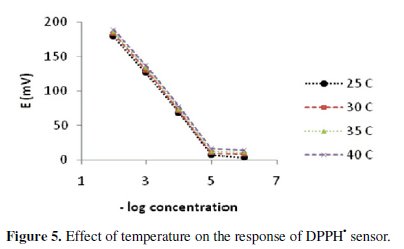
The limit of detection, slope and response time didn't significantly vary with variation of temperature, indicating reasonable thermal stability up to 40 °C.
Sensor selectivity
Table 2 shows the potentiometric selectivity coefficients of the proposed sensor in the presence of other interfering substances.
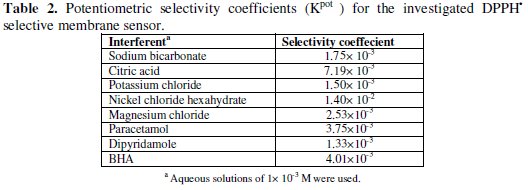
The results reveal that the proposed membrane sensor displays high selectivity.
Assessment of the antioxidant activity of paracetamol and dipyridamole in pure form, dosage forms and SIF
The radical scavenging activity was calculated as follows:
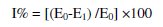
where E0 is the potential of the control and E1 is the potential in the presence of the test compound at different concentrations.
The IC50 values were calculated graphically by plotting the antioxidant drug concentration vs. the corresponding scavenging effect using the algorithm implemented in Microsoft Visual Basic® 6.0 (Microsoft Corporation, Redmond, WA, USA) (on the basis of probit, logit and angular regressions) as presented in Figs. 6, 7 and 8.
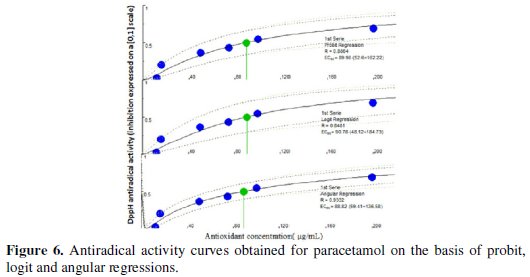
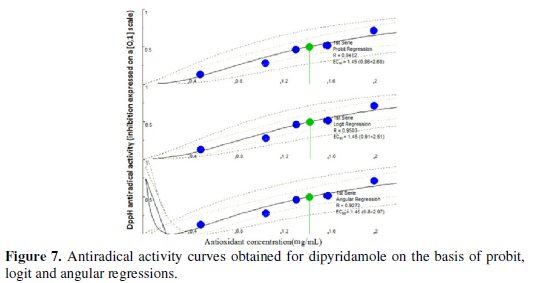
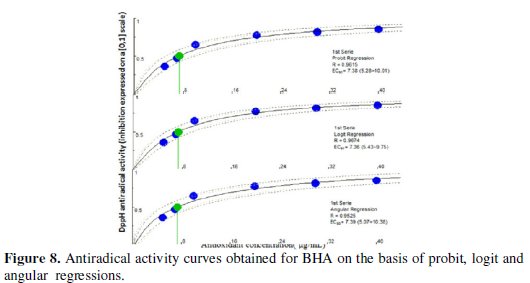
The proposed sensor was successfully used for evaluating the antioxidant activities. The percentage scavenging of BHA(IC50) = 7.38 μg/mL, paracetamol (IC50) = 89.98 μg/mL and dipyridamole (IC50) = 1.45 mg/mL. Probit model had been chosen as it is well adapting to the data obtained from the DPPH• assay and it generally gives the intermediate IC50 amongst the three regression models considered. Results arranged scavenging activity in the following order: BHA > paracetamol > dipyridamole. The AAI value [final concentration of DPPH• in μg mL-1 / IC50 (μg/mL)] of the standard antioxidant BHA is correlated well with that obtained from the application of the colorimetric method established by Gourine et al. [39] (Table 3).
The results proved the applicability of the proposed sensor for the evaluation of DPPH• scavenging activity of the studied drugs in their pharmaceutical formulations and in SIF.
Conclusions
The proposed membrane sensor was successfully used for assessment of DPPH• scavenging of paracetamol and dipyridamole in pure form and in dosage form in (SIF). It also offers moderate stability time, elimination of drug pretreatment or separation steps, wide pH range, low detection limit and direct determination of drugs scavenging effects in turbid and colored solutions without interference by pigments or excipients. In addition, DPPH• radical is stable, commercially available, and does not have to be generated before assay.
The use of the proposed sensor is particularly suited to fast response and low-cost screening of the antioxidant activity.
References
1. Halliwell B, Gutteridge J M C, Free Radicals in Biology and Medicine, third ed., Oxford University Press, New York, 1999. [ Links ]
2. Balazs L, Leon M. Neurochem Res. 1994;19:1131. [ Links ]
3. Lewen A, Matz P, Chan PH. Neurotauma. 2000;17:871. [ Links ]
4. Duh P D, Tu Y Y, Yen G C. Lebensmittel Wissenchaft und-Technologie. 1999;32:269. [ Links ]
5. Bautista A P, Meszaros K, Bojta J, et al. Leukocyte Biol. 1990;48:123. [ Links ]
6. Bautista A P, Spitzer J J. Am J Physiol. 1990;259:907. [ Links ]
7. CRC press, Taylor and Francis group, oxidative stress and inflammatory mechanisms in obesity, diabetes and metabolic syndrome, Lester Packer, Helmut Sies, 2007. [ Links ]
8. Magalhaes L M, Segundo M A, Reis S, et al. Anal Chim Acta. 2008;613:119. [ Links ]
9. Gulcin I. Life Sci. 2006;78:803. [ Links ]
10. Gourine N, Yousfi M, Bombarda I, et al. Ind Crops Products. 2010;31:203. [ Links ]
11. Beara IN, Lesjak MM, Cetojevic-Simin DD, et al. Food Res Int. 2012;49:501. [ Links ]
12. Tadhani MB, Patel VH, Subhash R. Food Compos Analysis. 2007;20:323. [ Links ]
13. Blois MS. Nature. 1958;181:199. [ Links ]
14. Milardovic S, Ivekovic D, Grabaric BS. Bioelectrochem. 2006;68:175. [ Links ]
15. Milardovic S, Ivekovic D, Ruwenjak V, et al. Electroanalysis. 2005;17:1847. [ Links ]
16. Ciesla L, Kryszen J, Stochmal A, et al. Pharm Biomed Anal. 2012;70:126. [ Links ]
17. Chandrasekar D, Madhusudhana K, Ramakrishna S, et al. Pharm Biom Anal. 2006;40:460. [ Links ]
18. Shi S, Ma Y, Zhang Y, et al. Sep Purif Tech. 2012;89:225. [ Links ]
19. Qiu J, Chen L, Zhu Q, et al. Food Chem. 2012;135:2366. [ Links ]
20. Yamaguchi T, Takamura H, Matoba T, et al. Biosci Biotech Biochem. 1998;62:1201. [ Links ]
21. Magalhaes L M, Santos M, Segundo MA, et al. Talanta. 2009;77:1559. [ Links ]
22. Ukeda H, Adachi Y, Sawamura M, Talanta. 2002;58:1279. [ Links ]
23. Polasek M, Skala P, Opletal L, et al. Anal Bioanal Chem. 2004;379:754. [ Links ]
24. Koleckar V, Opletal L, Brojerova E, et al. J Enzym Inhib Med Chem. 2008;23:218. [ Links ]
25. Magalhaes L M, Segundo M A, Reis S, et al. Anal Chim Acta. 2006;558:310. [ Links ]
26. Steinberg IM, Milardovic S. Talanta. 2007;71:1782. [ Links ]
27. Umezawa Y, Buhlmann P, Umezawa K, et al. Pure Appl Chem. 2000;72:1851. [ Links ]
28. Locatelli M, Gindro R, Travaglia F, et al. Food Chem. 2009;114:889. [ Links ]
29. Sharififar F, Moshafi M H, Mansouri S H, et al. Food Control. 2007;18:800. [ Links ]
30. Gulcin I, Beydemir S, Lici H A, et al. Pharm Res. 2004;49:59. [ Links ]
31. Loftsson T, Brewster M E. Pharm Sci. 1996;85:1017. [ Links ]
32. Adhikari B, Majumdar S. Prog Polym Sci. 2004;29:699. [ Links ]
33. Cunningham A J. Introduction to bioanalytical sensors. New York:Wiley;1998. p. 113. [ Links ]
34. Stefan R, Staden J V, Aboul-Enein H. Talanta. 1999;48:1139. [ Links ]
35. Foti M C, Daquino C, Geraci C. J Org Chem. 2004;69:2309. [ Links ]
36. Magalhaes L M, Segundo M A, Siquet C, et al. Microchim Acta. 2007;157:113. [ Links ]
37. Stasko A, Brezova V, Biskupic S, et al. Free Rad Res. 2007;41:379. [ Links ]
38. Ionita P. Chem Pap. 2005;59:11. [ Links ]
39. Gourine N, Yousfi M, Bombarda I, et al. Ind Crops Prod. 2010;31:203. [ Links ]
*Corresponding author. E-mail address: monahamdyph@yahoo.com
Received 16 May 2013; accepted 3 August 2014














