Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.31 no.6 Coimbra nov. 2013
https://doi.org/10.4152/pea.201306307
EIS Study of Amine Cured Epoxy-silica-zirconia Sol-gel Coatings for Corrosion Protection of the Aluminium Alloy EN AW 6063
I. Rute Fontinhaa,* , M. Manuela Saltaa , Mikhail L. Zheludkevichb and Mário G.S. Ferreirab
a LNEC - Laboratório Nacional de Engenharia Civil, Lisboa, Portugal
b UA - Universidade de Aveiro, CICECO, Departamento de Engenharia de Materiais e Cerâmica, Aveiro, Portugal
Abstract
The organic-inorganic hybrid sol-gel films, the structure of which comprises interconnected inorganic and organic networks have been reported as an environmentally friendly anti-corrosion pre-treatment for several metals, including aluminium alloys. In this paper, an epoxy-silica-zirconia hybrid sol-gel coating was synthesized from glycidoxypropyltrimethoxysilane (GPTMS) and zirconium npropoxide (TPOZ) precursors and applied to EN AW-6063 alloy by dip-coating. To promote the organic network formation through the epoxy group polymerization at room temperature, two types of amine crosslinkers were added during synthesis: diethylenetriamine (DETA), in different concentrations, and a tri-functional aminosilane. The evolution of the curing process and the corrosion behaviour of the coated aluminium alloy specimens were evaluated by Electrochemical Impedance Spectroscopy (EIS) in 0.5 M NaCl. The morphology and surface chemistry of the hybrid coatings were characterized by Energy Dispersive Spectroscopy (EDS) coupled with Scanning Electron Microscopy (SEM) and by Fourier Transform Infrared Spectroscopy (FTIR). The results obtained revealed that the sol-gel coatings with lower amine ratios required longer curing times, but showed the best anticorrosive performance with time. The increase in amine concentration has led to a more cross linked organic network, resulting in higher initial coatings resistance; however it has turned coatings more hydrophilic, prone to rapid degradation in water.
Keywords: sol-gel hybrid coating, silane, corrosion, EIS, aluminium.
Introduction
The aluminium alloys offer a unique combination of properties such as good mechanical resistance with a high strength to weight ratio, design flexibility and good corrosion resistance due to the spontaneous oxide layer formed on their surface, allied to a cradle-to-cradle life cycle. These properties make them widely used in several industries, including the building industry, which consumes around 30% of the aluminium products in Western Europe [1], in particular, those of 6000 series alloys. This type of alloys present good corrosion resistance in mild corrosive environments; however, develops pitting in marine highly polluted environments. Therefore, to fulfil long term service life requirements and to reduce maintenance needs, keeping an appropriate aesthetic appearance, aluminium building components are often organically coated which requires alloy surface pre-treatment and a conversion layer to improve adhesion. This pretreatment is often based on toxic Cr(VI) which, since 2007, has been under great restrictions due to environmental concerns, and should be banned from pretreatment industry in Europe until September of 2017 [2]. It could be estimated from European Aluminium Association data that around 1.12 Mton of aluminium alloys were powder coated in Europe, in 2011, mostly for building applications. This shows the importance of the development of a chromium-free technology for the aluminium pre-treatment industry. Presently, there are already several alternative pre-treatments in use; however, they lack the anticorrosive action of the Cr(VI) compounds.
Organic-inorganic hybrid silane based sol-gel coatings have been reported as a promising environmentally friendly alternative to Cr(VI) conversion layers for several metals, since they, not only exhibit barrier effect and compatibility with organic coatings, but also have the ability to incorporate corrosion inhibitors [3-12]. These types of coatings present combined mechanical and chemical properties typical of inorganic ceramics and of organic polymers. Among these, the epoxy-silane based hybrid sol-gel coatings are of particular interest due to the increased properties of flexibility, density and functional compatibility with organic coatings, achieved as a result of the epoxide organic group present [5,7-15]. During the synthesis of these coatings occurs simultaneously the formation of an organic network through epoxide rings opening and polymerization, and of an inorganic siloxane network through the hydrolysis and subsequent condensation of the silicon alkoxide groups [16]. Uncatalysed organic polymerization usually requires elevated temperature to complete the process. However, by the addition of amine crosslinking agents it is possible to promote the organic network formation at low temperature [17], with inherent energy savings.
The corrosion behaviour of amine-cured epoxy-silane sol-gel coatings has been studied by different authors [14,15,17-22]. The diethylenetriamine (DETA) is one of the most common epoxy crosslinkers used and an optimum amine concentration in terms of the best anticorrosive properties achieved by the coatings was reported by Vreugdenhil et al. [18] and by Davis et al. [17] of, respectively, 1.3 and 1 relative to the molar ratio ''epoxy group/amine reactive hydrogen''. Khramov et al. [19] also studied epoxy-silane sol-gel coatings crosslinked with amino-silanes and found significant improvement in these coatings corrosion performance in comparison to those of DETA crosslinked ones. In another study involving the addition of amino-silane crosslinkers [20], the above optimum molar ratio found ranged between 1 and 1.5. The aminosilanes present the advantage of contributing also to the inorganic network formation. Besides amino-silanes, other amines have been studied as alternative to DETA like di-amines of longer carbon chain [15] or branched amines [21,22], the resultant coatings showed better corrosion protection properties compared to those derived from formulations containing DETA as crosslinking agent. The epoxy-silane based coatings curing process (by heating or by the addition of crosslinking agents) is determinant for the establishment of both inorganic and organic networks of these hybrid coatings and should be appropriate to the precursors used in their synthesis. All the studies referred are focused on solely silane derived sol-gel coatings. In this work, a zirconium alkoxide precursor is used in addition to the epoxy-silane one to produce the hybrid sol-gel coating for corrosion protection of the aluminium alloy EN AW 6063. This type of sol-gel coatings usually is thermally cured [7,16]. The aim of this work is to study the corrosion properties of epoxy-silica-zirconia hybrid sol-gel coatings cured at room temperature by the addition of amine crosslinkers. Therefore, in the present work, epoxy-silica-zirconia hybrid sol-gel coatings were synthesized from glycidoxypropyltrimethoxysilane (GPTMS) and zirconium n-propoxide (TPOZ) precursors, applied to the aluminium alloy by dip-coating and cured at room temperature using two types of amine crosslinkers: diethylenetriamine (DETA), in different concentrations (GPTMS/amine-Hreactive molar ratios: 1.5 and 1), and a tri-functional amino-silane in that molar ratio of 1. A sol-gel coating prepared from the same precursors but without amine addition was also synthesized for comparison. The evolution of the curing process and the corrosion behaviour of the hybrid coated aluminium alloy specimens were evaluated by Electrochemical Impedance Spectroscopy (EIS). The morphology and surface chemistry of the hybrid coatings were also characterized by Energy Dispersive Spectroscopy (EDS) coupled to Scanning Electron Microscopy (SEM) and by Fourier Transform Infrared Spectroscopy (FTIR).
Experimental
Reagents and materials
Glycidoxypropyltrimethoxysilane (GPTMS), zirconium n-propoxide (TPOZ) 70% in propanol, 2-propanol, ethylacetoacetate, diethylenetriamine (DETA) and 3-[2-(2-aminoethylamino)ethylamino]propyl-trimethoxysilane (3A) were purchased from Aldrich and used as received. Nitric acid (65%, Merk) was used to acidify the water solution used to promote hydrolysis. Ultra-pure water (0.055 0.060 μS/cm) obtained from a Purelab Ultra System (Elga) was used. Aluminium test specimens, 3 cm × 7 cm × 1.1 cm, of commercial EN AW 6063 alloy [23] were used. Before coating deposition, the aluminium alloy samples were degreased with ethanol and then cleaned by immersion in an alkaline aqueous solution containing 50 g/L of P3 Almeco 18C (Henkel) for 10 min at 60 °C, followed by immersion in a 20% (wt) HNO3 solution for 15 min and finally rinsed with deionized water.
Sol-gel synthesis and coating deposition
The hybrid coating was synthesized from GPTMS and TPOZ precursors, based on the procedure described in [7], hydrolyzed separately, under acidic conditions at room temperature. The organo-siloxano sol was obtained by mixing GPTMS in 2-propanol (1:1 volume ratio) with diluted nitric acid and stirring it for 30 minutes. The inorganic sol was prepared by addition of TPOZ (70% in 2propanol) to the complexing agent ethylacetoacetate (1:1 volume ratio), stirring it for 20 minutes, and then, diluted nitric acid was added and stirring was extended for another 60 minutes. After this time, the two sols were mixed and stirred for 1 h, followed by 1 h ageing at room temperature. The amine crosslinking agent was added to the hybrid sol ten minutes prior coating the aluminium samples. The amount of amine added was calculated to yield a GPTMS/amine-Hreactive molar ratio of 1 and 1.5 for DETA, and of 1 for the amino-silane 3A. One coating was prepared without amine addition for comparison. The Zr/Si molar ratio in each final coating is 0.26. Table 1 resumes the different coatings prepared and respective identification.
The sol-gel coatings were applied to the previously cleaned aluminium EN AW 6063 alloy samples, by dipping and withdrawal at a speed of 18 cm/min, after a residence time of 100 s, using a dip-coater (Nima, model DC Small). After coating, the aluminium samples were left to dry at room temperature for 24 h (or 72 h in the case of the coating prepared without amine) and then stored in a desiccator before testing.
Experimental techniques
The evolution of the curing process and corrosion behaviour of the different aluminium alloy coated samples was evaluated by Electrochemical Impedance Spectroscopy (EIS) in neutral 0.5 M NaCl solution. The EIS tests were performed at room temperature, in a Faraday cage, with the solution exposed to air, with a Gamry Potentiostat REF600-06704, in the frequency range of 100 kHz-10 mHz, applying a 10 mV sinusoidal perturbation at OCP, with 10 to 7 points per decade logarithmically distributed. The test area was 1.34 cm2. A three-electrode cell was used, with a saturated calomel electrode (SCE) as reference, a platinum wire as counter-electrode and the coated aluminium alloy sample as working electrode. Two to four replicates were tested for each coating type. Gamry Echem Analyst software version 5.3 was used for impedance curves fitting to the appropriate equivalent circuits.
Surface coatings observation and chemical elemental analysis were carried out before immersion with a JEOL JSM-6400 scanning electron microscope with a coupled EDS detector (Inca-xSight, Oxford Instruments). Coatings chemical structure was carried out by specular reflectance Fourier Transform Infrared (FTIR) spectroscopy with a Nicolet Magna IR-550 spectrometer, between 4000- 450 cm-1 wavelengths, with a 4 cm-1 resolution.
Results and discussion
Coatings surface characterization by SEM/EDS
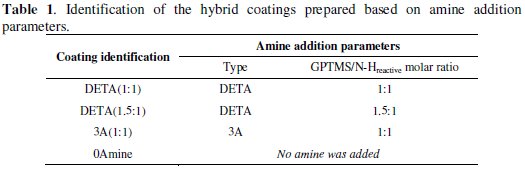
SEM observations of the hybrid sol-gel coatings synthesized revealed a very smooth homogeneous, crack-free surface for all coatings. However, there are a few pinhole-like defects which are more noticeable in the amino-silane (3A) cured coating. Figure 1 shows an example of one of these coating defects on an aluminium sample with the 3A cured coating in comparison with a defect-free surface of a DETA cured coated sample.
The EDS spectra presented in Fig. 1 reveal that in the defect area, the hybrid sol-gel coating is almost absent. Consequently, these areas may suffer early corrosion processes.
The results of several elemental chemical composition analysis by EDS carried out in the different synthesized hybrid coatings surface on defect-free areas indicate the following Al/Si ratios: 3A(1:1)-0.01; DETA(1:1)-0.2; 0Amine-0.8. Higher Al/Si ratios suggest higher coating thickness.
Coatings chemical structure characterization by FTIR
Figs. 2 and 3 display, respectively, the FTIR spectra obtained for the different amine cured hybrid coatings and for the hybrid coating prepared without amine.
FTIR peak/band assignment was done based on the literature.
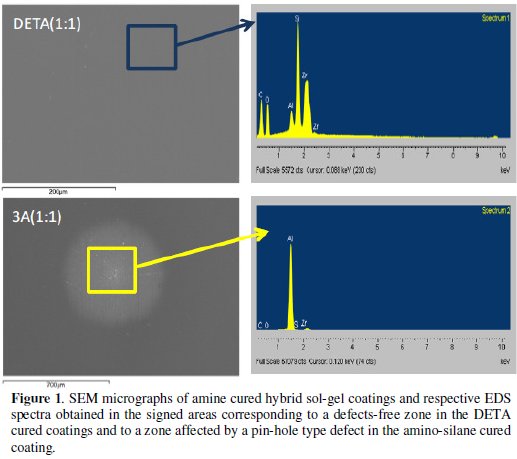
The FTIR spectra relative to the amine cured hybrid coatings (Fig. 2) were obtained 12 weeks after coating deposition on aluminium samples. These spectra exhibit practically the same peaks/bands although with some differences on their relative intensity. The band in the 3700-3100 cm-1 range is related with the presence of OH groups (from hydrolysis products, alcohol residuals and absorbed water) [16,24,26] and with primary and secondary N-H groups that also absorb in this range [18,22,27]. The peaks at 2931 cm-1 and 2870 cm-1 are assigned to C-H stretching vibration bands in the alkyl groups [16,26] and also to N-H vibrations [18]. The intermediate band at 1640 cm-1 is attributed to the bending vibration of water molecules absorbed [12,22]. The peak near 1600 cm-1, more notorious in the high amine cured coatings DETA(1:1) and 3A(1:1), can be assigned to N-H deformation modes in the -NH2 group, indicating that some unreacted crosslinking agent was left [18,22]. These groups add hydrophilicity to the coatings, confirmed by the presence of the absorbed water band. The intense peaks in the 1120-1010 cm-1 range are characteristic of vibration bands of Si-O- Si bonds [6,11,16,24-26] of the inorganic network, but the intense peak at 1120 cm-1 can also be assigned to the stretching vibrations of C-N-C bonds, namely, those formed in the organic polymerization reactions [18]. Thus, the extent of the inorganic network crosslinking is difficult to ascertain in the amine cured epoxysilane sol-gel coatings since the Si-O-Si band can be overlapped by the C-N-C band. The increased absorption observed below 800 cm-1 is also due to primary and secondary N-H groups.
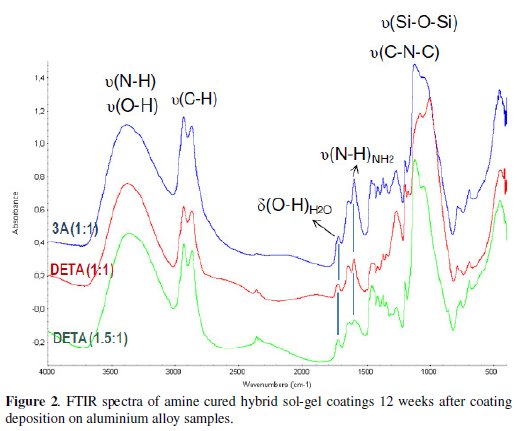
The epoxy ring characteristic absorption bands, namely the band at 1250 cm-1 (ring breathing), and the small peaks at 909 cm-1 (antisymmetric stretching) and at 856 cm-1 [16,18,22] that are visible in the hybrid coating prepared without amine addition spectra (Fig. 3), are not visible in the amine cured coatings FTIR spectra, suggesting an extensive organic network formation. The small band at 1270 cm-1, more intense in the high amine coating formulations is attributed to the stretching vibrations of C-O bond [27,28] in the ether, present in the opened epoxy ring products.
The FTIR spectra relative to the 0Amine coating (Fig. 3) were obtained 24 hours and 12 weeks after coating deposition on aluminium samples. The main peaks visible in both spectra are the ones related with the siloxane bonds (Si-O-Si), between 1100 cm-1 and 1050 cm-1, showing that the inorganic network should be well established at room temperature only 24 h after deposition, in opposition to the organic network, as evidenced by the presence of the epoxy ring absorption peaks. These peaks are less noticeable in the FTIR spectrum obtained after 12 weeks ageing, showing that the polymerization reactions involved in the organic network establishment proceed with time, although in a very slow rate. The inorganic network should have also improved with time, becoming more cross linked, as shown by the widening of the siloxane absorption band and the shifting of its maximum peak towards lower wavelengths [16]. The small peak at 1197 cm-1 near siloxane band in both spectra could be assigned to the stretching vibrations of Si-C bond [26].
The FTIR analysis results show that the amine addition was essential to achieve a higher extension of organic network formation in the hybrid coatings when compared to the coating prepared without amine. However, there is the risk of such extended organic network may imposing some constraints to the inorganic network formation due to geometrical reasons [17]. FTIR analyses have also indicated that some unreacted N-H groups are left in all amine cured coatings, what suggests that this crosslinking agent might be in excess. In this study a zirconium alkoxide was used as sol-gel precursor. Being a Lewis acid it also has the ability to catalyse epoxy ring opening reactions [16,29], reducing the number of epoxy rings available to react with amines. Therefore less amine than the amount added (calculated based on stoichiometric needs) would be necessary, justifying the unreacted N-H groups present in the sol-gel coating even after 12 weeks.
Electrochemical evaluation of coatings by EIS
To evaluate the corrosion behaviour, hybrid sol-gel coated aluminium samples were immersed in a 0.5 M NaCl solution for 15 days and EIS measurements were carried out during the immersion period. Before that, EIS measurements were carried out after 1 hour immersion in the same chloride solution at different times elapsed after coating deposition (curing time) to assess the evolution of the coatings' cure with time in terms of their barrier properties.
Evolution of cure with time
Figs. 4 to 6 show the impedance spectra obtained at different curing times for the aluminium alloy samples coated with the two types of amine (DETA, 3A) cured hybrid coatings and for the one without amine (0Amine).
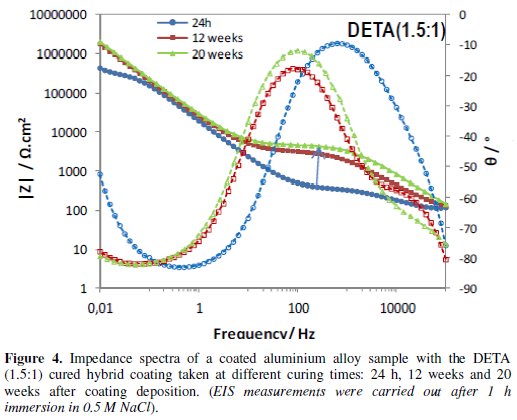
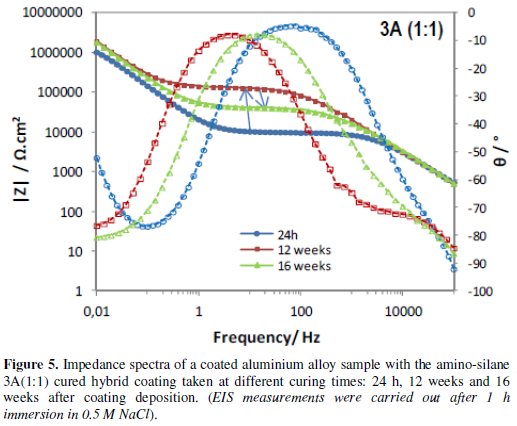
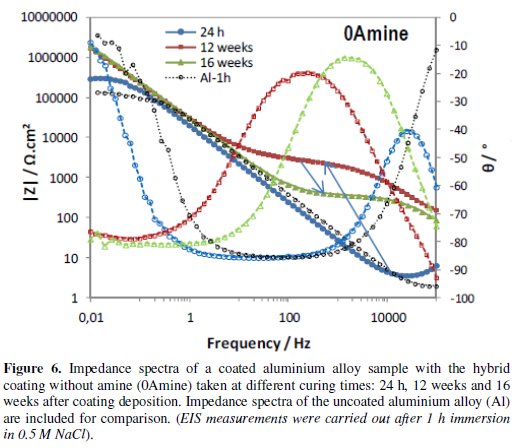
The impedance spectra of the uncoated aluminium alloy (Al) are included for comparison. At each curing time, EIS measurements were carried out after 1 h immersion in 0.5 M NaCl.
The EIS spectra obtained for the amine (DETA, 3A) cured coatings from the 24 hours curing time (Fig. 4 and 5) always present two time constants, which can be associated to the capacitive contribution of the sol-gel coating (in the 104 Hz-105 Hz range) and of the intermediate oxide layer (at ∼0.1 Hz) [7]. This intermediate oxide layer, constituted by Al-O-Si and Al-O-Zr bonds, results from condensation reactions between Zr-OH and Si-OH groups with Al-OH groups of the native oxide layer present in the metal surface [5,16]. Between the two capacitive regions, a resistive plateau can be observed in the 1 Hz -100 Hz range, associated to the coating resistance [7]. Its position at high impedance modulus indicates that these coatings present barrier properties 24 h after coating deposition. The same, however, does not apply to the coating prepared without amine addition, which EIS spectra (Fig. 6) obtained 24 h after coating deposition present only one broad time constant similar to the spectra obtained for the aluminium alloy sample without coating. In both cases, this relaxation process results only from the capacitance of the native oxide layer present in this type of alloy [30]. The absence coating impedance response indicates that the sol-gel coating does not have yet barrier properties, probably because the time elapsed was insufficient to complete the condensation reactions involved in the formation of the inorganic network, as also as to complete the reactions involved in the polymerization of the functional epoxy group necessary to establish the organic network, what is coherent with the FTIR analysis results. For longer curing times, EIS spectra already exhibit both capacitive and resistive contributions of the sol-gel coating, similarly to what was observed for the amine cured coatings. The 0Amine coating resistive plateau, however, is positioned at lower impedance values than for the amine cured coatings, indicating inferior barrier properties. According to EIS measurements all coatings improve barrier properties with time until 12 weeks, as the hybrid network becomes more crosslinked. For longer curing times (16 or 20 weeks) the amino-silane cured coating and the one prepared without anime suffer some kind of degradation, leading to a decrease in their barrier properties.
Evolution of anticorrosive properties
The continuous immersion in the 0.5 M NaCl solution started after the times used to evaluate the curing process (16 to 20 weeks after coating deposition). Figs. 7 and 8 present the typical impedance spectra obtained at the beginning and at the end of the immersion time (15 days) in the chloride solution, for the coated aluminium EN AW 6063 alloy samples with amine cured coatings and with the coating prepared without amine addition (0Amine).
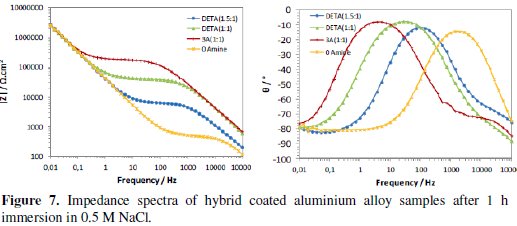
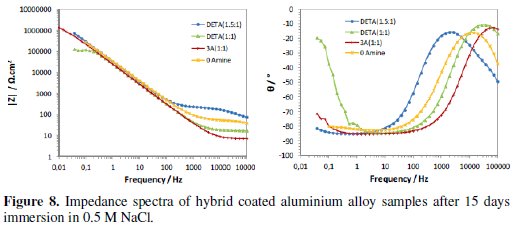
Initially the two time constants associated to the sol-gel coating at medium-high frequencies and to the intermediate oxide layer at low frequencies are visible (Fig. 7). The relative position of the resistive plateau associated to the coating resistance in terms of impedance modulus value allows the ranking of the different coatings' barrier properties: the amino-silane cured coating is the one with the best barrier properties and the coating prepared without amine addition has the worst ones. No signs of corrosion activity were visible in the EIS spectra obtained for this short immersion time.
With immersion time, the EIS spectra of all coatings evidence a marked decrease in the impedance at medium-high frequencies range (104Hz-10Hz), showing a great loss of sol-gel coatings' barrier properties, more pronounced for those with higher amine ratios, especially the amino-silane (3A) cured coating (Fig. 8). This decay is caused by water and chloride ions penetration into coating, hence, these two coatings should be more hydrophilic than the other two, what is in accordance with FTIR analysis results. At the end of the immersion time in the chloride solution, the DETA(1.5:1) cured coating is the only one showing some barrier ability.
The differences in the impedance response of the different sol-gel coated samples at the end of immersion time are also visible in the impedance spectra at low frequencies (<0.1 Hz), namely, in the lay-out of the phase angle curves (Fig. 8). Impedance response in this frequency region is related to the intermediate oxide layer properties and the onset of corrosion processes. The increase of the phase angle observed in the EIS spectra of the coatings with higher amine content suggests a less capacitive (thus more resistive) behaviour of this layer, also meaning that these two coatings are less corrosion resistant.
On the whole, the EIS corrosion evaluation indicates the DETA(1.5:1) cure coating as the one with best anticorrosive properties, followed by the coating prepared without amine addition. In spite of its initial lower barrier properties, the 0Amine coating was less affected by immersion in the chloride solution and showed better corrosion protective ability than the higher amine cured coatings. The amino-silane (3A) cured coating initially presented the highest barrier properties, probably due to a more crosslinked organic and inorganic networks leading to a higher coating thickness (as estimated by the SEM/EDS analysis), but suffered the fastest degradation with immersion and at the end has practically lost its barrier ability. The same was observed for the DETA(1:1) cured coating. These coatings are the ones with the higher amine content that, according to FTIR analysis results, was not fully consumed in the epoxy ring opening reactions, what has turned these coatings more hydrophilic, thus prone to rapid degradation in water.
The EIS results are coherent with visual observation of the immersed coated aluminium alloy samples displayed in the photos taken after immersion in the chloride solution (Fig. 9).
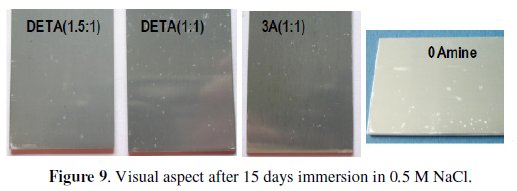
The high amine content coatings exhibit more pin-hole type defects, evidenced by the whitish stains resultant from the metallic substrate oxidation and incipient pitting corrosion development. These coatings also became yellowish with immersion time (not perceptible in the photos) what is a sign of their chemical degradation.
Conclusions
Epoxy-zirconia-silica sol-gel coatings were synthesized, deposited on aluminium EN AW 6063 alloy samples and cured at room temperature by the addition of amine crosslinking agents. The results obtained revealed that these coatings achieve appropriate barrier properties 24 h after coating deposition. Furthermore, these barrier properties improve significantly for longer curing times (12 weeks), showing that the curing process takes several weeks to complete. It was observed that the amount of amine added influenced the coatings protective properties, namely, their barrier properties. The ones with less amine require longer times to finish cure, but once cured, show the best anticorrosive performance with time. The best corrosion performance was achieved by a coating cured with DETA added in the GPTMS/N-Hreac molar ratio of 1.5:1. It was found that amine additions in the GPTMS/N-Hreac molar ratio of 1:1 were excessive, possibly because the zirconium alkoxide present in the coatings lowered the number of epoxy rings available to react. The amine in excess turned coatings more hydrophilic, which was detrimental to long term coating anticorrosive efficacy, as evidenced by the EIS results.
References
1. European Aluminium Association, Annual Report 2010 - Activity Report, EAA, 2011. [ Links ]
2. Meirsschaut S. ESTAL and the Challenges to the Surface Treatment on Aluminium as a result of European Legislation, ESTAL Congress Preparing Aluminium for the Future, Krakow, Poland, 25th-27th September, 2013. [ Links ]
3. Twite RL, Bierwagen GP. Prog Org Coat. 1998;33:91-100. [ Links ]
4. Palanivel V, Zhu D, van Ooij WJ. Prog Org Coat. 2003;47:384-392. [ Links ]
5. Zheludkevich ML, Salvado IMM, Ferreira MGS. J Mater Chem. 2005;15:5099-5111. [ Links ]
6. Rosero-Navarro NC, Pellice SA, Castro Y, et al. Surf Coat Tech. 2009;203:1897-1903. [ Links ]
7. Zheludkevich ML, Serra R, Montemor MF, et al. Electrochim Acta. 2005;51:208-217. [ Links ]
8. Raps D, Hack T, Wehr J, et al. Corros Sci. 2009;51:1012-1021. [ Links ]
9. Fedel M, Olivier M, Poelman M, et al. Prog Org Coat. 2009;66:118-128. [ Links ]
10. Lamaka SV, Zheludkevich ML, Yasakau KA, et al. Prog Org Coat. 2007;58:127-135. [ Links ]
11. Tavandashti NP, Sanjabi S. Prog Org Coat. 2010;69:384-391. [ Links ]
12. Alvarez P, Colazzo A, Hernandez M, et al. Prog Org Coat. 2010;67:152-160. [ Links ]
13. Feng Z, Liu Y, Thompson GE, et al. Electrochim Acta. 2010;55:3518-3527. [ Links ]
14. Gupta G, Pathak SS, Khana AS. Prog Org Coat. 2012;74:106-114. [ Links ]
15. Metroke TL, Kachurina O, Knobbe ET. Prog Org Coat. 2002;44:295-305. [ Links ]
16. Oliver MS, Blohowiak KY, Dauskardt RH. J Sol-Gel Sci Techn. 2010;55:360-368. [ Links ]
17. Davis SR, Brough AR, Atkinson A. J Non-Cryst Solids. 2003;315:197-205. [ Links ]
18. Vreugdenhil AJ, Balbyshev VN, Donley MS. J Coating Technol. 2001;73:35-43. [ Links ]
19. Khramov AN, Balbyshev VN, Voevodin NN, et al. Prog Org Coat. 2003;47:207-213. [ Links ]
20. Pathak SS, Khanna AS. Prog Org Coat. 2008;62:409-416. [ Links ]
21. Pathak SS, Khanna AS, Sinha TJM. Prog Org Coat. 2007;60:211-218. [ Links ]
22. Roussi E, Tsetsekou A, Tsiourvas D, et al. Surf Interface Anal. 2010;42:299-305. [ Links ]
23. EN 573-3 -Aluminium and aluminium alloys -Chemical composition and form of wrought products -Part 3:Chemical composition, (2003) CEN, Brussels. [ Links ]
24. Ivanova Y, Gerganova TS, Dimitriev Y, et al. Thin Solid Films. 2006;515:271-278. [ Links ]
25. Bae J-Y, Yang SC, Jin JH, et al. J Sol-Gel Sci Techn. 2011;58:114-120. [ Links ]
26. An infrared Spectroscopy Atlas for the Coatings Industry - 4th Ed., Vols. I and II, D. R. Brezinski Ed., Federation of Societies for Coatings Technology, Philadelphia, 1991. [ Links ]
27. Giresh KB, Jena KK, Allauddin S, et al. Prog Org Coat. 2010;68:165-172. [ Links ]
28. Pantoja M, Dıaz-Benito B, Velasco F, et al. Appl Surf Sci. 2009;255:6386-6390.
29. Hoebbel D, Nacken M, Schmidt H. J Sol-Gel Sci Techn. 2000;19:305-309. [ Links ]
30. Zheludkevich ML, Poznyak SK, Rodrigues LM, et al. Corros Sci. 2010;52:602-611. [ Links ]
*Corresponding author. E-mail address: rfontinha@lnec.pt
Received 3 December 2013; accepted 30 December 2013














