Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.31 no.4 Coimbra ago. 2013
https://doi.org/10.4152/pea.201304221
Inhibition of Copper Corrosion in 2 M HNO3 by the Essential Oil of Thyme Morocco
S. Houbairia,* , M. Essahlia and A. Lamiria
University Hassan 1st, Faculty of Science and Techniques, Laboratory of Applied Chemistry and Environment, B.P. 577, Settat, Morocco.
Abstract
The main objective of this work is to study the behavior of copper corrosion in nitric acid medium (2 M) for the evaluation and comparison of the corrosive power of the essential oil of Thymus Satureoides. To do this, we used weight loss and polarization techniques. The results show that the recovery rate of copper corrosion decreases in the presence of the essential oil tested. In a second step, we performed tests on the major component of the oil and the results obtained showed that the activity of the essential oil of thyme is clearly not related to it's major constituent. On the other hand, it is clear from this study that the inhibition efficiency increases with the concentration of inhibitors to reach 89.04% at 1200 ppm for the essential oil of thyme and 69.72% at 1600 ppm for borneol. The adsorption isotherm and the activation energy were also determined.
Keywords: copper, corrosion, inhibition, essential oil of Thymus Satureoides, natural inhibitor.
Introduction
Corrosion is due to an electrochemical or chemical action of the environment on metals and alloys. This has important implications in various fields, especially in industries. Replacement of corroded parts, accidents and pollution risks are frequent events which sometimes have severe economic impacts.
Corrosion inhibitors constitute means of protection against metal corrosion. They have the distinction of being the only intervention in the corrosive environment, making it a control method easy and inexpensive to implement. For fifty years, numerous studies on these compounds have led to offer products or mixtures of specific products corresponding to the given corrosion systems (metal-corrosive environment couple). Each case of corrosion, however, remains a special one. In general, for each material there is a family of inhibitors conducive to a satisfactory corrosion protection.
In the case of copper, several studies have examined the behavior of this metal and its alloys in aggressive media in the presence of organic inhibitors containing hetero atoms [1-7].
However, these products are generally obtained by chemical synthesis and therefore have a negative impact on the environment. This has led many researchers around the world to use new non-polluting natural molecules to the environment.
In addition, several studies have been made to study the inhibitory effect of various natural substances against the corrosion of materials, particularly in the steel industry: oil of wormwood [8-9-10], eucalyptus [11], aqueous extracts of coffee [12], the essence of black cumin [13], aqueous extracts of fruit peel [14], extract Khillah [15] pulegone [16], oil of L. angustifolia Lavender [17], essential oil of cedar [18], rosemary [19], limonene [20]. The encouraging results obtained by natural compounds as inhibitors of steel corrosion in acidic solutions led us to consider other natural substances against corrosion of copper.
The aim of this study is to compare the inhibitory effect of essential oil of thyme on the corrosion of copper in 2 M HNO3 and search the compound responsible for the activity obtained by gravimetric measurements and polarization.
Experimental conditions
Inhibitor
Extraction of the essential oil
The essential oil was obtained by steam distillation of water using a Clevengertype distiller for 2 hours and 30 minutes. The yield of essential oil of Thymus Satureoides is 1.1%. The essential oil yield was calculated on the basis of the dry matter.
After extraction, a portion of the oil was used for analysis of the chemical composition; the other part was used for tests of anti-corrosion activity. The oil, after extraction, was recovered and stored in a dark bottle at 4 °C before use. The borneol was provided by the company SOMAPROL.
Study of the chemical composition and identification of compounds
The thyme essential oil was analyzed by gas phase chromatography coupled with mass spectrometry.
Corrosive solution
A 2 M HNO3 nitric acid solution obtained by diluting concentrated acid 67% brand SIGMA-ALDRICH with distilled water has been used. The environment is not deaerated.
Gravimetric tests
Gravimetric tests were performed maintaining the desired temperature of the electrolyte with a thermostat brand FRIGITHERM with an error of de 0.1 °C. The electrolyte volume was 30 mL. The samples have rectangular form with surface areas of 9.6 cm2 with an error of 2 mm. Prior to measurements, they undergo a mechanical polishing with abrasive paper of up to 1200 increasing size, followed by degreasing with acetone, washing with distilled water and drying in air. Each value of the gravimetric test is the average of at least three tests.
Electrochemical measurements
The electrochemical experiments were performed in a pyrex cell with three electrodes: copper (1 cm 2) as working electrode, platinum as an auxiliary electrode and a saturated calomel electrode, SCE, as a reference electrode. The current-potential curve is obtained by operating in potentiodynamic mode; the potential applied to the sample varies continuously with a scanning rate of 30 mV / min. We chose a relatively low rate of scanning so as to remain in a quasi- stationary regime. The measurements are performed with an assembly comprising a PGZ100 potentiostat-galvanostat, associated with "voltamaster4" software. Before curve plot, the working electrode is maintained at a potential of -800 mV for 15 minutes. The tests were carried out maintaining the temperature at 25 °C ± 0.1 °C of the electrolyte with a thermostat FRIGITHERM mark.
Results and discussion
Analyzing the chemical composition of the essential oil
The essential oil of thyme Thymus Satureoides belongs to the botanical family lipped and has the following major components: borneol (35.9%) (Fig. 1), carvacrol (17.8%), camphene (10.2%), α-thujone (2.4%) and α-terpineol (0.6%).
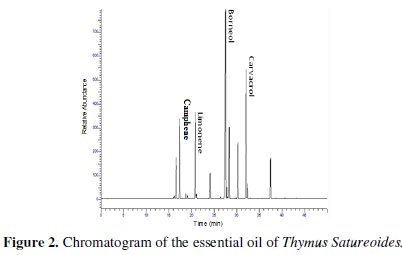
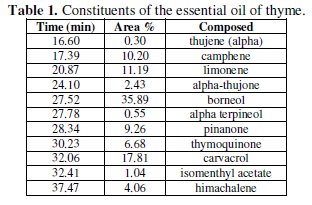
Analysis of the chemical composition of the essential oil was performed by gas chromatography coupled with mass spectrometry. The chromatogram obtained is shown in Fig. 2 the retention times and the relative percentages of the various constituents of the essential oil are shown in Table 1.
Weight loss measurement
The corrosion rate of the copper is determined by weight loss measurement after 1 hour immersion in 2 M HNO3 with and without addition of inhibitors at different concentrations. The inhibition efficiency (IE %) of the compounds is calculated from the following equation:

where W0 and W are, respectively, the corrosion rates of copper in 2 M HNO3 without and with addition of the compound tested. The results of the study are summarized in Table 2 for the case of essential oil and in Table 3 for the case of borneol.
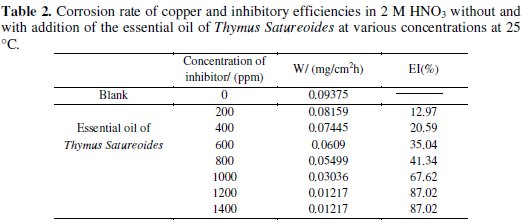
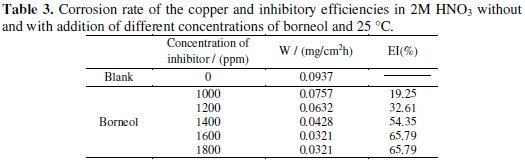
From analysis of the two tables, we can see that the increase of the concentration of the inhibitors is accompanied by a decrease in the corrosion rate. This decrease is significant even at low concentrations (600 ppm) for the essential oil. The inhibition efficiency reached 87.02% at 1200 ppm for the essential oil of Thymus Satureoides, and 65.79% at 1600 ppm for the major product (borneol) of this essential oil. Hence we can say that the inhibitory effect of the essential oil of Thymus Satureoides is not due to its main chemical component (borneol) alone, but to a synergy between its different chemical compounds.
This decrease in corrosion rate (w) of copper is probably due to the adsorption of these compounds on the metal surface [21]. This behavior could be attributed to the strong interaction of the inhibitors with the metal surface [22].
Electrochemical measurements
Case of the essential oil of Thymus Satureoides
The polarization behavior of copper in 2 M HNO3 with and without addition of the inhibitor is shown in Fig. 3.
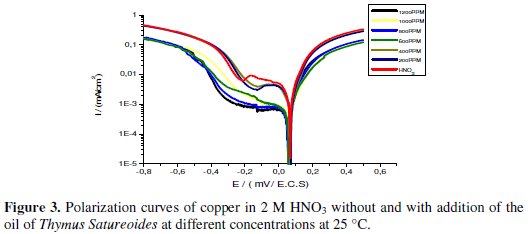
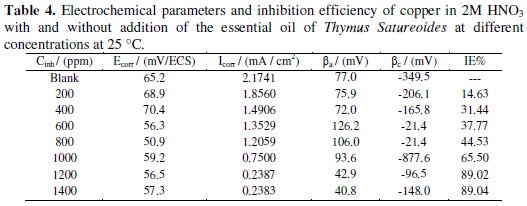
The electrochemical parameters, values of corrosion current (Icorr), corrosion potential (Ecorr), cathodic Tafel slope (βc), anodic Tafel slope (βa) and the efficiency of inhibition (IE %) are given in Table 4.
The inhibition efficiency IE% of the compounds tested is defined by the following equation:

where Icorr and I'corr, respectively, represent corrosion current densities determined by extrapolation of the straight Tafel corrosion potential without and with addition of the inhibitor.
Examination of Fig. 3 and Table 4 allows us to conclude that the addition of the tested compound causes a slight shift of the corrosion potential but with a tendency towards the cathode values. This displacement is accompanied by a net decrease of the densities of anodic and cathodic current which is more marked when the concentration of the inhibitor increases until a critical concentration at which the value of 0.2387 mA / cm2 is obtained, corresponding to an efficiency of 89.02%.
This decrease of the current can be explained by the inhibiting action of this inhibitor, caused by the adsorption of the chemical compounds of the essential oil on the surface of the active electrode sites, creating a barrier that slows the dissolution of copper in the anodic sites and the release of hydrogen by blocking the hydrogen reduction at the cathodic sites.
This result suggests that the reaction mechanism is virtually unaffected by the addition of the inhibitor. In the light of these results we noted the mixed nature of the inhibitor used with predominant cathodic effectiveness [23].
Case of borneol
The polarization behavior of copper in 2 M HNO3 with and without addition of the inhibitor is shown in Fig. 4.
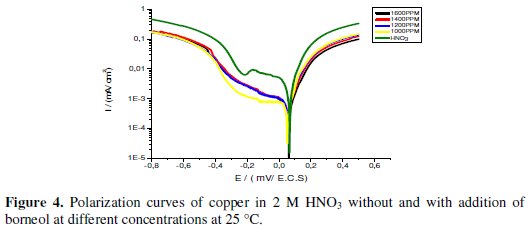
The electrochemical parameters, values of corrosion current (Icorr), corrosion potential (Ecorr), cathodic Tafel slope (βc), anodic Tafel slope (βa) and the efficiency of inhibition (IE %) are given in Table 5.
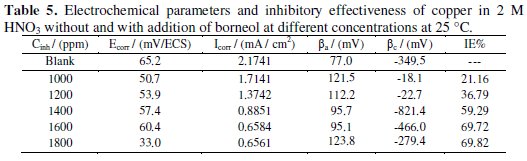
The analysis of Fig. 4 and Table 5 shows that the addition of this inhibitor results in a slight shift of the corrosion potential with a tendency towards cathodic values. This shift accompanied by a decrease in the cathodic and anodic current is due to the inhibitory action of a mixed character of borneol. And the inhibition efficiency calculated by electrochemical measurement of the essential oil (Thymus Satureoides 89.02% to 1200 ppm) is higher than that measured in the presence of its major product alone (69.72% borneol to 1600 ppm).
Following these findings, we note that the results relating to gravimetric measurements and those relating to electrochemical measurements are in good agreement.
Adsorption isotherms
The adsorption isotherms are very important for understanding the mechanism of the electrochemical reactions of metals [24]. Knowledge of the type of adsorption and the determination of the thermodynamic parameters characterizing the adsorption often helps to elucidate the mode of action of these inhibitors.
The most frequently used isotherms are Langmuir, Frumkin and Temkin ones [25].
The recovery rate (Θ) at different concentrations for the inhibitors tested in 2 M HNO3 was assessed from measurements of weight loss.
The curve representing ln (Θ / Θ-1) as a function of ln [C], where C is the concentration of the inhibitor, is a straight line (Fig. 5) for the essential oil of Thymus satureoides, and (Fig. 6) for borneol, indicating that the adsorption of the two inhibitors on the surface of the copper plate is made according to the Langmuir model:

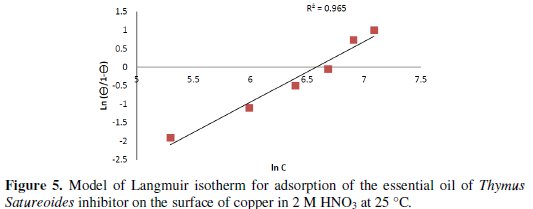
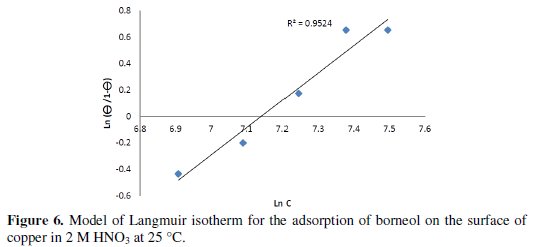
The strong correlation (R2 = 0.965 for the essential oil of Thymus Satureoides and R2 = 0.952 for borneol) for the plot of the Langmuir adsorption isotherm tested inhibitors confirms the validity of this approach.
Based on the Langmuir isotherm model, it is possible to approach to the representation of adsorption phenomena, which is based on three assumptions, explaining that the adsorption is localized and does not give rise to the formation of a monolayer, and all sites are equivalent and the surface is uniform, and that there is no interaction between the adsorbed molecules. This allows to consider a constant adsorption energy [25].
Effect of temperature
It is well known that increasing the temperature always aggravates the problem of corrosion in the absence of an effective inhibitor which must necessarily be stable and resistant to both the increase in the concentration of the acid and the rise of temperature.
The study of the influence of temperature on the rate of inhibition leads to several information including the mechanism of inhibition and activation energy. Given the importance of this factor, we conducted trials of weight loss of copper in 2 M HNO3 with the addition of the essential oil of Thymus satureoides and of borneol at different temperatures between 10 and 40 °C. The values of the rate corrosion and inhibitory efficacy as a function of temperature are given in Table 6.
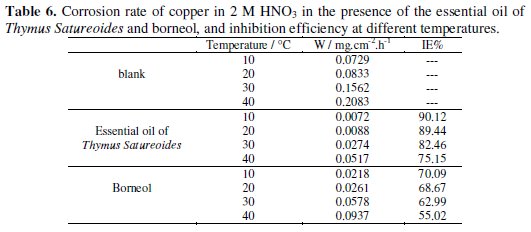
The overall results showed that the corrosion rate of copper increases with temperature. However, the inhibition efficiency is changing in the opposite direction for the two cases. This implies that these inhibitors are adsorbed on the substrate by electrostatic bonds (weak bonds). This type of temperature-sensitive links cannot fight effectively against corrosion when the temperature increases [26].
In the case of the acid corrosion, many authors [27] use of the Arrhenius equation to account for the effect of temperature (T) on the rate of corrosion and therefore believe that the logarithm of the rate of corrosion W is a linear function of T-1. We can calculate the activation energy from the following relationship:
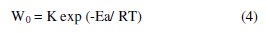
and
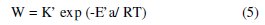
where K and K' are constants (Arrhenius pre-exponential parameter), and the Ea and E'a activation energies, respectively in the absence and presence of the inhibitor.
Fig. 7 shows the Arrhenius plot of coordinates in the corrosion rate of copper in HNO3 in the absence and presence of the essential oil of Thymus satureoides at 1200 ppm and at 1600 ppm of borneol.
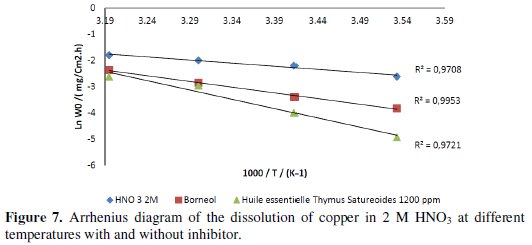
The variation of the logarithm of the corrosion rate as a function of T-1 in the three cases (Fig. 7) provides lines showing that Arrhenius law is enforced. The values of the activation energy obtained from straight lines are given in Table 7.

From the results of Table 7 it is clear that in the presence of each of the two inhibitors tested in solution, Ea increases. It is observed that the activation energy increases with the addition of the inhibitor for the two inhibitors tested, while the IE% decreases as the temperature increases. This behavior is reported as a characteristic phenomenon of physisorption inhibition to the metal surface. The recovery rate, lower at higher temperatures, suggests that at these temperatures the rate of destruction of the film physically adsorbed increases faster than its rate of formation.
This phenomenon can also be explained by the fact that the process of corrosion of copper in the presence of the inhibitor does not depend only on the reaction that takes place on the surface of bare metal, but also on the dissemination of Cu2+ ions through the layer of the adsorbed inhibitor [26-27].
The value of activation energy derived from the Arrhenius straight is 19.66 kJ.mol-1 in the absence of the inhibitor; this value is in agreement with the literature [28], and at a concentration of 1200 ppm for essential oil and 1600 ppm for borneol, i.e., when the rate of recovery is maximum, the value of the activation energy in the presence of this oil is 58.93 kJ.mol-1, while it is only 36.21 kJ mol-1 for borneol alone. This confirms that the physical adsorption of the essential oil of Thymus Satureoides is stronger than that of borneol, noting that its action is due to a synergy of its chemical compounds and the formation of a more adherent surface film thus making it more effective than that formed in the presence of its major constituent alone.
General discussion
Addition of this inhibitor in a corrosive nitric medium (2 M), results in a significant reduction of the corrosion rate.
Moreover, the hydrogen overvoltage may decrease or increase depending on the nature of the metal, the electrolyte, and polarization conditions [29]. In this case the hydrogen overvoltage of copper is much greater in the presence of the inhibitor.
The essential oil of thyme can thus be considered among the inhibitors of corrosion which provoke the increase in the overvoltage of hydrogen [30], leading to the blockage of the cathodic reduction of hydrogen.
Furthermore, the influence of the inhibitor on the nobility of the corrosion potential is due to the acceleration of the formation of the protective layer into the nitric acid medium.
The comparison of electrochemical results obtained in this study shows that the addition of the essential oil of Thymus Satureoides has a more pronounced effect than that of its major constituent, borneol alone.
Also, we found that the maximum efficiency of 89.02% is achieved at 1200 ppm of the total oil, while it is only 69.72% at a higher concentration of 1600 ppm in the case of borneol.
Moreover, with the same concentrations, the corrosion current in the presence of borneol decreases to a value of 0.6561 mA/cm2, remaining a value much higher than that obtained with the total essential oil which is in the order of 0.2383 mA/cm2.
Regarding the Tafel slopes, in the presence of inhibitors, their values change, but this does not necessarily mean a change in the mechanism of the reaction. Indeed, when the recovery rate increases with the inhibitor concentration, the active sites electrode is reduced and the adsorbed film may have an ohmic behavior, which is manifested by a change in the value of βc and βa. So while having a good corrosion inhibiting efficiency which slows down the corrosion process, the reduction of nitric acid, the release of hydrogen and the dissolving of copper following the same mechanism takes place in the absence of the inhibitor at the free sites of the metal.[29]
Conclusions
Based on the above results, the following conclusions can be drawn:
• The essential oil of Thymus Satureoides proved to be an effective inhibitor for the corrosion of copper in 2M HNO3. The study also showed that this activity is mainly due to a synergy between the chemicals constituting the Thymus Satureoides oil, and not just to its major constituent (borneol) alone.
• The effectiveness of inhibitors increases with the inhibitor concentration to reach 89.02% at 1200 ppm for the essential oil of Thymus Satureoides, and 69.72% at 1600 ppm for the major constituent alone.
• The displacement of the value of the corrosion potential with electrochemical results shows that the inhibitors tested act as mixed type inhibitors with predominant cathodic effectiveness for the essential oil.
• The increase in temperature affects the inhibitory efficacy, as it decreases with the temperature increase.
• The two inhibitors are physisorbed onto the surface of copper according to the Langmuir isotherm model.
References
1. Fouda A S, Gomah S, Moussa M N. Corr Prot Mater. 2003;22:21. [ Links ]
2. Lee W J. Mater Sci Eng A. 2003;348:217. [ Links ]
3. Mostafa H A, Zaghloul E I, Moussa M N. Port Electrochim Acta. 2002;20:63. [ Links ]
4. Mostafa H A, El-Maskoud S A A, Moussa M N H. Port Electrochim Acta. 2001;19:109. [ Links ]
5. El-Maskoud S A A, El-Shafei A A, Mostafa H A, et al. Mater Corros. 1992;46:468. [ Links ]
6. Chakraborty S B, Bandyopadhyay T K, Chaudhuri S R. Bull Electrochem. 1992;8:111. [ Links ]
7. Fouda A S, Mohamed A K. J Electrochem Soc India. 1990;39:244. [ Links ]
8. Benabdellah M, Benkaddour M, Hammouti B, et al. Appl Surf Sci. 2006;252:6212. [ Links ]
9. Bouyanzer A, Hammouti B. Pigment Resin Tech. 2004;33:287. [ Links ]
10. Ouachikh O, Bouyanzer A, Bouklah M, et al. Surf Rev Lett. 2009;16:49. [ Links ]
11. Bouyanzer A, Majidi L, Hammouti B. Bull Electrochem. 2006;22:321. [ Links ]
12. Torres V V, Amado R S, Sa C F, et al. Corros Sci. 2011;53:2385. [ Links ]
13. Abdallah M, Al Karanee S O, Fatah A A A. Chem Eng Comm. 2010;197:1446. [ Links ]
14. Rocha J, Gomes J C P, Elia E. Corros Sci. 2010;52:2341. [ Links ]
15. El-Etre A Y, Khillah. Appl Surf Sci. 2006;252:8521. [ Links ]
16. Faska Z, Bellioua A, Bouklah M, et al. Monatsh Chem. 2008;139:1417. [ Links ]
17. Halambek J, Berkovic K, Vorkapic-Furac J. Corros Sci. 2010;52:3978. [ Links ]
18. Bouyanzer A, Majid L, Hammouti B. Phys Chem News. 2007;37:70. [ Links ]
19. Bendahou M, Benabdellah M, Hammouti B. Pigment Resin Tech. 2006;95. [ Links ]
20. Chaieb E, Bouyanzer A, Hammouti B, et al. Acta Phys Chim Sin. 2009;25. [ Links ]
21. Muralidharan S, Phani K L N, Pitchumani S, et al. J Electrochem Soc. 1995;142:1478. [ Links ]
22. Chaudhary R S, Sharma S. Indian J Chem Technol. 1999;6:202. [ Links ]
23. Fouda A S, Wahed H A. Arab J Chem. 2011. [ Links ]
24. Hackerman N, McCafferty E. Proceedings of the 5th International Congress on Metallic Corrosion. Houston, TX; 1974. p. 542. [ Links ]
25. Bilgic S, Caliskan N. Appl Surf Sci. 1999;152:107. [ Links ]
26. Fouda A S, Mohamed A K. J Electrochem Soc India. 1990;39:244. [ Links ]
27. Fiala A. These d'etat. Universite Mentouri-Constantine; 2007. [ Links ]
28. Mostafa A B. Corros Prevention Control. 1980; 70. [ Links ]
29. L'hydrogene dans les aciers. http://thesis.sangoru.com/these_docs/I_hydrogen_LR_these.pdf. [ Links ]
30. Schmidt R. Presses polytechniques et universitaires romandes. 1999. [ Links ]
31. Etude cinetique. http://sotofab.free.fr/theseIII.html. [ Links ]
32. Bilel M. These d'etat. Universite Mentouri-Constantine; 2011. [ Links ]
*Corresponding author. E-mail address: sara.houbairi@gmail.com
Received 30 July 2013; accepted 31 August 2013














