Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.32 no.5 Coimbra set. 2014
https://doi.org/10.4152/pea.201405315
The Inhibition of Mild Steel Corrosion in an Acidic Medium by the Aqueous Extract of Leaves of Polyalthia Longifolia
O. Chinyem* , D.E. Ogbeifun and M.O. Edemab
Department of Chemistry, University of Benin, Benin City, Edo State, Nigeria
Abstract
The inhibitive effect of aqueous extract of leaves of Polyalthia longifolia on the corrosion of mild steel was studied using the gravimetric (weight loss) method at the temperatures 303 and 318 K. The results show that the aqueous extract of Polyalthia longifolia leaves inhibited the corrosion of mild steel in dilute H2SO4 solution. It was found that the inhibition efficiency increased with inhibitor concentration and decreased with rise in temperature. Values of the activation energy of the inhibited corrosion reaction range between 45.40 kJ/mol and 62.22 kJ/mol. This is much higher than the 25.74 kJ/mol obtained for the blank. The adsorption of the extract was spontaneous and occurs according to Flory-Huggins adsorption isotherm. The corrosion inhibition of Polyalthia longifolia leaves extract was attributed to the adsorption of phytochemical molecules present in the extract onto the surface of the mild steel. Physical adsorption mechanism has been proposed for the adsorption of the inhibitor.
Keywords: Polyalthia longifolia, mild steel, corrosion, inhibitor, adsorption.
Introduction
Mild steel is the most common form of steel. It provides material properties that are acceptable for many industrial applications. The failure of mild steel structures in contact with aqueous solutions attributed to corrosion is not new to the scientific community [1]. The attempt by scientists to solve this problem has often resulted in the use of certain compounds as corrosion inhibitors in mild steel-corrodent systems [1]. The use of inhibitors during industrial processes such as acid cleaning, prickling, descaling, etching, etc., has proven to be one of the best methods of protecting metals against corrosion [2-4]. Several inhibitors in use are either synthesized from cheap raw materials or chosen from compounds having hetero-atoms in their aromatic systems or long carbon chain [5].
Unfortunately, many of these inhibitors used are toxic to our environment. Thus, the search for green corrosion inhibitors became essential.
Green corrosion inhibitors are biodegradable and do not contain heavy metals or other toxic compounds [6]. The use of naturally occurring substances to inhibit the corrosion of metals in acid and alkaline environment has been reported by several researchers [8-31]. The present study is aimed at investigating the adsorption and inhibitive properties of aqueous extract of leaves of Polyalthia longifolia for the corrosion of mild steel in dilute H2SO4 solution. Polyalthia longifolia (Indian mast/Masquerade tree) is a lofty evergreen tree. Though found natively in India and Sri-lanka, P. longifolia has been introduced in many tropical countries around the world including Nigeria. It is a member of the Annonaceae (sugar apple family). The weeping, branching habit of its 25 foot tall tree gives it a narrow columnar shape. The leaves are glossy-green, long, narrow and have attractive wavy edges. The tree in actual sense is commonly seen as a lofty column, very graceful with its downward - sweeping branches and shining, green foliage; but sometimes wide - spreading slender branches issue from the straight trunk and form a compact symmetrical crown. The bark is smooth and dark-grayish brown.
The plant has been used in traditional system of medicine for the treatment of fever, skin diseases, diabetes, hypertension and helminthiasis [33]. A number of biologically active compounds have been isolated from this plant [34-36]. The plant extract and isolated compounds were studied for various biological activities like antibacterial activity, cytotoxicity, antifungal activity [37-40].
Experimental
A mild steel sheet of purity 97% Fe was mechanically press-cut into coupons, each having a dimension of 4.5 × 4 × 0.12 cm. Each coupon was degreased with absolute ethanol, dipped in acetone and allowed to dry in air. The treated coupons were then stored in moisture - free desiccators before their use for corrosion studies. All other reagents used for the study were obtained from BDH and were of Analar grade. Deionised and doubly distilled water was used throughout.
Extraction of plant
Fresh leaves of Polyalthia longifolia were obtained from the premises of the University of Benin, Benin City, Nigeria. The leaves were air-dried and ground to powder. 200 g of the ground sample were boiled in 1000 mL of distilled water for 20 mins and filtered. The filtrate was evaporated over a hot water bath to yield the dried-leaf decoction extract. Different concentrations were prepared by dissolving 0.1, 0.2, 0.3 and 0.4 g of the extract in 1 L of 0.5 M H2SO4.
Phytochemical analysis
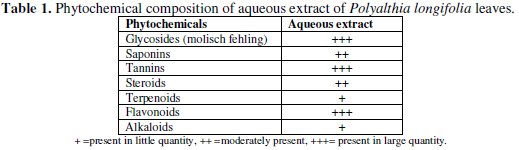
Qualitative chemical tests were conducted for the above extract of Polyalthia longifolia leaves to identify the various phytochemical constituents [41] (Table 1).
Three 250 mL beakers which separately contained 0.1, 0.25 and 0.5 M H2SO4 solutions were placed in a water bath maintained at 318 K. Into each of these beakers was suspended a previously weighed mild steel coupon. Another set of three 250 mL beakers each containing 0.1, 0.25 and 0. 5 M H2SO4 solutions was maintained at 303 K. Into each of these was also suspended a previously weighed mild steel coupon.
250 mL of each test solution containing different inhibitor concentration was immersed in a water bath and maintained at 318 K. Into each of these beakers was also suspended a previously weighed mild steel coupon.
These coupons were retrieved at 24 h interval progressively for 168 h (7 days). Each retrieved coupon was washed several times in 20% NaOH containing 200 g/ L of zinc dust until clean, dried in acetone and reweighed [1]. The weight loss was evaluated in grams. A reading report represents the average of three readings recorded on a Mettler Toledo analytical balance to the nearest 0.0001 g.
The difference in weight for a period of 168 h was taken as total weight loss. From the weight loss results, the inhibition efficiency (%I) of the inhibitor, degree of surface coverage (Θ) and corrosion rate (CR) were calculated using eq. 1, 2 and 3, respectively [42]
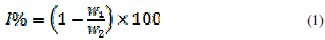


where W1 and W2 are the weight loss (g/dm3) for mild steel in the presence and absence of the inhibitor in H2SO4 solution, respectively, θ is the degree of surface coverage of the inhibitor, A is the area of mild steel (cm2), t is the time of immersion (h) and W is the weight loss of the mild steel after time t.
Results and discussion
Effect of concentration and temperature
Figs. 1 and 2 show the variation of weight loss with time for corrosion of mild steel in 0.1 M, 0.25 M and 0.5 M H2SO4 at 303 and 318 K, respectively.
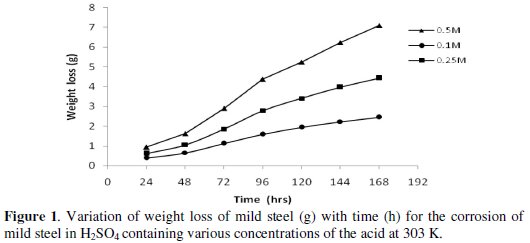
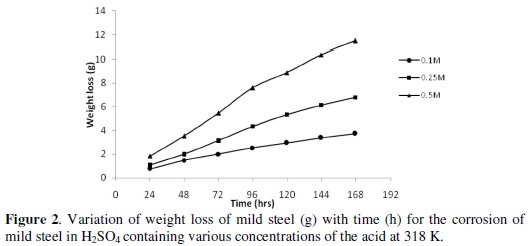
Inspection of the figures reveals that the weight loss of mild steel in H2SO4 increases with time and corrodent concentration. At 318 K, the values obtained for weight loss are relatively higher than those obtained at 303 K, indicating that the rate of corrosion of mild steel in H2SO4 increased with temperature.
Figs. 3 and 4 show the variation of weight loss with time for the corrosion of mild steel in 0.5 M H2SO4 (control) and in various amounts of P. longifolia in 0.5 M H2SO4 at 303 K and 318 K, respectively.
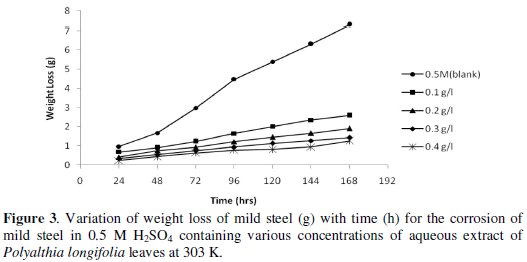
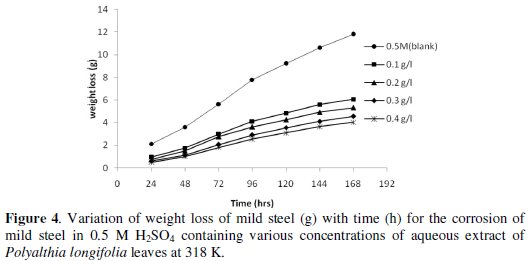
It is evident that the weight loss of mild steel in H2SO4 increases with increase in the time of contact, but decreases with increase in the concentration of aqueous extract of leaves of P. longifolia, indicating that the extract inhibits the corrosion of mild steel in H2SO4.
In the presence of the inhibitor, at 318 K, the values obtained for weight loss were relatively higher than the values obtained at 303 K, indicating that the rate of corrosion of mild steel in H2SO4 increases with temperature. This may be due to the competition between the forces of adsorption and desorption.
Corrosion rates of the mild steel sample in 0.5 M H2SO4 in the absence and presence of different concentrations of the aqueous extract of P. longifolia leaves were determined. The results obtained are represented in Table 2.
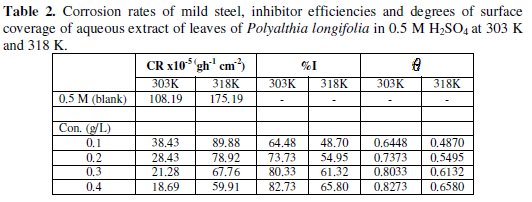
The corrosion rate decreases with increase in concentration of the extract. This indicates that the aqueous extract of P. longifolia inhibits the corrosion of mild steel in H2SO4 and the extent of corrosion inhibition depends on the amount of the extract present.
It can be seen from Table 2 that the inhibition efficiency of P. longifolia varies with its concentration. Maximum value of inhibition efficiency (82.73%) was obtained at extract concentration of 0.4 g/L, while the least value was obtained at extract concentration of 0.1 g/L.
Fig. 5 shows the variation of the inhibition efficiency against the different extract concentrations at 303 K and 318 K.
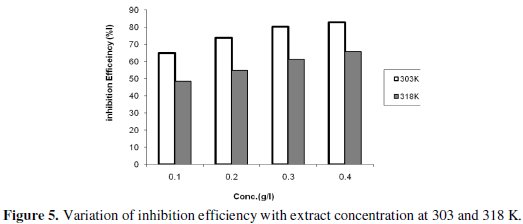
It is observed that the inhibition efficiency increases with increasing extract concentration but decreases with increase in temperature, suggesting that the extract is a corrosion inhibitor and that the mechanism of adsorption is physical. For a physical adsorption mechanism, inhibition efficiency of an inhibitor decreases with temperature, while for a chemical adsorption mechanism, values of inhibition efficiency increase with temperature [43-45]. The degree of protection increases with increase of the surface fraction occupied by adsorbed molecules. As the extract concentration increased, the number of the adsorbed molecules on the surface increased.
Parameter θ, which is estimated from the inhibition efficiency values, could be used to represent the fraction of the surface occupied by the adsorbed molecule [9].
Thermodynamic and adsorption consideration
Values of activation energy (Ea) for the corrosion of mild steel in presence and absence of different extract concentrations have been calculated using Arrhenius equation [31, 36].

Taking logarithm of both sides of eq. 4, eq. 5 is obtained
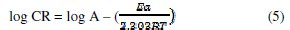
where CR is the corrosion rate of mild steel, A is the Arrhenius constant or pre-exponential factor, Ea is the activation energy of the reaction, R is the gas constant and T is the temperature. Considering a change in temperature from 303 K (T1) to 318 K (T2), the corresponding values of corrosion rates at these temperatures are ρ1 and ρ2, respectively. Inserting these parameters into eq. 5, eq. 6 is obtained
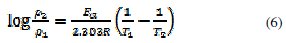
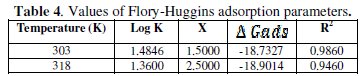
Values of activation energy (Ea) calculated from eq. 6 for the aqueous extract are recorded in Table 3.
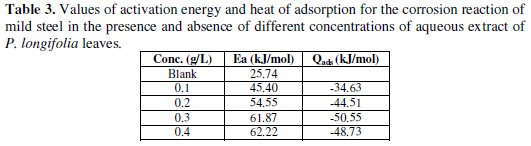
These values were found to range from 45.40 kJ/mol to 62.22 KJ/mol with an average value of 56.01 kJ/mol. The result obtained indicates that the adsorption of the extract is consistent with the mechanism of physical adsorption. For a physical adsorption mechanism, the activation energy should be less than 80 kJ/mol [7, 37-40]. Also, the values of Ea obtained in the presence of the aqueous extract of P. longifolia leaves were higher than the value of 25.74 kJ/mol obtained for the blank, indicating that the extract inhibited the corrosion of mild steel in dil. H2SO4. The activation energies were also observed to increase with increasing the concentration of the extract, indicating that there is increasing ease of adsorption of inhibitors with increasing the concentration [7].
Values of heat of adsorption of P. longifolia leaves extract on mild steel surface were calculated using eq. 7.
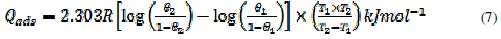
where θ1 and θ2 are the degrees of surface coverage at temperature T1 (303 K) and T2 (318 K), respectively. Values of Qads calculated from eq. 7 are recorded in Table 3. These values are negative, indicating that the adsorption of P. longifolia leaves extract on mild steel surface is exothermic. The negative values also show that the adsorption and hence the inhibition efficiency decreases with rise in temperature. Similar observation has also been reported by other workers [7, 37-39, 41].
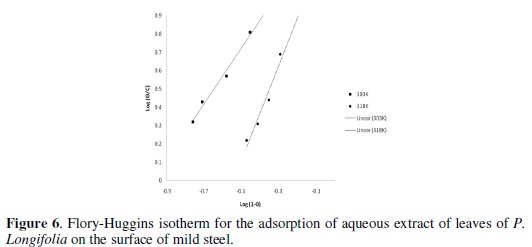
The adsorption characteristics of the inhibitor were also studied by fitting the data obtained for degree of surface coverage into different adsorption isotherms. The tests revealed that the adsorption of aqueous extract of leaves of Polyalthia longifolia on the surface of mild steel is best described by Flory-Huggins adsorption isotherm.
The assumption of Flory-Huggins adsorption isotherm can be expressed by eq. 8 [36],
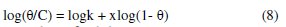
where x is the number of inhibitor molecules occupying one site (or the number of water molecules replaced by one molecule of the inhibitor), C is the concentration of the inhibitor, θ is the degree of surface coverage and K is the equilibrium constant of adsorption. Linear plots were obtained when log(θ/C) was plotted against log(1-θ), confirming the applicability of Flory-Huggins isotherm to the adsorption of aqueous extract of leaves of Polyalthia longifolia on the surface of mild steel. Values of the adsorption parameters deduced from Flory-Huggins plots are represented in Table 4.
The equilibrium constant of adsorption of aqueous extract of leaves of Polyalthia longifolia on the surface of mild steel is related to the free energy of adsorption (ΔGads according to eq. 9 [36, 42-43]
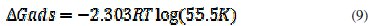
where R is the gas constant, T is the temperature and K is the equilibrium constant of adsorption.
Values of free energy of adsorption on mild steel surface were calculated from the plot of the isotherm (Fig. 6).
Calculated values of ΔGads are recorded in Table 4.
These values are negative and ranged from -18.7327 kJ/mol at 303 K and 18.9014 kJ/mol at 318 K. This indicates that the adsorption of the aqueous extract of P. longifolia leaves is spontaneous and occurs via physical adsorption mechanism. Generally, values of ΔGads up to -20 KJ/mol are consistent with electrostatic interaction between the charged metal and charged molecules, which signifies physical adsorption, while for chemical adsorption, the values are more negative than -40 kJ/mol [7, 37-39, 41, 44].
Phytochemical analysis of aqueous extract of Polyalthia longifolia leaves shows that it contains tannins, saponins, glycosides, flavonoids, etc. Plant materials that have been successfully utilized for corrosion inhibition of most metals have been reported to contain such chemicals [45].
Conclusions
Our present report shows that the aqueous extract of leaves of Polyalthia longifolia can be used as an inhibitor for mild steel corrosion. The inhibition action is performed via adsorption of the extract onto the mild steel surface according to the Flory-Huggins adsorption isotherm. The adsorption of the inhibitor is spontaneous and exothermic and follows the physical adsorption mechanism.
In view of the above conclusion, the use of the aqueous extract of leaves of Polyalthia longifolia as an inhibitor is recommended.
References
1. Ita B I, Abakedi O U, Onuchukwu A I. J Chem Soc Nigeria. 2007;32:179. [ Links ]
2. Ashassi-Sorkabi H, Ghalebsaz-Jeddi N. Mater Chem Phys. 2005;92:480. [ Links ]
3. Emregul K C, Atakol O. Mater Chem Phys. 2003;82:188. [ Links ]
4. Ekop A S, Eddy N O. Australian J Basic Appl Sci. 2008;2:1258. [ Links ]
5. Abdallah M. Corros Sci. 2002;44:717. [ Links ]
6. Eddy N O, Ebenso E E. Afri J Pure Appl Chem. 2008;2:046. [ Links ]
7. EI-Etre A Y. Corros Sci. 1998;40:1845. [ Links ]
8. EI-Etre A Y. Corros Sci. 2003;45:2485. [ Links ]
9. EI-Etre A Y. Appl Surface Sci. 2006;252:8521. [ Links ]
10. Ebenso E E, Ekpe U J, Ibok U J. Discov Innov. 1998;10:52. [ Links ]
11. Ebenso E E, Ibok U J, Ekpe U J, et al. Trans SAEST. 2004;39:117. [ Links ]
12. Ebenso E E, Ekpe U J. West Afri J Biol Appl Chem. 41:21. [ Links ]
13. Ekpe U J, Ebenso E E Ibok U J. J W Afri Sci Assoc. 1994;37:13. [ Links ]
14. Umoren S A, Obot I B, Ebenso E E, et al. Anti-Corros Methods Mater. 2006;53:277. [ Links ]
15. Umoren S A, Obot I B, Ebenso E E. E-Journal Chem. 2008;5:355. [ Links ]
16. Umoren S A, Obot I B, Ebenso E E et al. Port Electrochim Acta. 2008;26:199. [ Links ]
17. Umoren S A, Obot I B, Ebenso E E et al. Port Electrochim Acta 2008;26:267. [ Links ]
18. Umorem S A, Ogbobe O, Igwe I E et al. Corros. Sci. 2008;40:1998. [ Links ]
19. Umoren S A, Ebenso E E. Pigment Resin Technol. 2008;37:137. [ Links ]
20. Abdallah M. Port Electrochim Acta. 2004;22:161. [ Links ]
21. Okafor P C, Ekpe U J, Ebenso E E, et al. Bull Electrochem. 2005;21:347. [ Links ]
22. Okafor P C, Osabor V I, Ebenso E E. Pigment Resin Techol. 2007;36:299. [ Links ]
23. Okafor P C, Ikpi M I, Uwah I E, et al. Corros Sci. doi:10.1016/j.corsci.2008.05.009.(press). [ Links ]
24. EI-Etre A Y, Abdallah M. Corros Sci. 2000;42:731. [ Links ]
25. Chetouani A, Hammouti B, Benkaddour M. Pigment Resin Techol. 2004;33:26. [ Links ]
26. Oguzie E E. Mater Chem Phys. 2006;99:441. [ Links ]
27. Oguzie E E. Pigment Resin Technol. 2006;35:334. [ Links ]
28. Oguzie E E. Corros Sci. 2007;49:1527. [ Links ]
29. Oguzie E E, Onuchukwu A I, Okafor P C, et al. Pigment Resin Technol. 2006;35:63. [ Links ]
30. Oguzie E E, Onuoha G N, Ejike E N. Pigment Resin Technol. 2007;36:44. [ Links ]
31. Kirtikar K R, Basu B D. Indian medicinal plants. Dehradhun: International Book Distributors; 1995. p. 562. [ Links ]
32. Wu Y C, Duh C Y, Wang S K, et al. J Nat Prod. 1990;53:1327. [ Links ]
33. Zhao G X, Jung J H, Smith D L, et al. Planta Med. 1991;57:380. [ Links ]
34. Chakrabarty M, Amarnath C A. J Nat Prod. 1992;55:256. [ Links ]
35. Murthy M M, Bindu M S M H, Annapurna J. Fitoterapia. 2005;76:336. [ Links ]
36. Stevigny C, Bailly C, Quertin-Leclercq J. Curr Med Chem Anticancer Agents. 2005;6:581. [ Links ]
37. Nair R, Chanda S. Indian J Pharmacol. 2006;38:142. [ Links ]
38. Jain A K, Jain A, Jain S, et al. Plant Arch. 2006;6:841. [ Links ]
39. Sofowora A. Medicinal plant and traditional medicine in Africa. John Wiley and Sons Ltd; 1982. p. 128-145. [ Links ]
40. Eddy N O, Mamza P A P. Port Electrochim Acta. 2009;27:443. [ Links ]
41. Ebenso E E. Mater Chem Phys. 2003;79:58. [ Links ]
42. Ebenso E E. Bull Electrochem. 2003;19:209. [ Links ]
43. Ebenso E E. Bull Electrochem. 2004;20:551. [ Links ]
44. Sheatty D S, Shetty P, Nayak H V S. J Chidean Chem Soc. 2006;51:849. [ Links ]
45. Bhajiwala H M, Vashi R T. Bull Electrochem. 2001;17:441. [ Links ]
46. El-Etre A Y, Abdallah M, El-Tantawy Z E. Corros Sci. 47 (2005) 385.
47. Odoemelam S A, Eddy N O. J Surface Sci Technol. 2008;24:1. [ Links ]
48. Bilgic S, Sahin M. Mater Chem Phys. 2001;70:290. [ Links ]
49. Ebenso E E, Eddy N O, Odiongenyi A O. Afri J Pure Appl Chem. 2008;4:107. [ Links ]
*Corresponding author. E-mail address: chinyemogor@yahoo.com
Received 3 June 2014; accepted 2 September 2014














